Abstract
It has been claimed that inflorescences of Scabiosa tschiliensis Grunning (ST) may prevent liver diseases because of its higher chlorogenic acid. There was scant information on the phytochemical profiles and antioxidant activities of the whole plant from different growing stages. The changes of active-compounds and antioxidant activities of ST from three growing stages were studied. Total phenolic and flavonoid contents were analyzed and ranged from 0.00 to 140.03 mg GAE/g and 9.10 to 460.01 mg RE/g, respectively. The pre-flowering stage ethyl acetate (PFSEA) fraction of ST appeared to contain the highest content of chlorogenic acid, and demonstrated the highest DPPH radical-scavenging activity with the IC50 value of 8.47 ± 0.23 µg/mL which was nearly equal to the IC50 value of vitamin C (7.60 ± 0.61 µg/mL). Principal component analysis suggested that the PFSEA fraction of ST might be a desirable antioxidant natural resource due to the highest potential antioxidant properties.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2666-9) contains supplementary material, which is available to authorized users.
Keywords: Scabiosa tschiliensis Grunning, Different growing stages, Antioxidant activity, Phenolics, Principle component analysis
Introduction
Owing to the existence of phenolics and flavonoids, which have multiple pharmacological activities for their antioxidant abilities, plants have been used as good resources of natural antioxidants (Cai et al. 2004; Lin et al. 2011). For the wide distribution, broad diversity and low toxicity, natural antioxidants in traditional Chinese medicine plants have become a hot topic recently (Andrade et al. 2011; Qin et al. 2008). For instance, rosemary and aloe extracts are used in food industry as commercial antioxidants, due to the presence of abundant phenolics (Hu et al. 2003; Kuhlmann and Röhl 2006).
Scabiosa tschiliensis Grunning, belonging to the Dipsacaceae family, grows on the mountains at an altitude of 300 to 1500 meters. In China, S. tschiliensis is widely distributed in the west of Hebei Province and the Inner Mongolia autonomy district. Wang et al. (2013) reported that properties of Scabiosa genus were anti-inflammation, antioxidant and enhancement of the immune and cardiovascular system. The flowers have been used to treat liver toxicity, fever, headache and other diseases in Inner Mongolia (Anonymous 1977; Wang et al. 2013).
Ma et al. (2015) reported the bioactivities and chemicals of the inflorescences of S. tschiliensis, however there was no research on antioxidant activities of whole plant of the S. tschiliensi. As we all know, the contents of effective constituents were influenced by growing stage and extraction solvent. Therefore it is necessary to estimate the quality of S. tschiliensis from different growing stages and extraction solvents. This variation may help in the development of S. tschiliensis as a potential antioxidant resource. Quantitative analysis of effective constituents in S. tschiliensis samples collected from three stages and five extraction solvents were studied. The total phenolic and flavonoid contents, inhibiting of lipid peroxidation and radical-scavenging activities were also determined. The active chemicals and antioxidant properties of the S. tschiliensis samples were used as descriptors of principal component analysis (PCA) for differentiating the analyzed S. tschiliensis samples.
Materials and methods
Samples
Scabiosa tschiliensis (whole plants) were gathered from Lingshan (1400–1500 msl) in the Beijing area of China, from June to October 2008, and identified by Prof. Lin Yang (College of Life and Environmental Sciences, Minzu University, China). Fresh samples were dried at ambient temperature and ground into fine powder using laboratory mill (FW100, Taisite Instrument Co. Ltd., Tianjin, China).
Chemicals
Quercitrin, ferulic acid, chlorogenic acid, isorhamnetin, vanillic acid, icariin, caffeic acid, rutin, L-epicatechin, quercetin, 2,2′-azinobis(3-ethyl-benzothiazoline-6-sulphonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma (St. Louis, MO, USA). Ascorbic acid, dimethyl sulphoxide (DMSO), thiobarbituric acid (TBA), Folin–Ciocalteu reagent, trichloroacetic acid (TCA) and other reagents were purchased from Beijing Chemical Works (Beijing, China). All the solvents for HPLC use were of HPLC grade.
Sample extraction and fractionation
The milled whole plant of S. tschiliensis during pre-flowering stage (300 g) was extracted with 95% ethanol (5 L) for 7 days at room temperature (four times). The extract was decanted, filtered and concentrated in a rotary evaporator. The ethanol extract (EE) was further to be isolated according to our previous study (Liu et al. 2013) and the resulting four fractions were water (WA), n-butanol (NB), ethyl acetate (EA) and petroleum ether (PE) soluble fractions. These fractions were stored in the dark (4 °C) until processing. Preparation procedures of S. tschiliensis during the other two stages were consistent with those of S. tschiliensis during pre-flowering stage (PFS). The yield was determined as follows:
| 1 |
Total phenolic content
The total phenolic content (TPC) was carried out in accordance with our previous study (Liu et al. 2013) TPC was calculated using a standard curve of gallic acid and expressed as mg gallic acid.
Total flavonoid content
The total flavonoid content (TFC) was analyzed according to our previous study (Guo et al. 2015). TFC was calculated using a standard curve of rutin and showed as mg rutin equivalents/g of dry extract (mg RE/g).
HPLC analysis of phenolic compounds
Samples were analyzed using an analytical HPLC unit (Shimadzu) equipped with a Waters 1525 pump, a Waters 2996 PAD detector and a BDS HYPERSIL C18 column (5 µm particle size, 250 × 4.6 mm i.d. Shimadzu, Japan), material and thermostated at 25 °C. The mobile phase were solvent A (acetonitrile) and solvent B (water, 2.0% formic acid). The gradient conditions were: 95–90% B in 0–20 min; 90–70% B in 20–25 min; 70–70% B in 35–50 min; 70–95% B in 50–55 min; 95–95% A in 55–60 min. Flow was adjusted to 1.0 mL/min and the injection volume was 10 μL. Chromatographic data were recorded at 280 nm. Spectral data from all peaks were collected from 200 to 600 nm. Phenolic compounds quantification was obtained by external standard calibration curves using authentic standards.
DPPH assay
The assay was carried out in accordance to our previously study (Liu et al. 2013). Results were expressed as IC50 values, which is defined as the concentration required to scavenge 50% of DPPH free radicals. The inhibition percentage (I%) of DPPH radical-scavenging activity of each sample was determined with the equation:
| 2 |
where A c is the absorbance of the control reaction, and A t is the absorbance of the test sample. All samples were analysed in triplicate.
Hydroxyl radical-scavenging activity assay
The assay was carried out in accordance to our previously study (Liu et al. 2013). The inhibition percentage (I%) of OH radical-scavenging activity of each sample was calculated with the Eq. (2).
ABTS assay
In accordance with the method of Gursoy et al. (2009) with a slight variation, ABTS radical-scavenging activity was determined. 7.0 mM ABTS and 2.45 mM K2S2O8 were mixed to prepare the stock solution and stored for 16 h (25 ± 2 °C) in the dark. Then, the solution was diluted until an absorbance was 0.70 ± 0.02 at 734 nm. 150 μL ABTS solution was mixed with 50 μL of sample solution at difference concentrations. After reacting for 30 min (25 ± 2 °C), the absorbance was measured (734 nm). The inhibition percentage (I%) of ABTS radical-scavenging activity of each sample was calculated with formula (2).
Inhibition of lipid peroxidation
The Inhibition of lipid peroxidation assay was performed in accordance with the method reported by a previous study (Tai et al. 2011) with some modification. Mice (25–30 g) were fasted for 16 h (25 ± 2 °C; humidity: 40–60%), and decapitated. 10% homogenate (8.5 mgprot/mL) of liver tissues were prepared. 200 μL of homogenate was mixed with 100 μL of sample solution and 100 μL of 250 μ M freshly prepared FeSO4. The mixture was fostered for 1 h (37 °C). Then, 1.0 mL (0.67%, w/v) of TBA and 1.0 mL of TCA (15%, w/v) were added to the mixture, boiling in a water bath for 40 min and centrifuging at 3500 rpm for 10 min. The absorbance was measured at 532 nm. The inhibition percentage (I%) of lipid peroxidation activity was calculated with the Eq. (2).
Statistical analysis
Values are given as the mean values ± standard deviation of three measurements. One-way ANOVA was performed to test difference, followed by Tukey’s HSD Test. Significance was considered with p ≤ 0.05. Pearson’s correlation test and principal comment analysis (PCA) were also performed. These analyses were carried out using SPSS v. 22.0 program (IBM Corp., Armonk, NY, USA).
Results and discussion
Extraction yields
The crude extracts yields of different materials from S. tschiliensis were in the order of: pre-flowering stage (PFS) > flowering stage (FLS) > fruiting stage (FRS) (Table 1). Among solvent-partitioned fractions, the WA fraction from S. tschiliensis (FLS and FRS) showed the highest yield followed by the NB, EA and PE fractions. Comparative to the other fractions, the NB fractions from S. tschiliensis (PFS) showed higher yield. It indicated that the contents of the crude extracts were distributed unevenly among different growing stage and the mainly constituents of S. tschiliensis were polar compounds.
Table 1.
Yields of crude extract as % (w/w) of dried different materials and solvent-partitioned fractions as % (w/w) of crude extract from S. tschiliensis
| Samples | Yields (%)a | ||
|---|---|---|---|
| PFS | FLS | FRS | |
| Crude extract | 17.44 ± 0.81 | 15.88 ± 0.54 | 13.47 ± 0.35 |
| PE fraction | 4.72 ± 0.26 | 6.77 ± 0.63 | 10.01 ± 0.43 |
| EA fraction | 20.21 ± 065 | 16.92 ± 0.75 | 17.75 ± 0.34 |
| NB fraction | 39.90 ± 0.54 | 37.38 ± 0.94 | 24.35 ± 0.54 |
| WA fraction | 35.17 ± 0.93 | 38.53 ± 0.62 | 47.9 ± 0.32 |
PFS pre-flowering stage, FLS flowering stage, FRS fruiting stage
aValues are the mean ± standard deviation (n = 3)
Total flavonoid and phenolic contents
As we all know, phenolic and flavonoid compounds play an important role in the antioxidant capabilities of plants (Sarikurkcu et al. 2010). The relationship between anti-oxidation effects and TFC and TPC of the crude extracts and solvent-partitioned fractions of S. tschiliensis were analyzed.
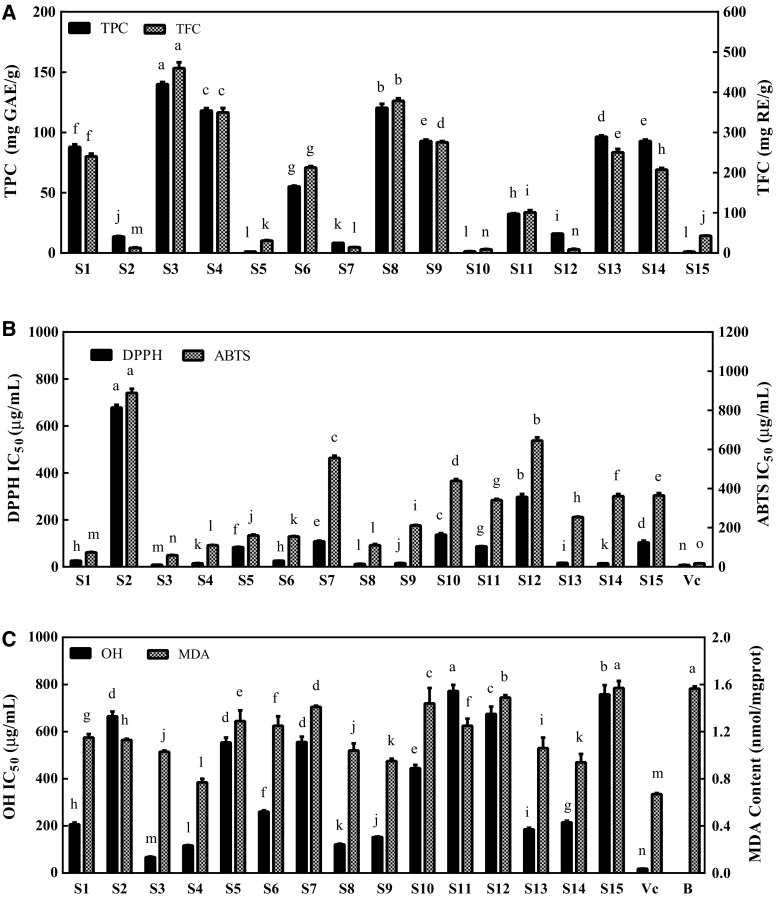
TPC values are displayed in Fig. 1a. TPC values of the S. tschiliensis were distributed unevenly among different growing stage. The highest amount of the TPC in the S. tschiliensis was found in the PFS (88.02 mg GAE/g), which decreased significantly (p < 0.05) in the other two growing stages (FLS and FRS) to 55.21 and 32.43 mg GAE/g. TPC of the solvent-partitioned fractions of S. tschiliensis were ranged from 0.00 to 140.03 mg GAE/g. The result demonstrated that the decreasing order of TPC values in the S. tschiliensis at three growing stages was EA fraction, NB fraction, crude extract, PE fraction and WA fraction. Tai et al. (2011) also demonstrated that TPC of other fractions were lower than that of EA fraction from the edible flower of Sophora viciifolia.
Fig. 1.
Antioxidant activity of different fractions derived from S. tschiliensis during three stages are assessed using Total phenolic and flavonoid content assays (a), DPPH and ABTS scavenging activity assay (b), OH scavenging activity assay and MDA content (c). Data represent the mean ± SD (n = 3). Values of the various sampless with the same letter (a, b, c, etc.) are not significantly different (p > 0.05), tested using Least Significant Difference (LSD) test. Abbreviations: total phenolic content (TPC), total flavonoid content (TFC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydroxide radical (OH), 2,2′-azinobis(3-ethyl-benzothiazoline-6-sulphonic acid) (ABTS), malondialdehyde (MDA). Vc, ascorbic acid; B, blank; S1, PFSEE; S2, PFSPE; S3, PFSEA; S4, PFSNB; S5, PFSWA; S6, FLSEE; S7, FLSPE; S8, FLSEA; S9, FLSNB; S10, FLSWA; S11, FLSEE; S12, FRSPE; S13, FRSEA; S14, FRSNB; S15, FRSWA
The data from Fig. 1a also showed that the TFC of crude extracts from the S. tschiliensis were ranged from 101.23 to 240.61 mg RE/g, in the same order as TPC, and the TFC of the solvent-partitioned fractions of S. tschiliensis varied from 9.1 to 460.0 mg RE/g. The TFC of the S. tschiliensis (FLS and FRS) increased in the same order as TPC: WA fraction < PE fraction < crude extract < NB fraction < EA fraction. However the sequence of TFC of the S. tschiliensi (PFS) was not in agreement with the sequence of TPC, and the WA fraction had slight higher levels of the TFC than the PE fraction. Among all the fractions, EA fractions showed the higher TFC than other fractions. The TFC of EA fraction from Spatholobus suberectus Dunn was much higher than that of other fraction (Cheng et al. 2011), which was in agreement with our finding in this study.
The results above implied that TPC and TFC of the crude extracts of the S. tschiliensis (PFS) were higher than those of crude extracts during the other two stages. Some studies in the literature (Gruz et al. 2011) also revealed that plants exhibited remarkable fluctuation in TPC at different stages. The S. tschiliensis (PFS) were collected in June at a high latitude site (1400–1500 m) that has the lowest annual average temperature among the three stages. Zhang et al. (2013) indicated that low temperature stress might increase antioxidant-activities and secondary metabolite content. The damage to cells might be caused by low temperature stress through perturbing the scavenging systems that quench oxygen and arousing reactive oxygen species (ROS). According to Pennycooke et al. (2005), the low temperature stress could cause the synthesis of certain phenolic compounds. Therefore the highest TPC and TFC of S. tschiliensis (PFS) may be caused by the low average temperature. A good correlation between the TPC and the TFC (r = 0.975, p < 0.001) was showed. It suggested that most phenolic compounds in the fraction of the S. tschiliensis are flavonoid compounds.
Constituents comparison of S. tschiliensis from different stages
HPLC analysis of the constituents of the S. tschiliensis samples showed 6 principal compounds presenting in plant extracts (Table 2). PFS, FLS and FRS showed similar chromatogram profile but varied in phytochemical contents. The most abundant phenolics found in the S. tschiliensis samples were chlorogenic acid, fumalic acid and rutin. Caffeic acid, icariin and quercitrin were at lower concentrations, and caffeic acid and quercitrin were not detected in FRS (Table 2). The highest content of chlorogenic acid was in PFS followed by FLS and FRS. The most abundant compound was fumalic acid from 10.30 ± 0.61 to 15.41 ± 0.84 mg/g. The second higher content component was rutin from 3.45 ± 0.16 to 15.02 ± 0.9 mg/g. Among samples at different stages, the PFS has the highest contents of chlorogenic acid, fumalic acid and rutin with 26.81 ± 0.62, 15.41 ± 0.84 and 15.02 ± 0.95 mg/g, respectively. The annual average precipitation and temperature of PFS are lower than those of other stage samples, which cause the accumulation of phenolic compounds. Chlorogenic acid, with higher content than other compounds (Table 2), might be the main active compound, and the content of chlorogenic acid in S. tschiliensis (PFS) is higher than the values found in the literatures (Mudge et al. 2016), which is also higher than the values in the inflorescences of S. tschiliensis reported by Ma et al. (2015). Thus, S. tschiliensis (PFS) is an important natural plant resource of chlorogenic acid.
Table 2.
The content of the active compounds in different Scabiosa tschiliensis samples
| Batch No | Samples | Chlorogenic acid | Caffeic acid | Fumalic acid | Rutin | Quercitrin | Icariin |
|---|---|---|---|---|---|---|---|
| S1 | PFSCE | 26.81 ± 0.62 | 5.80 ± 0.21 | 15.41 ± 0.84 | 15.02 ± 0.95 | 2.01 ± 0.01 | 7.70 ± 0.31 |
| S2 | PFSPE | – | – | – | – | – | – |
| S3 | PFSEA | 45.35 ± 2.63 | 5.84 ± 0.20 | 11.32 ± 0.61 | 54.8 ± 3.17 | 6.10 ± 0.11 | 8.21 ± 0.21 |
| S4 | PFSNB | 33.42 ± 1.12 | – | 22.85 ± 1.5 | 15.05 ± 0.60 | – | – |
| S5 | PFSWA | 9.15 ± 0.51 | – | – | – | – | – |
| S6 | FLSCE | 16.12 ± 0.90 | 5.93 ± 0.35 | 14.13 ± 0.65 | 8.2 0 ± 0.23 | 1.10 ± 0.09 | 6.80 ± 0.49 |
| S7 | FLSPE | 4.23 ± 0.04 | – | 8.24 ± 0.36 | 2.45 ± 0.24 | – | – |
| S8 | FLSEA | 26.42 ± 2.12 | 5.93 ± 0.25 | 12.12 ± 0.74 | 16.4 ± 0.61 | 5.51 ± 0.32 | 6.91 ± 0.37 |
| S9 | FLSNB | 32.35 ± 1.43 | 6.12 ± 0.30 | 21.61 ± 1.53 | 16.7 ± 0.83 | – | – |
| S10 | FLSWA | 5.6 1 ± 0.35 | – | – | – | – | – |
| S11 | FRSCE | 13.32 ± 0.55 | – | 10.3 ± 0.61 | 3.45 ± 0.16 | – | 7.71 ± 0.39 |
| S12 | FRSPE | 6.33 ± 0.35 | – | 7.8 1 ± 0.36 | 1.41 ± 0.10 | – | – |
| S13 | FRSEA | 13.95 ± 0.45 | – | 10.23 ± 0.53 | 6.2 1 ± 0.24 | – | 7.22 ± 0.36 |
| S14 | FRSNB | 16.43 ± 0.61 | – | 14.22 ± 0.68 | 3.70 ± 0.31 | – | – |
| S15 | FRSWA | 4.5 ± 0.15 | – | – | – | – | – |
Concentration unit is mg/g dry weight; result presented as mean ± SD from three replicates
The highest contents of chlorogenic acid and rutin were 45.35 ± ± 2.6 and 54.8 ± 3.1 mg/g in PFSEA sample. According to Narita and Inouye (2015), the average content of chlorogenic acid in 12 commercial roasted coffee beans was 26.6 mg/g, so the chlorogenic acid in PFSEA is 1.7 times the value found in the literature. Meanwhile, the content of rutin in PFSEA sample is higher than the value in buckwheat and Amaranthus paniculatus leaves showed in Kraujalis et al. (2015) and Kreft et al. (2006). Both chlorogenic acid and rutin have antioxidant, anti- inflammatory, anti-diabetic, and neuroprotective effects (Chua 2013; Marques and Farah 2009; Ong et al. 2013). Then, PFSEA sample might be potentially applied in antioxidant, anti- inflammatory, anti-diabetic, liver-protective, and neuroprotective effects.
DPPH radical scavenging activity
DPPH-radicals scavenging activity was used to evaluate the antioxidant capacities of the crude extracts and solvent-partitioned fractions from S. tschiliensis. The result showed a dose-dependent DPPH-scavenging activity (5–200 µg/mL) of the crude extracts and solvent-partitioned fractions of S. tschiliensis. For further estimation of the antioxidant activity, the IC50 was calculated. As shown in Fig. 1b, the IC50 values of crude extracts from the S. tschiliensis were ranged from 25.68 ± 1.21 to 86.79 ± 1.29 µg/mL, and the crude extracts of the S. tschiliensis (PFS) possessed the strongest DPPH-scavenging activity, showing an IC50 of 25.68 ± 1.21 µg/mL. Crude extract of S. tschiliensis (PFS) showed significantly higher DPPH-scavenging activity than some tested herbs of Scabiosa genus, such as Scabiosa comosa (302.8 µg/mL) and Scabiosa arenaria (170 µg/mL) (Besbes Hlila et al. 2013; Hlila et al. 2015). The results also showed that the EA fractions from S. tschiliensi possessed the strongest DPPH-scavenging activity, which was similar with the report by Besbes Hlila et al. (2013) on Tunisian Scabiosa arenaria. The EA fraction of S. tschiliensis (PFS) possessed the strongest DPPH-scavenging activity among the crude extracts and solvent-partitioned fractions, showing an IC50 of 8.47 ± 0.23 µg/mL nearly equal to the IC50 of Vc (7.60 ± 0.61 µg/mL). Little correlations (r = −0.574 or −0.523, p < 0.05) were observed between DPPH-scavenging activity and TPC or TFC of the extracts. This suggested that the strong DPPH-scavenging activity of the extracts from S. tschiliensis was caused by other compounds rather than phenolic and flavonoids compounds.
ABTS scavenging activity
The ABTS·+ radical formed from the reaction ABTS-e → ABTS·+ reacts quickly with the electron/hydrogen donors to form colorless ABTS (Tai et al. 2011), and the ABTS assay is generally applied to screen both lipophilic and hydrophilic antioxidants from vegetables, foods and fruits (Costantini et al. 2014). As showed in Fig. 1b, significant differences among the IC50 values of the ABTS radical scavenging abilities of all the samples were found (p < 0.05). Meanwhile, among the crude extracts and solvent-partitioned fractions from S. tschiliensis, which showed a dose-dependent ABTS-scavenging activity (5–200 µg/mL) in Fig. 1b, the highest ABTS radical scavenging ability was showed in the EA fraction of S. tschiliensis (PFS) (IC50 = 58.76 ± 1.78 µg/mL), and the lowest ABTS radical scavenging activities were displayed in the PE fractions. The EA and NB fraction demonstrated higher ABTS·+ scavenging activity than other fractions, which was according to the reference by Li et al. (2008) on Lysimachia clethroides. The correlations with S. tschiliensis were: r = 0.658 between ABTS·+ and TPC, r = 0.747 between ABTS·+ and TFC. The results can be concluded that the antioxidant-compounds are rich in EA and NB fraction, and the ABTS radical scavenging activity of S. tschiliensis was not limited to phenolics and flavonoids.
Hydroxyl radical scavenging activity
ROS are different kinds of activated oxygen containing free radicals and nonradical species. Hydroxyl radical (OH) is one of the most important free radicals in living cells (Karuppagounder et al. 2013). At concentration from 6.25 to 250 µg/mL, the samples also exhibited dose-dependent hydroxyl radical-scavenging activities. As shown in Fig. 1c, the IC50 values of crude extracts from the S. tschiliensis were ranged from 206.47 to 772.45 µg/mL, and the crude extract of S. tschiliensis (PFS) possessed the strongest hydroxyl scavenging activity, showing an IC50 of 206.47 ± 8.01 µg/mL. The EA action (PFS) showed the highest hydroxyl radical scavenging activity (67.64 ± 2.61 µg/mL), which was similar with the reference by Zhang et al. (2011) on leaves of Eucommia ulmoides Oliv. Significant correlations between the contents of antioxidant component and the IC50 values of hydroxyl radical-scavenging activity (TPC, r = 0.881; TFC, r = 0.896; p < 0.001) were demonstrated. The results showed that the hydroxyl radical scavenging activity of S. tschiliensis depended on the phenolic and flavonoids constituents.
Lipid peroxidation inhibition
Another parameter evaluated was the inhibitory effect of samples from S. tschiliensis tested on lipid peroxidation in rat liver microsomes exposed to oxidative stress in the presence of peroxyl radical. Our results showed (Fig. 1c) that all samples except FRSWA provided a protective effect towards lipid oxidative damage at a dose of 0.1 mg/mL. And the NB fractions of S. tschiliensis during the three stages possessed lowest TBARS values, showing an MDA Content 0.77 ± 0.30, 0.95 ± 0.21 and 0.94 ± 0.92 nmol/mgprot, respectively. Among all the samples, NA fraction produced by S. tschiliensis (PFS) showed the highest lipid-peroxidation-inhibition activity, which is close to that of VC. Zhang et al. (2011) reported the prominent lipid-peroxidation-inhibition activity of ferulic acid, and the high content of ferulic acid could lead to the high lipid-peroxidation-inhibition activity of NB fraction of S. tschiliensis (PFS). It was observed that TBARS had a certain correlation with the content of antioxidant-compounds (TPC, r = 0.836; TFC, r = 0.775; p < 0.001). These results suggested that the phenolic constituents of S. tschiliensis might be responsible for the lipid-peroxidation-inhibition activity.
Principal component analysis
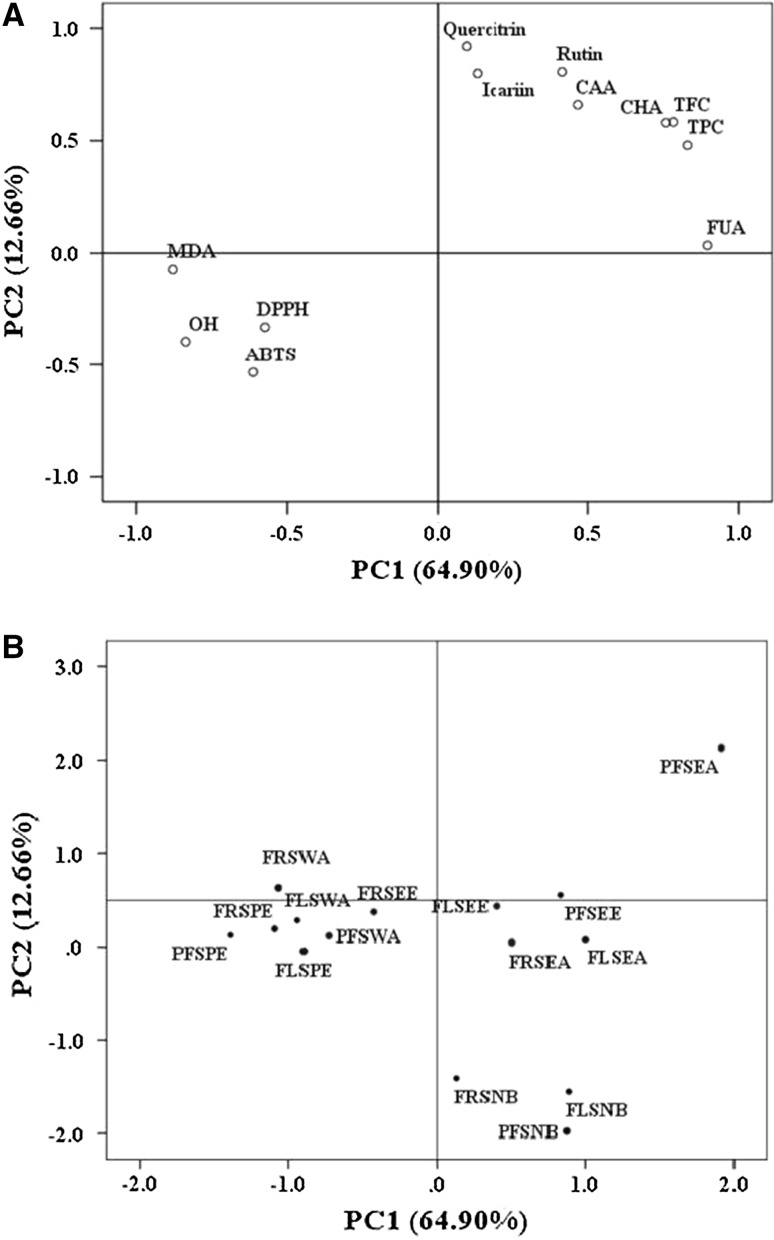
Principal component analysis (PCA) was used as the method getting the inter-relationships overview among TFC, TPC, the measured-antioxidant activities and the contents of active-compounds. Figure 2a shows the loading plot of the first and second principal components (PC1and PC2) accounted for 64.90 and 12.66% of the variance, respectively.
Fig. 2.
Principal component analysis loading plot of TPC, TFC, antioxidant activity and active compounds from of different fractions derived from S. tschiliensis during three stages (a). Principal component analysis score plot of different S. tschiliensis samples (b). Abbreviations: CHA chlorogenic acid, CAA caffeic acid, FUA Fumalic acid, PFS pre-flowering stage, FLS flowering stage, FRS fruiting stage, EE ethanolic extract, PE petroleum ether, EA ethyl acetate, NB n-butanol, WA water
It showed a well correlation between the first principal component (PC1) and TPC, TFC, OH, ABTS, CHA and rutin with the loadings 0.976, 0.947, −0.897, −0.812, 0.955 and 0.832 respectively. It also displayed the correlation with loadings of 0.652 between the second principal component (PC2) and quercitrin. Good correlations between TPC, TFC and OH were showed, which accorded with those from Pearson’s correlation analysis. Moreover, DPPH, OH and ABTS radical scavenging activities and MDA content displayed similar loadings on PC 1, which showed the four properties were closely related to antioxidant activity. Moreover, phenolic compounds could be good antioxidants because of the high loadings of TPC and TFC on PC1. The content of CHA which was relevant well to OH, DPPH and ABTS radical scavenging activities and MDA content, was higher than other chemicals. Thus, chlorogenic might be the main antioxidant compound in S. tschiliensis. Furthermore, some other reports (Hernández-Herrero and Frutos 2015; Jeszka-Skowron et al. 2015) also showed a good antioxidant activity of rutin and this is also similar to our correlation analysis results.
PCA can also provide an overview of the similarities and differences among the 15 S. tschiliensis samples. There were positive correlations between the PFSEA fraction and PC1 and PC2, which was in the upper-right of Fig. 2b. A high content of total flavonoids and phenolics and high antioxidant activity were revealed in PFSEA. The relationships among FRSNB, PFSNB and FLSNB were close, which demonstrated that they have similar contents of total flavonoid and phenolic and antioxidant activities. PFSPE showed the largest negative value of PC 1, which demonstrated that PFSPE had the lowest contents of total flavonoid and phenolic and antioxidant activities.
Conclusion
S. tschiliensis Grunning is a kind of potentially rich plants with satisfactory health promoting properties. The contents of the crude extracts were distributed unevenly in different growing stage and the mainly constituents of S. tschiliensis were polar compounds. The PFSEA fraction showed good antioxidant activities and high total flavonoid and phenolic contents. Its IC50 of DPPH radical-scavenging activity was 8.47 ± 0.23 µg/mL, which was nearly equal to the that of ascorbic acid (7.60 ± 0.61 µg/mL). The prominent antioxidant activities of the EA fraction from S. tschiliensis (PFS) were indicated by these results. Furthermore, six compounds of different S. tschiliensis samples were quantified. Chlorogenic acid was the most abundant components, and S. tschiliensis could be accepted as a natural plant resource of the compound. The EA fraction of S. tschiliensis (PFS) has high contents of chlorogenic acid, fumalic acid and rutin. PCA indicates that EA fraction of S. tschiliensis (PFS) with the best characters might be a desirable natural source of antioxidant. The useful information of the research could be used to the application and collection of the S. tschiliensis resource in food production.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by YLDX Project (ydzxxk201619), “111″ Project (B08044) and National Major Scientific Equipment Program (2012YQ03026108).
Footnotes
Junli Wang, Kun Liu and Xiaoxu Li have contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2666-9) contains supplementary material, which is available to authorized users.
References
- Andrade TdJAdS, Araújo BQ, Citó AMdGL, da Silva J, Saffi J, Richter MF, Ferraz AdBF. Antioxidant properties and chemical composition of technical Cashew Nut Shell Liquid (tCNSL) Food Chem. 2011;126:1044–1048. doi: 10.1016/j.foodchem.2010.11.122. [DOI] [Google Scholar]
- Anonymous (1977) Chinese traditional medicine dictionary. Shanghai People’s Public Health Publishing House, Shanghai
- Besbes Hlila M, Omri A, Ben Jannet H, Lamari A, Aouni M, Selmi B. Phenolic composition, antioxidant and anti-acetylcholinesterase activities of the Tunisian Scabiosa arenaria. Pharm Biol. 2013;51:525–532. doi: 10.3109/13880209.2012.746713. [DOI] [PubMed] [Google Scholar]
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Fu Y, Wang Z, Yang D, Chen J, Wang D. Determination on the contents of condensed tannins in Spatholobus suberectus Dunn. extracts and primary study on their anti-tumor activities. Acta Sci Nat Univ Sunyatseni/Zhongshan Daxue Xuebao. 2011;50:75–80. [Google Scholar]
- Chua LS. A review on plant-based rutin extraction methods and its pharmacological activities. J Ethnopharmacol. 2013;150:805–817. doi: 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Costantini S, Colonna G, Castello G. A holistic approach to study the effects of natural antioxidants on inflammation and liver cancer. In: Zappia V, Panico S, Russo LG, Budillon A, Della Ragione F, editors. Advances in nutrition and cancer. Berlin: Springer; 2014. pp. 311–323. [DOI] [PubMed] [Google Scholar]
- Gruz J, Ayaz FA, Torun H, Strnad M. Phenolic acid content and radical scavenging activity of extracts from medlar (Mespilus germanica L.) fruit at different stages of ripening. Food Chem. 2011;124:271–277. doi: 10.1016/j.foodchem.2010.06.030. [DOI] [Google Scholar]
- Guo M, Xu D, Wang J, Zhang R, Huang J. Establishment of regeneration system and flavonoids analysis of Scabiosa tschiliensis Grunning. J Hebei Univ. 2015;35:379–384. [Google Scholar]
- Gursoy N, Sarikurkcu C, Cengiz M, Solak MH. Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food Chem Toxicol. 2009;47:2381–2388. doi: 10.1016/j.fct.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Hernández-Herrero JA, Frutos MJ. Influence of rutin and ascorbic acid in colour, plum anthocyanins and antioxidant capacity stability in model juices. Food Chem. 2015;173:495–500. doi: 10.1016/j.foodchem.2014.10.059. [DOI] [PubMed] [Google Scholar]
- Hlila MB, Mosbah H, Mssada K, Jannet HB, Aouni M, Selmi B. Acetylcholinesterase inhibitory and antioxidant properties of roots extracts from the Tunisian Scabiosa arenaria Forssk. Ind Crop Prod. 2015;67:62–69. doi: 10.1016/j.indcrop.2015.01.009. [DOI] [Google Scholar]
- Hu Y, Xu J, Hu Q. Evaluation of Antioxidant Potential of Aloe vera (Aloe barbadensis Miller) Extracts. J Agric Food Chem. 2003;51:7788–7791. doi: 10.1021/jf034255i. [DOI] [PubMed] [Google Scholar]
- Jeszka-Skowron M, Krawczyk M, Zgoła-Grześkowiak A. Determination of antioxidant activity, rutin, quercetin, phenolic acids and trace elements in tea infusions: influence of citric acid addition on extraction of metals. J Food Compos Anal. 2015;40:70–77. doi: 10.1016/j.jfca.2014.12.015. [DOI] [Google Scholar]
- Karuppagounder SS, Madathil SK, Pandey M, Haobam R, Rajamma U, Mohanakumar KP. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Kraujalis P, Venskutonis PR, Ibáñez E, Herrero M. Optimization of rutin isolation from Amaranthus paniculatus leaves by high pressure extraction and fractionation techniques. J Supercrit Fluid. 2015;104:234–242. doi: 10.1016/j.supflu.2015.06.022. [DOI] [Google Scholar]
- Kreft I, Fabjan N, Yasumoto K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006;98:508–512. doi: 10.1016/j.foodchem.2005.05.081. [DOI] [Google Scholar]
- Kuhlmann A, Röhl C. Phenolic antioxidant compounds produced by in vitro. cultures of rosemary (Rosmarinus officinalis.) and their anti-inflammatory effect on lipopolysaccharide-activated Microglia. Pharm Biol. 2006;44:401–410. doi: 10.1080/13880200600794063. [DOI] [Google Scholar]
- Li C, Song Y, Liu Y, Kang W. Antioxidant activity of extracts from Lysimachia clethroides Fine Chemicals. 2008;25:1191–1193. [Google Scholar]
- Lin L, Cui C, Wen L, Yang B, Luo W, Zhao M. Assessment of in vitro antioxidant capacity of stem and leaf extracts of Rabdosia serra (MAXIM.) HARA and identification of the major compound. Food Chem. 2011;126:54–59. doi: 10.1016/j.foodchem.2010.10.060. [DOI] [Google Scholar]
- Liu K, Wang J, Zhao L, Wang Q. Anticancer, antioxidant and antibiotic activities of mushroom Ramaria flava. Food Chem Toxicol. 2013;58:375–380. doi: 10.1016/j.fct.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Ma JN, Bolraa S, Ji M, He QQ, Ma CM (2015) Quantification and antioxidant and anti-HCV activities of the constituents from the inflorescences of Scabiosa comosa and S. tschilliensis. Nat Prod Res:1-5. doi:10.1080/14786419.2015.1027701 [DOI] [PubMed]
- Marques V, Farah A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2009;113:1370–1376. doi: 10.1016/j.foodchem.2008.08.086. [DOI] [Google Scholar]
- Mudge E, Applequist WL, Finley J, Lister P, Townesmith AK, Walker KM, Brown PN. Variation of select flavonols and chlorogenic acid content of elderberry collected throughout the Eastern United States. J Food Compos Anal. 2016;47:52–59. doi: 10.1016/j.jfca.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita Y, Inouye K. Chapter 21—chlorogenic acids from coffee. In: Preedy VR, editor. Coffee in health and disease prevention. San Diego: Academic Press; 2015. pp. 189–199. [Google Scholar]
- Ong KW, Hsu A, Tan BKH. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem Pharmacol. 2013;85:1341–1351. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Pennycooke JC, Cox S, Stushnoff C. Relationship of cold acclimation, total phenolic content and antioxidant capacity with chilling tolerance in petunia (Petunia × hybrida) Environ Exp Bot. 2005;53:225–232. doi: 10.1016/j.envexpbot.2004.04.002. [DOI] [Google Scholar]
- Qin XJ, He W, Hai CX, Liang X, Liu R. Protection of multiple antioxidants Chinese herbal medicine on the oxidative stress induced by adriamycin chemotherapy. J Appl Toxicol. 2008;28:271–282. doi: 10.1002/jat.1276. [DOI] [PubMed] [Google Scholar]
- Sarikurkcu C, Tepe B, Semiz DK, Solak MH. Evaluation of metal concentration and antioxidant activity of three edible mushrooms from Mugla, Turkey. Food Chem Toxicol. 2010;48:1230–1233. doi: 10.1016/j.fct.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Tai Z, et al. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011;126:1648–1654. doi: 10.1016/j.foodchem.2010.12.048. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Rapid micropropagation system in vitro and antioxidant activity of Scabiosa tschiliensis Grunning. Plant Growth Regul. 2013;69:305–310. doi: 10.1007/s10725-012-9765-4. [DOI] [Google Scholar]
- Zhang Q, Su YQ, Zhang JF. Comparative evaluation of antioxidant activity of aqueous extract and its different solvent fractions from Eucommia ulmoides oliv. leaves. Food Science. 2011;32:23–27. [Google Scholar]
- Zhang DY, et al. Variation of active constituents and antioxidant activity in pyrola [P. incarnata Fisch.] from different sites in Northeast China. Food Chem. 2013;141:2213–2219. doi: 10.1016/j.foodchem.2013.05.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.