Abstract
Sixteen honeydew and 15 floral honeys from Lebanon were analyzed for pollen spectra and physicochemical parameters. A total of 37 families and 67 taxa were recorded with the honeybees producing honeydew honey exhibiting a more diverse foraging behavior than those making floral honeys. The honeydew and floral honeys exhibited differences in moisture content, pH, electrical conductivity, color, protein and Maillard reaction products. The honeydew honeys contained more total phenols, had higher antioxidant contents, and displayed higher antioxidant capacities than the floral samples in the Trolox equivalent antioxidant capacity, oxygen radical absorbance capacity, inhibition of superoxide dismutase activity and protection of red blood cells against hemolysis assays. The honey samples exhibited higher antioxidant capacities, in the aforementioned assays, than their corresponding methanol-extractable phenol fractions although the differences did not reach statistical significance in the floral samples. The relative antioxidant capacity indices which integrate measures of antioxidant capacity from the different assays of the honey samples and their corresponding extracts exhibited similar patterns (r = 0.9774, 0.9937) thereby indicating that the antioxidative behavior of the entire honeys is mirrored by their methanol-extractable phenolic fractions.
Keywords: Lebanese Honey, Honeydew, Pollen, Phenolics, Antioxidants
Introduction
Honey is a natural sweet substance produced by honeybees through foraging of different plants, transforming the collected material in their bodies into a liquid and depositing the liquid in the cells of honeycombs. The properties of honey are shaped to a large extent by the plants foraged by the honeybees. Within this context, two major types of honey are recognized: Floral honey which is derived from the nectar of flowering plants and honeydew honey which is obtained from the secretions of plants and/or excretions of plant-sucking insects. Honeydew honey is produced by honeybees through collecting the sugar-containing substances, excreted by insects after sucking and transforming the sap of conifer trees (e.g. fir, oak, chestnut, elm, hazel), and subsequent maturing in the honeycombs (Diez et al. 2004). Honeydew and floral honeys differ in a host of chemical, physicochemical and sensory properties and functionality (Manzanares et al. 2011). Honey has been used by humans for millennia as food and medicine (Ajibola 2015). Honey is prized by humans as food due to its sweet taste, pleasant flavor and high sugar content (~80%) which serves as a ready source of energy. The medicinal properties of honey are varied and include antibacterial, anti-inflammatory, wound healing, immunity boosting and scavenging of reactive oxygen species (Ajibola 2015). The medicinal effects of honey are largely modulated by its antioxidative components. The antioxidant activity of honey is attributed to a number of substances including enzymes (catalase, glucose oxidase, peroxidase) (Al et al. 2009), proteins (Chua et al. 2015), amino acids (Perez et al. 2007), carotenoids (Erejuwa et al. 2012), Maillard reaction products (MRPs) (Brudzynski and Miotto 2011) and phenols (Alvarez-Suarez et al. 2012). The phenolic fraction of honey comprises diverse classes of compound and is largely responsible for the observed differences in the antioxidant activities of honeys (da Silva et al. 2016). The attribution of the antioxidant activity of honey to its phenolic fraction is based on the consistent and high positive correlations between antioxidant capacity and the total phenols (TP), study of the antioxidant activities of the phenol fractions separated on XAD-type hydrophobic resins and profiling of the compounds found in the separated phenol fractions (Ferreira et al. 2009; Gheldof et al. 2002). The methanol fraction eluted from XAD-2 resins is comprised chiefly of flavonoids, phenolic acids and some phenolic polymers (Alvarez-Suarez et al. 2010) and has been reported to exhibit marked differences in antioxidant activity among honeys with contrasting antioxidant capacities (Dong et al. 2013). Notwithstanding the presumed role of the phenolic fraction in modulating the antioxidant capacity of honey, little is known about the contribution of the other antioxidant principles of honey viz. enzymes, amino acids, proteins, carotenoids and MRPs. A plausible approach for resolving the contributions of the phenolic fraction and the other antioxidant principles to the antioxidant functionality of honey would entail comparison of the antioxidant capacities of whole honeys and their phenolic fractions.
The antioxidant capacity of foods is usually determined by chemical tests which measure the different facets of antioxidants’ action (radical scavenging, metal reducing, quenching of superoxides) and biological tests which measure the protective effects of antioxidants against cellular damage by free radicals. A holistic measure of the antioxidant capacity of foods entails quantification of the different mechanisms through which antioxidants exert their effects by a combination of different assays (Tabart et al. 2009). Accordingly, the antioxidant capacities of honeys and their methanolic fractions were determined by measuring their antioxidant contents and the Trolox equivalents antioxidant capacity (TEAC), oxygen radical absorbance capacity (ORAC) and superoxide anion scavenging capacity by the superoxide dismutase (SOD) activity assays and protection of red blood cells (RBCs) against hemolysis by free radicals.
There is a long history of apiculture in the Eastern Mediterranean and reports indicate that honey has been used for millennia in the Levant for healing purposes (Lev 2003). Apart from the honeys from Greece (Karabagias et al. 2016) and Turkey (Can et al. 2015), little or no information exists on honeys from the other Eastern Mediterranean countries. Geographically, Lebanon rises steeply from the coast to mountains 3088 m high giving rise to diverse microclimates which support one of the highest densities of floral diversity in the Mediterranean basin renowned for being one of the most biologically diverse regions of the world (MOE 2001). However, no studies have reported on the properties of honeys from Lebanon. Therefore, the objectives of the present work are to: (a) characterize the physicochemical and melissopalynological properties and antioxidant capacity of floral and honeydew honeys from Lebanon, and (b) investigate the contribution of the methanolic fraction to the antioxidant functionality of honey through critical assessment of the antioxidant capacities of whole honeys and their methanolic phenol fractions.
Materials and methods
Honey samples
Thirty one locally-produced honeys were collected from the different regions of Lebanon. The honeys were harvested between April and October of 2012 and, as stated by the beekeepers, 15 were floral and 16 honeydew. The samples (~1 Kg) were placed in glass containers and stored in the dark at 20 °C.
Chemicals
Chemicals, XAD-2 resin, and WST-based SOD determination kit were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Chemical and physicochemical analyses of honey samples
Moisture, proline and hydroxymethylfurfural (HMF) contents and pH and electrical conductivity of the honey samples were determined according to the International Honey Commission (IHC 2009).
The protein content was determined by the Bradford method (Bradford 1976) and expressed in mg bovine serum albumin/100 g honey (Cimpoiu et al. 2013).
The color intensity was determined by measuring the absorbance of an aqueous solution of honey (50% w/v) at 720 and 560 nm and expressed as the net absorbance (A560–A720) (Beretta et al. 2005).
The MRPs contents were determined by measuring the absorbance of an aqueous solution of honey (50% w/v) at 720 and 450 nm and expressed as the net absorbance (A450–A720) (Brudzynski and Miotto 2011).
Pollen analysis
Analysis of pollen and honeydew elements (HDE), comprising fungal elements and yeasts, was carried out according to Louveaux et al. (1978) using a LEICA DFC300 FX microscope interfaced to a DFC 300 FX digital TV camera (Heerbrugg, Switzerland). The family and, whenever possible, the species, and type of plant (nectariferous or nectarless) foraged by the honeybees were determined by counting a minimum of 500 pollen grains. The frequencies of the different pollen types were grouped into the following classes: predominant pollen (>45%), secondary pollen (16–45%), minor important pollen (3–15%) and minor pollen (1–3%). The numbers of HDE were determined and the honeydew indices (number of HDE/number of pollen grains from nectariferous plants) of the honey samples were calculated. The pollen diversity of the honey samples was assessed by computing the Shannon–Weaver index . ln p i where p i is the proportion of pollen i in the sample (Ramírez-Arriaga et al. 2011).
Isolation of the methanolic fraction from honey
The methanolic fractions of the honeys were isolated according to Dong et al. (2013). A solution of honey in acidified water (pH 2.1 with HCl) was passed through a column of Amberlite XAD-2 resin (25 × 2 cm) and after washing with acidified water and deionized water, the phenolic fraction was eluted with methanol. The methanol fraction was evaporated to dryness at 40 °C, under reduced pressure, weighed and stored at −20 °C until analyzed.
Total phenols and antioxidant assays of honeys and methanolic fractions
Determination of total phenols
The total phenols (TP) contents were determined by the Folin–Ciocalteu reagent as described by Saxena et al. (2010). Honey (60 µL; 0.05 g/mL) or methanolic extract (60 µL; 1 mg/mL) were used in the determinations and TP contents were expressed as mg of gallic acid equivalents/100 g honey.
Determination of antioxidant content
The antioxidant contents of honey and the methanolic extracts were determined according to their ability to reduce the DPPH radical as described by Meda et al. (2005). When working with the methanolic extracts, an appropriate dilution of the dried extract in methanol containing an equivalent amount of phenolics as the honey solution was used. The antioxidant content was expressed as mg of ascorbic acid equivalent antioxidant content (AEAC)/100 g honey.
Trolox equivalents antioxidant capacity (TEAC) assay
The TEAC assay which measures the ability of hydrogen-donating compounds to decolorize the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) cation (ABTS+•) was carried out according to Oddo et al. (2008). TEAC was expressed in µM Trolox equivalents/100 g honey.
Oxygen radical absorbance capacity (ORAC) assay
The ORAC assay which measures the degree of protection of fluorescent probes (fluorescein) by antioxidants against decay by free radicals generated by 2, 2′-azobis (2-methylpropionamidine) dihydrochloride was carried out according to Gillespie et al. (2007). The ORAC values were expressed in μM Trolox equivalents/g of honey.
Scavenging of superoxide anion by inhibition of SOD assay
The assay was carried out using the WST kit as per the manufacturer’s recommendations. The superoxide anion generated by xanthine oxidase reduces WST (2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl) 2H-tetrazolium, monosodium salt) to WST-1 formazan which absorbs strongly at 450 nm. In the presence of SOD the rate of formation of the WST-1 formazan chromophore is decreased through reduction of the superoxide anion. Hydrogen-donating antioxidants inhibit SOD action by competing for reduction of the superoxide anions. The antioxidant capacity of the honey samples and their methanolic extracts were expressed as % inhibition of SOD.
Protection of red blood cells (RBCs) against hemolysis assay
The protection of RBCs against hemolysis by honey or methanolic extracts was carried out according to Tabart et al. (2009). RBCs were obtained from healthy individuals after informed consent. The protection of RBCs against hemolysis was expressed in mM TE/100 g honey.
The relative antioxidant capacity indices (RACI) (Sun and Tanumihardjo 2007) of the honeys and their extracts were computed by averaging the z-scores (, where x is the raw value, µ the mean and σ the standard deviation) of the antioxidant content, ABTS, ORAC, SOD and hemolysis of RBCs assays.
Determinations were carried out in triplicate.
The study was approved by the Institutional Review Board of the University.
Statistical analysis
The chemical/physicochemical parameters and the Shannon–Weaver diversity indices of the honeydew and floral honeys were compared by unpaired two-tailed t tests. The antioxidant assays of the honey samples and methanolic extracts were subjected to one way ANOVA and means were separated by the Fisher-Hayter version of the least significant difference test when the F values were significant (Williams and Abdi 2010). Comparisons were made at α-level ≤ 0.05 unless otherwise indicated. Statistical analyses were carried out with Microsoft Excel®.
Results and discussion
Pollen analysis of honeys
Sixty seven types of pollen belonging to 37 families were identified in the honeys (Table 1). Among the floral honeys, 2 samples are considered monofloral with one being vetch honey containing >45% Vicia sativa pollen and the other citrus honey with the underrepresented citrus pollen being present at 15% (Louveaux et al. 1978) (Table 1). The preponderance of multifloral honeys (87%) in the floral samples is indicative of a highly heterogeneous foraging behavior of the honeybees presumably due to the absence of expansive regions of land cultivated by a major crop and/or supporting major vegetation. The high frequency of multifloral honeys was also related to the high floral diversity in Lebanon (MOE 2001) and the practice of moving the beehives to different areas in the country to exploit the different flowering periods. Further, the floral and honeydew honeys exhibited broad spectra of pollens with Shannon–Weaver indices of 5.4 ± 1.14 and 6.1 ± 0.66, respectively. The greater diversity (P = 0.05) of the pollen in the honeydew samples is due to the dearth of pollen in the syrupy secretions of the trees which drives the honeybees to forage multiple types of plants to satisfy their need for pollen. The honeydew samples also contained more pollen from nectarless plants than their floral counterparts possibly because these pollens were deposited through wind on the syrupy materials on trees which served as the starting material for honey processing by the bees (Manzanares et al. 2011). The HDE indices of the honeydew samples ranged between 0.92 and 1.38 with mean value of 1.04 ± 0.11 which was lower than the cut-off ≥3 for honeydew honeys according to the classification scheme of Louveaux et al. (1978). HDE indices of 0.16 (Escuredo et al. 2012) and 0.3 (Manzanares et al. 2011) have been reported for Spanish honeydew honeys thereby suggesting that the HDE index was not a robust indicator of honeydew honey.
Table 1.
Pollen types and their frequencies in honeydew (n = 16) and floral honeys (n = 15)
| Family | Pollen type | Honeydew honey | Floral honey | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N/NL | P (%) | S (%) | M (%) | T (%) | P (%) | S (%) | M (%) | T (%) | ||
| Anacardiaceae | Pistacia lentiscus | N | 0 | 0 | 19 | 31 | 0 | 0 | 0 | 0 |
| Apiaceae | Coriandrum sativum | N | 0 | 0 | 25 | 63 | 0 | 7 | 27 | 53 |
| Eryngium paniculata | N | 0 | 0 | 25 | 25 | 0 | 7 | 47 | 27 | |
| Asteraceae | Artemisia | NL | 0 | 0 | 6 | 19 | 0 | 0 | 7 | 0 |
| Cichorium intybus | N | 0 | 0 | 31 | 50 | 0 | 0 | 20 | 40 | |
| Cirsium libanoticum | N | 0 | 0 | 13 | 38 | 0 | 0 | 7 | 33 | |
| Helianthus | N | 0 | 0 | 0 | 13 | 0 | 0 | 33 | 20 | |
| Inula viscosa | N | 0 | 0 | 19 | 13 | 0 | 0 | 13 | 27 | |
| Arecaceae | Trachycarpus | N | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 27 |
| Betulaceae | Alnus | NL | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 |
| Betula | NL | 0 | 0 | 13 | 38 | 0 | 0 | 0 | 0 | |
| Carpinus | NL | 0 | 0 | 6 | 25 | 0 | 0 | 0 | 0 | |
| Boraginaceae | Cynoglossum | N | 0 | 0 | 25 | 13 | 0 | 0 | 20 | 13 |
| Echium vulgare | N | 0 | 0 | 0 | 13 | 0 | 0 | 33 | 27 | |
| Myosotis sylvatica | N | 0 | 0 | 19 | 56 | 0 | 0 | 0 | 20 | |
| Brassicaceae | Brassica species | N | 0 | 25 | 75 | 0 | 0 | 0 | 87 | 7 |
| Caprifoliaceae | Cephalaria dipsacoides | N | 0 | 0 | 44 | 31 | 0 | 0 | 0 | 27 |
| Knautia arvensis | N | 0 | 0 | 0 | 25 | 0 | 0 | 33 | 40 | |
| Lonicera | N | 0 | 0 | 44 | 38 | 0 | 0 | 0 | 27 | |
| Convolvulaceae | Convolvolus arvensis | N | 0 | 0 | 0 | 44 | 0 | 0 | 53 | 40 |
| Corylaceae | Corylus | NL | 0 | 0 | 6 | 13 | 0 | 0 | 0 | 0 |
| Cucurbitaceae | Cucumis sativus | N | 0 | 6 | 38 | 6 | 0 | 7 | 53 | 33 |
| Ecballium elaterium | N | 0 | 0 | 25 | 38 | 0 | 0 | 33 | 33 | |
| Cupressaceae | Thuja | NL | 0 | 0 | 6 | 13 | 0 | 0 | 0 | 0 |
| Cyperaceae | Cyperus papyrus | NL | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 |
| Ephedraceae | Ephedra frustillata | N | 0 | 0 | 0 | 44 | 0 | 0 | 0 | 7 |
| Ericaceae | Rhododendron | N | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 40 |
| Fabaceae | Astragalus | N | 0 | 0 | 6 | 6 | 0 | 0 | 13 | 20 |
| Melilotus alba Medicus | N | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | |
| Onobrychis viciifolia | N | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 7 | |
| Robinia pdeudoacacia L. | N | 0 | 0 | 13 | 19 | 0 | 0 | 0 | 33 | |
| Trifolium repens | N | 0 | 0 | 94 | 6 | 0 | 0 | 53 | 27 | |
| Vicia sativa | N | 0 | 0 | 13 | 38 | 7 | 13 | 27 | 20 | |
| Fagaceae | Castanea sativa | N | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 |
| Qurecus Alba | NL | 0 | 6 | 69 | 13 | 0 | 0 | 13 | 20 | |
| Gramineae | Zea mays | NL | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 14 |
| Iridaceae | Crocus | N | 0 | 0 | 88 | 6 | 0 | 0 | 53 | 13 |
| Lamiaceae | Cistus | N | 0 | 0 | 6 | 0 | 0 | 0 | 20 | 7 |
| Phlomis bruguieri | N | 0 | 0 | 13 | 63 | 0 | 0 | 27 | 13 | |
| Rosmarinus officinalis | N | 0 | 0 | 0 | 19 | 0 | 0 | 7 | 0 | |
| Origanum syriacum | N | 0 | 0 | 6 | 44 | 0 | 0 | 0 | 13 | |
| Liliaceae | Asparagus acutifolius | N | 0 | 0 | 0 | 19 | 0 | 0 | 7 | 27 |
| Mimosoideae | Acacia dictyophleba | NL | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 |
| Malvaceae | Hibiscus pedunculatus | N | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Myrtaceae | Eucalyptus | N | 0 | 0 | 44 | 31 | 0 | 0 | 53 | 33 |
| Myrtus communius | N | 0 | 0 | 19 | 31 | 0 | 0 | 33 | 40 | |
| Oleaceae | Fraxinus ornus | N | 0 | 0 | 6 | 50 | 0 | 0 | 27 | 13 |
| Ligustrum vulgare | N | 0 | 0 | 6 | 13 | 0 | 0 | 13 | 13 | |
| Onagraceae | Epilobium montanum | N | 0 | 0 | 19 | 38 | 0 | 0 | 0 | 0 |
| Papaveraceae | Papaver syriacum | NL | 0 | 0 | 0 | 38 | 0 | 0 | 0 | 0 |
| Pinaceae | Pinus | NL | 0 | 0 | 0 | 38 | 0 | 0 | 0 | 1 |
| Plumbaginaceae | Armeria maritima | N | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 13 |
| Polygonaceae | Rumex | NL | 0 | 0 | 19 | 6 | 0 | 0 | 0 | 0 |
| Ranunculaceae | Ranunculus | N | 0 | 0 | 13 | 56 | 0 | 0 | 20 | 7 |
| Rosaceae | Crataegus monogyna | N | 0 | 0 | 63 | 25 | 0 | 0 | 27 | 53 |
| Eryobtrya japonica | N | 0 | 0 | 38 | 13 | 0 | 0 | 33 | 27 | |
| Filipendula | N | 0 | 0 | 13 | 38 | 0 | 0 | 27 | 47 | |
| Prunus mume | N | 0 | 0 | 13 | 6 | 0 | 0 | 5 | 26 | |
| Prunus persica | N | 0 | 0 | 6 | 56 | 0 | 0 | 7 | 27 | |
| Rubus fruticosus | N | 0 | 6 | 88 | 0 | 0 | 0 | 87 | 7 | |
| Rutaceae | Citrus aurantium | N | 0 | 0 | 38 | 19 | 0 | 7 | 20 | 20 |
| Citrus sinensis | N | 0 | 0 | 75 | 19 | 0 | 7 | 53 | 33 | |
| Salicaceae | Populus alba | NL | 0 | 0 | 0 | 19 | 0 | 0 | 0 | 0 |
| Salix alba | NL | 0 | 0 | 56 | 44 | 0 | 0 | 27 | 33 | |
| Taxaceae | Taxus baccata | N | 0 | 0 | 0 | 19 | 0 | 0 | 0 | 7 |
| Tiliaceae | Tilia platyphyllus | N | 0 | 0 | 6 | 13 | 0 | 0 | 0 | 20 |
| Trilliaceae | Trillium nivale | N | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 13 |
P predominant pollen (>45%), S secondary pollen (16–45%), M minor important pollen (3–15%), T minor pollen (1–3%), N nectariferous plant, NL nectarless plant
Chemical and physicochemical parameters of honey samples
The moisture contents of the honey samples were lower than the cut-off value at ≤20% (da Silva et al. 2016) with the exception of one floral sample which had a marginally higher value of 20.2% (Table 2). Moisture is the major determinant of honey stability and is largely determined by the practices of the honey producer during harvesting. The honeydew samples had lower moisture (P < 0.000) than their floral counterparts possibly to confer on them a more viscous consistency desired by the local consumers.
Table 2.
Chemical and physicochemical parameters of honeydew (n = 16) and floral (n = 15) honeys
| Parameter | Honeydew mean ± SD (range) |
Floral mean ± SD (range) |
Probability |
|---|---|---|---|
| Moisture (g/100 g) |
15.20 ± 0.83 (13.72–16.64) |
17.12 ± 1.70 (14.49–20.20) |
0.000 |
| Hydroxymethylfurfural (mg/kg) |
4.33 ± 4.93 (0.14–17.05) |
8.72 ± 7.34 (2.49–28.38) |
0.059 |
| Proline (mg/kg) |
758.69 ± 266.36 (169.39–1083.08) |
627.66 ± 283.25 (171.20–1139.48) |
0.203 |
| Protein (mg BSAa/100 g) |
109.38 ± 18.12 (77.11–140.42) |
137.91 ± 49.47 (66.08–242.88) |
0.039 |
| pH | 5.26 ± 0.27 (4.48–5.67) |
4.02 ± 0.28 (3.67–4.51) |
0.000 |
| Electrical conductivity (mS/cm) |
1.32 ± 0.20 (0.86–1.65) |
0.54 ± 0.23 (0.22–0.93) |
0.000 |
| Total phenols (mg GAEb)/100 g) |
100.10 ± 21.72 (69.53–130.30) |
57.33 ± 10.10 (33.93–71.80) |
0.000 |
| Antioxidant content (mg AEACc/100 g honey) |
55.59 ± 6.78 (46.13–66.62) |
34.05 ± 14.24 (8.28–50.78) |
0.000 |
| Maillard reaction products (A450) (AUd) |
1.54 ± 0.21 (1.20–1.80) |
0.93 ± 0.27 (0.38–1.44) |
0.000 |
| Color (A560–A720) (AUd) |
0.34 ± 0.05 (0.25–0.40) |
0.21 ± 0.07 (0.07–0.33) |
0.000 |
a BSA bovine serum albumin
b GAE gallic acid equivalents
c AEAC ascorbic acid equivalent antioxidant capacity
d AU absorbance units
HMF contents of the honeys were lower than the limit of 40 mg/kg (da Silva et al. 2016). HMF is an indicator of honey freshness and its content increases upon thermal treatment applied on honey and/or storage at high temperatures (Bath and Singh 2001; Kędzierska-Matysek et al. 2016; Singh and Bath 1998). Conflicting results on HMF contents of honeydew and floral honeys have been reported with some workers reporting higher values for honeydew honeys (Manzanares et al. 2011) while others noting no differences (Escuredo et al. 2012). No differences (P = 0.059) in HMF contents were observed between honeydew and floral honeys with the maximum value registered for HMF being 28.38 mg/kg thereby indicating that all the samples are considered fresh.
The proline contents of the samples were higher than the proposed limit of 180 mg/kg (da Silva et al. 2016) for properly ripened honey except for one floral (169.39 ± 3.52 mg/kg) and a honeydew sample (171.20 ± 7.86 mg/kg). Values < 180 mg/kg have been reported for some certified honeys (Hermosín et al. 2003). Proline is derived from the salivary secretions of the honeybees during conversion of the nectar to honey. The honeydew and floral samples did not exhibit differences (P = 0.203) in proline contents in contrast to the higher values reported for honeydew honeys (Manzanares et al. 2011).
The proteins of honey are mainly enzymes secreted by honeybees during processing of the nectar to honey and enzymes and proteins derived from the collected nectar and pollen, and royal jelly proteins (Iglesias et al. 2006). The protein contents of the samples were similar to those reported (da Silva et al. 2016) with the honeydew honeys containing more proteins (P = 0.039) as noted by other workers (Cimpoiu et al. 2013).
All samples had pH values < 7 consistent with the acidic nature of honey. The honeydew honeys had higher pH (P < 0.000) than the floral samples in line with other findings (Manzanares et al. 2011).
Honeydew samples exhibited higher electrical conductivity than the cut-off level ≥80 mS/cm (da Silva et al. 2016). The electrical conductivity of honey is governed by the levels of minerals and the content and ionization of organic acids in the product. The higher electrical conductivity of honeydew honeys is due to their higher mineral contents (Escuredo et al. 2012) and their relatively-high pH values which promote greater ionization of amino and organic acids. The higher electrical conductivity of honeydew honeys corroborates earlier reports (Escuredo et al. 2012).
Honeydew honeys contained higher levels of TP (P < 0.000) than the floral samples. The phenols in honey are grouped into flavonoids and phenolic acids and range from monomers to high molecular weight polymers (da Silva et al. 2016). The TP of the floral samples are higher than those reported for multifloral honeys from Turkey (29.54 ± 12.71 mg GAE/100 g) (Can et al. 2015) and lower than those from Burkina Faso (70.67 ± 16.75 mg GAE/100 g) (Meda et al. 2005) while the TP of the honeydew samples were comparable to those of Romanian honeys (Al et al. 2009).
Honeydew honeys exhibited higher antioxidant contents (P < 0.000) than the floral samples. This finding is consistent with the reported increase of antioxidant content with increasing darkness of the honey color (Meda et al. 2005). The antioxidant contents of the floral samples were higher than those reported for Indian (15–30 mg AEAC/100 g) (Saxena et al. 2010) and Burkina Fasan (10.2–37.9 mg AEAC/100 g) (Meda et al. 2005) multifloral honeys.
The MRPs were present at higher levels (P < 0.000) in the honeydew honeys. The MRPs are formed through interactions of free amino acids/proteins and reducing sugars during ripening of honey and comprise diverse groups of compound including pyrazines, furans and high molecular weight melanoidins (Brudzynski and Miotto 2011). Honeydew honeys contain higher levels of free amino acids (Hermosín et al. 2003) and proteins (Table 2) than floral honeys and would, therefore, provide more conducive conditions for the Maillard reaction.
Honeydew honeys had darker colors (P < 0.000) consistent with their intrinsic deep brown and black colors. As noted earlier, honeydew honeys contain higher concentrations of phenols and MRPs with chromophores which absorb strongly in the visible range. The higher absorbance of honeydew honeys has been reported recently (Flanjak et al. 2016).
The honeydew honeys exhibited higher electrical conductivity, pH, TP and darker colors consistent with these being the most discriminatory parameters between the honeydew and floral types of honeys. These findings along with the characteristic pollen spectra and feedback from the beekeepers strongly support the indicated grouping of the samples as honeydew and floral honeys.
Total phenols and antioxidant assays of honeys and methanolic fractions
The TP and antioxidant capacity of the methanol-extractable phenolic fractions were compared to those of the entire honeys at iso-phenol levels to assess the contribution of the methanol-extractable phenol fraction to the overall antioxidant activity of the honeys. The TP, as determined by the Folin–Ciocalteau reagent, of the honey extracts were lower than those of the corresponding honeys though the differences did not reach statistical significance (P > 0.05) (Table 3). The lower TP of the extracts could be attributed to the removal of some water-soluble phenols in the early eluates during isolation of the methanolic fraction (Gheldof et al. 2002) and/or the presence of other reducing substances in the honey that react strongly with the Folin–Ciocalteau reagent (Ferreira et al. 2009).
Table 3.
Total phenols, antioxidant contents, ABTS, ORAC, SOD and RBC assays (mean ± SD; range) of honeydew (n = 16) and floral (n = 15) honeys and their methanolic extracts
| Assay | Honeydew honey |
Floral honey |
Honeydew honey extracts |
Floral honey extracts |
|---|---|---|---|---|
| Total phenols (mg GAE/100 g honey) |
100.11 ± 21.72a
(69.53–130.30) |
57.33 ± 10.10bd
(33.93–71.80) |
92.13 ± 19.60a
(67.12–119.18) |
52.31 ± 9.23cd
(31.93–66.63) |
| Antioxidant content (mg AEAC/100 g honey) |
55.59 ± 6.78a
(46.13–66.62) |
34.05 ± 14.24bd
(8.28–50.78) |
45.74 ± 6.15c
(37.44–55.88) |
27.44 ± 11.53d
(6.69–40.56) |
| TEAC/ABTS (µM TE/100 g honey) |
0.35 ± 0.04a
(0.27–0.40) |
0.22 ± 0.10bc
(0.05–0.37) |
0.26 ± 0.03c
(0.17–0.30) |
0.17 ± 0.07bd
(0.04–0.28) |
| ORAC (µM TE/g honey) |
0.30 ± 0.05a
(0.21–0.39) |
0.23 ± 0.10bc
(0.07–0.41) |
0.20 ± 0.03cd
(0.15–0.28) |
0.14 ± 0.07d
(0.04–0.24) |
| SOD (% inhibition) |
54.78 ± 7.86a
(46.39–69.69) |
34.36 ± 14.02bc
(9.27–54.44) |
42.50 ± 6.98c
(30.56–55.31) |
27.93 ± 11.77bd
(7.25–48.40) |
| RBC protection against hemolysis (mM TE/100 g honey) |
0.33 ± 0.14a
(0.11–0.51) |
0.21 ± 0.12bc
(0.06–0.42) |
0.26 ± 0.13abc
(0.07–0.45) |
0.17 ± 0.10c
(0.04–0.36) |
GAE gallic acid equivalents, AEAC ascorbic acid equivalent antioxidant capacity; TEAC/ABTS trolox equivalents antioxidant capacity/2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid, TE trolox equivalents, ORAC oxygen radical absorbance capacity, SOD superoxide dismutase, RBC red blood cells. Averages of triplicate determinations
a, b, c, dEntries with different superscripts are different (P < 0.05) by the least significant difference test
The antioxidant capacity of foods is determined by different tests to capture the different mechanisms through which antioxidative compounds exert their action. These tests should reflect the ability of antioxidants to quench radicals by hydrogen transfer, reduce radicals through electron transfer and protect cells against oxidative damage (Tabart et al. 2009). In the present work, the antioxidant capacity of the whole honeys and their methanolic extracts was determined by a combination of hydrogen transfer (ORAC) and electron transfer (TEAC/ABTS, SOD) assays, antioxidant contents, and protection of RBCs against hemolysis by free radicals.
The honeydew honeys exhibited higher antioxidant activity than their floral counterparts (P < 0.05) in all the antioxidant assays (Table 3). The higher antioxidant capacity of the honeydew samples could be attributed to their higher contents of total phenols (Table 3), MRPs (Table 2), and their higher pH (Table 2) which promotes ionization of the indigenous organic acids thereby leading to stronger chelation of metals and concomitant potentiation of the antioxidant activity of phenolic compounds. Honeydew honeys have been recently reported to exhibit higher antioxidant capacity than floral honeys by the DPPH and ferric reducing antioxidant power (FRAP) assays (Flanjak et al. 2016).
The honey samples had higher antioxidant activity than their corresponding extracts in the ABTS, ORAC and SOD assays, and antioxidant contents, and while the differences were significant (P < 0.05) in the honeydew samples, the differences in floral samples did not reach statistical significance except in the ORAC assay (Table 3). The superior antioxidant capacity of the honey samples could be related to the contribution of the components removed in the early elutions of the Amberlite XD-2 column during extraction of the methanolic fraction. To this end, the water-soluble phenolics in the early eluates from the column have been reported to possess strong antioxidant properties by the ORAC test (Gheldof et al. 2002). Further, the proteins of honey which are removed in the early washings have been reported to possess antioxidant activity in the DPPH and FRAP (Chua et al. 2015) and ORAC (Gheldof et al. 2002) assays. Moreover, the carotenoids of honey are known to possess electron transfer and hydrogen transfer capacities and to potentiate the antioxidant activity of honey phenolics (Han et al. 2012).
The honey samples and their extracts protected RBCs against oxidative damage but the differences in the protective effects did not reach statistical significance (P > 0.05) (Table 3). The observed lack of differences in the potency of honeys and their extracts might be related to the nature of phenolic compounds operative in the protection of RBCs against damage. Work with pure compounds (Tabart et al. 2009) and honey extracts (Alvarez-Suarez et al. 2012) strongly suggests that flavonoids and phenolic acids incorporate into the hydrophobic core of the cell membrane where they act as fillers imparting rigidity to the membrane in addition to scavenging the free radicals involved in the oxidation of lipids. In addition to their protective effects on cell membranes, the flavonoids diffuse to the cytosol where they curtail the activity of the oxidative enzyme SOD and prevent depletion of glutathione thereby protecting the cellular organelles against damage by free radicals. The methanolic extracts of honeys are chiefly comprised of flavonoids and phenolic acids (Alvarez-Suarez et al. 2012; Can et al. 2015) and the observed similarities in the potency of honey and extracts in protecting RBCs suggest that these compounds are largely responsible for the functionality honey with the other antioxidant principles of honey (high molecular weight phenols, MRPs, proteins, carotenoids, ascorbic acid) assuming marginal roles in this assay. The magnitudes of the protective effects of honeys on RBCs against oxidative damage were similar to those reported for South African honeys (Serem and Bester 2012).
The antioxidant assays for foods and other biological materials are based on different principles, expressed in different units and, very often, are measured by different versions of the same assay in different laboratories. These shortcomings render comparisons of antioxidant capacities determined under different conditions impractical. Accordingly, indices which integrate the different modes of action of antioxidants as determined by different assays under different operating conditions have been proposed (Sun and Tanumihardjo 2007; Tabart et al. 2009). Within this framework, data from the different antioxidant assays for the honeys and their corresponding extracts were normalized and the resulting z-scores were averaged to yield the relative antioxidant capacity indices (RACI) (Sun and Tanumihardjo 2007). The RACIs of the honeys and their corresponding extracts exhibited very similar patterns (Fig. 1) with correlation coefficients of 0.9774 (P < 0.001) and 0.9937 (P < 0.001) for the honeydew and floral pairs, respectively. These findings indicate that the antioxidant activities of honeydew and floral honeys are very closely mimicked by their methanol-extractable phenolic fractions. Recently, very strong relationships (r = 0.9043–0.9978) between TEAC values (ABTS assay) of honey and its methanolic extract have been reported for a set of 56 honey samples (Sancho et al. 2016).
Fig. 1.
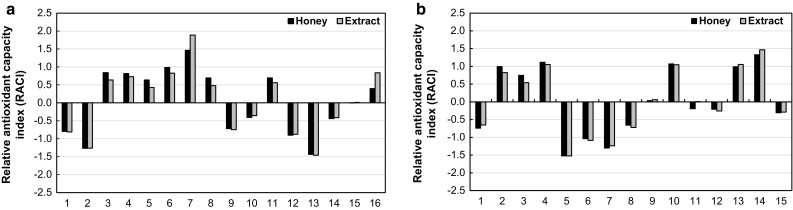
Relative antioxidant capacity indices (RACI) of honeys and their corresponding methanol-extractable phenolic fractions (a honeydew honey; b floral honey)
Conclusion
The nectar honeys were overwhelmingly multifloral reflective of the high floral biodiversity, absence of large swaths of land supporting a major crop/vegetation in Lebanon and the practice of moving beehives to different areas to exploit the different flowering periods in the country. In addition to having higher pH and darker colors, honeydew honeys contain more total phenols and Maillard reaction products and possess higher antioxidant capacities and ability to protect red blood cells against oxidative damage than floral honeys. Further, the Lebanese honeys exhibited, in general, comparable antioxidant potential to honeys from other parts of the world thereby making them a useful source of dietary antioxidants. At iso-phenol levels, the methanol-extractable phenolic fractions obtained by fractionation of honeydew and floral honeys exhibited lower antioxidant capacity than the entire honeys. The antioxidant capacity of entire honeys is modulated by flavonoids, phenolic acids, higher molecular weight polyphenols, proteins, carotenoids, ascorbic acid and Maillard reaction products and synergistic effects among these constituents. However, when compared at iso-phenol levels the methanol-extractable fraction comprising chiefly the flavonoids and phenolic acids exhibited similar antioxidant functionality including the ability to protect red blood cells against oxidative damage as the entire honey in both honeydew and floral honeys. The observed correspondence in antioxidative behavior suggests that the methanolic fraction could effectively mediate the therapeutic effects of honey. Further, the methanolic fraction serves as a concentrated source of food-grade flavonoid and phenolic acids thereby extending the range of applications of honey in food systems.
Acknowledgements
Funding of the work was provided by the University Research Board of the American University of Beirut. The expert help of Ms. Zainab Rizk in the pollen analyses and Mr. Samson Atamian in collecting the samples is gratefully appreciated.
Compliance with ethical standards
The study has been approved by the Institutional Review Board of the American University of Beirut.
Conflict of interest
The authors declare that they have no competing interests.
References
- Ajibola A. Novel insights into the health importance of natural honey. Malays J Med Sci. 2015;22:7–22. [PMC free article] [PubMed] [Google Scholar]
- Al ML, Daniel D, Moise A, Bobis O, Laslo L, Bogdanov S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009;112:863–867. doi: 10.1016/j.foodchem.2008.06.055. [DOI] [Google Scholar]
- Alvarez-Suarez JM, Gonzalez-Paramas AM, Santos-Buelga C, Battino M. Antioxidant characterization of native monofloral Cuban honeys. J Agric Food Chem. 2010;58:9817–9824. doi: 10.1021/jf1018164. [DOI] [PubMed] [Google Scholar]
- Alvarez-Suarez JM, Giampieri F, Gonzalez-Paramas AM, Damiani E, Astolfi P, Martinez-Sanchez G, Bompadre S, Quiles JL, Santos-Buelga C, Battino M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem Toxicol. 2012;50:1508–1516. doi: 10.1016/j.fct.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Bath KP, Singh N. Effect of microwave heating on hydroxymethylfurfural formation and browning in Helianthus annuus and Eucalyptus lanceolatus honey. J Food Sci Technol. 2001;38:366–368. [Google Scholar]
- Beretta G, Granata P, Ferrero M, Orioli M, Facino RM. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal Chim Acta. 2005;533:185–191. doi: 10.1016/j.aca.2004.11.010. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brudzynski K, Miotto D. The relationship between the content of Maillard reaction-like products and bioactivity of Canadian honeys. Food Chem. 2011;124:869–874. doi: 10.1016/j.foodchem.2010.07.009. [DOI] [Google Scholar]
- Can Z, Yildiz O, Sahin H, Turumtay EA, Silici S, Kolayli S. An investigation of Turkish honeys: their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015;180:133–141. doi: 10.1016/j.foodchem.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Chua LS, Lee JY, Chan GF. Characterization of the proteins in honey. Anal Lett. 2015;48:697–709. doi: 10.1080/00032719.2014.952374. [DOI] [Google Scholar]
- Cimpoiu C, Hosu A, Miclaus V, Puscas A. Determination of the floral origin of some Romanian honeys on the basis of physical and biochemical properties. Spectrochim Acta A. 2013;100:149–154. doi: 10.1016/j.saa.2012.04.008. [DOI] [PubMed] [Google Scholar]
- da Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R. Honey: chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Diez MJ, Andres C, Terrab A. Physicochemical parameters and pollen analysis of Moroccan honeydew honeys. Int J Food Sci Technol. 2004;39:167–176. doi: 10.1046/j.0950-5423.2003.00769.x. [DOI] [Google Scholar]
- Dong R, Zheng Y, Baojun XuB. Phenolic profiles and antioxidant capacities of Chinese unifloral honeys from different botanical and geographical sources. Food Bioprocess Technol. 2013;6:762–770. doi: 10.1007/s11947-011-0726-0. [DOI] [Google Scholar]
- Erejuwa OO, Sulaiman SA, Wahab MSA. Honey: a novel antioxidant. Molecules. 2012;17:4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escuredo O, Fernandez-Gonzalez M, Seijo MC. Differentiation of blossom honey and honeydew honey from Northwest Spain. Agriculture. 2012;2:25–37. doi: 10.3390/agriculture2010025. [DOI] [Google Scholar]
- Ferreira ICFR, Aires E, Barreira JCM, Estevinho LM. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem. 2009;114:1438–1443. doi: 10.1016/j.foodchem.2008.11.028. [DOI] [Google Scholar]
- Flanjak I, Kenjeric D, Bubalo D, Primorac L. Characterisation of selected Croatian honey types based on the combination of antioxidant capacity, quality parameters, and chemometrics. Eur Food Res Technol. 2016;242:467–475. doi: 10.1007/s00217-015-2557-0. [DOI] [Google Scholar]
- Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 2002;50:5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- Gillespie KM, Chae JM, Ainsworth EA. Rapid measurement of total antioxidant capacity in plants. Nat Protoc. 2007;2:867–870. doi: 10.1038/nprot.2007.100. [DOI] [PubMed] [Google Scholar]
- Han RM, Zhang JP, Skibsted LH. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules. 2012;17:2140–2160. doi: 10.3390/molecules17022140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosín I, Chicón RM, Cabezudo MD. Free amino acid composition and botanical origin of honey. Food Chem. 2003;83:263–268. doi: 10.1016/S0308-8146(03)00089-X. [DOI] [Google Scholar]
- Iglesias MT, Martín-Alvarez PJ, Polo MC, de Lorenzo C, Pueyo E. Protein analysis of honeys by fast protein liquid chromatography: application to differentiate floral and honeydew honeys. J Agric Food Chem. 2006;54:8322–8327. doi: 10.1021/jf061900n. [DOI] [PubMed] [Google Scholar]
- IHC (2009) Harmonised Methods of the International Honey Commission (2009) http://www.ihc-platform.net/ihcmethods2009.pdf. Accessed 10 May 2016
- Karabagias IK, Dimitriou E, Kontakos S, Kontominas MG. Phenolic profile, colour intensity, and radical scavenging activity of Greek unifloral honeys. Eur Food Res Technol. 2016 [Google Scholar]
- Kędzierska-Matysek M, Florek M, Wolanciuk A, Skałecki P, Litwińczuk A. Characterisation of viscosity, colour, 5-hydroxymethylfurfural content and diastase activity in raw rape honey (Brassica napus) at different temperatures. J Food Sci Technol. 2016;53:2092–2098. doi: 10.1007/s13197-016-2194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev E. Traditional healing with animals (zootherapy): medieval to present-day Levantine practice. J Ethnopharmacol. 2003;85:107–118. doi: 10.1016/S0378-8741(02)00377-X. [DOI] [PubMed] [Google Scholar]
- Louveaux J, Maurizio A, Vorwohl G. Methods of melissopalynology. Bee World. 1978;59:139–157. doi: 10.1080/0005772X.1978.11097714. [DOI] [Google Scholar]
- Manzanares AB, Hernandez ZG, Galdon BR, Rodriguez ER, Romero CD. Differentiation of blossom and honeydew honeys using multivariate analysis on the physicochemical parameters and sugar composition. Food Chem. 2011;126:664–672. doi: 10.1016/j.foodchem.2010.11.003. [DOI] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- MOE (2001) Ministry of Environment. Lebanon State of the environment report. http://www.moe.gov.lb/getattachment/The-Ministry/Reports/State-Of-the-Environment-Report-2001/Chap-10-Biodiversity.pdf.aspx. Accessed 10 May 2016
- Oddo LP, Heard TA, Rodríguez-Malaver A, Pérez RA, Fernández-Muiño M, Sancho MT, Sesta G, Lusco L, Vit P. Composition and antioxidant activity of Trigona carbonaria honey from Australia. J Med Food. 2008;11:789–794. doi: 10.1089/jmf.2007.0724. [DOI] [PubMed] [Google Scholar]
- Perez RA, Iglesias MT, Pueyo E, Gonzalez M, De Lorenzo C. Amino acid composition and antioxidant capacity of Spanish honeys. J Agric Food Chem. 2007;55:360–365. doi: 10.1021/jf062055b. [DOI] [PubMed] [Google Scholar]
- Ramírez-Arriaga E, Navarro-Calvo LA, Díaz-Carbajal E. Botanical characterization of Mexican honeys from a subtropical region (Oaxaca) based on pollen analysis. Grana. 2011;50:40–54. doi: 10.1080/00173134.2010.537767. [DOI] [Google Scholar]
- Sancho MT, Pascual-Mate A, Rodrıguez-Morales EG, Oses S, Escriche I, Periche A, Fernandez-Muino MA. Critical assessment of antioxidant-related parameters of honey. Int J Food Sci Technol. 2016;51:30–36. doi: 10.1111/ijfs.12988. [DOI] [Google Scholar]
- Saxena S, Gautam S, Sharma A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010;118:391–397. doi: 10.1016/j.foodchem.2009.05.001. [DOI] [Google Scholar]
- Serem JC, Bester MJ. Physicochemical properties, antioxidant activity and cellular protective effects of honeys from southern Africa. Food Chem. 2012;133:1544–1550. doi: 10.1016/j.foodchem.2012.02.047. [DOI] [Google Scholar]
- Singh N, Bath KP. Relationship between heating and hydroxymethylfurfural formation in different honey types. J Food Sci Technol. 1998;35:154–156. [Google Scholar]
- Sun T, Tanumihardjo SA. An integrated approach to evaluate food antioxidant capacity. J Food Sci. 2007;72:159–165. doi: 10.1111/j.1750-3841.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Tabart J, Kevers C, Pincemail J, Defraigne JO, Dommes J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009;113:1226–1233. doi: 10.1016/j.foodchem.2008.08.013. [DOI] [Google Scholar]
- Williams LJ, Abdi H. Fisher’s least significant difference (LSD) test. In: Salkind N, editor. Encyclopedia of research design. Thousand Oaks: Sage; 2010. pp. 491–494. [Google Scholar]


