Abstract
Spices are well known for their taste and flavor imparting properties. Green cardamom (Elletaria cardamomum), a herb spice belongs to family Zingiberaceae. In current study, GC–MS analysis of green cardamom essential oil (CEO) resulted in identification of twenty-six compounds with α-terpinyl acetate (38.4%), 1,8-cineole (28.71%), linalool acetate (8.42%), sabinene (5.21%), and linalool (3.97%) as major bioactive components. Present study also described the antimicrobial properties like zone of inhibition, minimum inhibitory concentration against microbial strains with special emphasis on quorum sensing inhibition. Disk diffusion assay showed that C. albicans and S. mutans were the most sensitive microorganisms followed by S. aureus, L. monocytogenes, B. cereus and S. typhimurium sensor strains, respectively. Whilst P. aeruginosa was found most resistant strain as CEO did not inhibited its growth. The minimum inhibitory concentration (MIC) values of CEO against tested strains were 10 ± 0.00 mg/mL against S. typhimurium, S. aureus and 5 ± 0.00 mg/mL against S. mutans, C. albicans strains, respectively. Regarding quorum sensing inhibition the tested concentrations 0.625 and 0.313 mg/mL of CEO inhibited violacein production with very little effect on growth of C. violaceum. Conclusively, study proved that quorum sensing inhibition values of CEO were much lower compared to MIC revealed values. Hence, cardamom bioactive constituents can effectively be used to develop novel antimicrobial drugs against conventional antibiotics.
Keywords: Green cardamom, GC–MS, Antimicrobial potential, Quorum sensing inhibition
Introduction
Spices exert therapeutic effects on human health including antimicrobial, anti-inflammatory, hypolipidemic, anti-mutagenic and anti-carcinogenic roles (Rahmatullah et al. 2009; Shukla et al. 2010). As spices also contained natural antioxidants so, their use exhibit health effects to swift positive changes in biological processes of cells like immune-competence, drug metabolism, induction of abnormal cell death (apoptosis), cell proliferation and differentiation (Kapoor et al. 2008; Sharma et al. 2011).
Among spices, cardamom (Elettaria cardamomum), perennial herbaceous plant of order Zingiberaceae, also known as green cardamom is used for prevention and control of several infections. It is commonly grown in tropical regions over the globe such as South-Western parts of the Indian Peninsula, Sri Lanka, Thailand, Vietnam, Mexico and Tanzania. In addition to provide distinctive flavor and fragrance in various foods, it is used as folk medicine, food preservative, and flavor modifier. In the fourth century, cardamom was used as a medicinal herb in India and was an item of Roman and Greek trade. It was mostly utilized as an aromatic stimulant, carminative, astringent, diuretic and anti-inflammatory in South Asia. It has also been used for the treatment of asthma, cardiac disorders, indigestion (stomatitis), rectal diseases and congestive jaundice as well as an insect controlling agent (Jamal et al. 2005; Verma et al. 2009; Sharma et al. 2011; Savan and Kucukbay 2013).
Cardamom fruit pods contained tiny, brown aromatic seeds that give pungent and sweet taste. It is commonly used as breath freshener to maintain oral health as antimicrobial agent that provides remedy for dental caries (Aneja and Radhika 2009). Furthermore, various clinical trials have confirmed that cardamom exhibits hypocholesterolemic, hypoglycemic, anti-inflammatory and antimutagenic properties against highly prevalent diseases (Hossain et al. 2008; Amma et al. 2010; Sharma et al. 2011; El-Yamani 2011).
Various methods have been reported for the fractionation and quantification of cardamom bioactive components. Mostly, spectrophotometer is used for simple analyses depending on the color development of the sample. However, for detailed categorization of bioactive components, gas chromatography mass spectroscopy (GC–MS) is recommended (Amma et al. 2010). Numerous studies have depicted that cardamom essential oil contains bioactive components, which represent its beneficial impact, such as α-terpinyl acetate (21.3–44.3%), 1,8-cineole (10.7–28.4%) and linalool (6.4–8.6%) (Menon et al. 1999; Singh et al. 2008; Savan and Kucukbay 2013).
According to various studies, cardamom extract possessed reducing power due to the presence of phenolic contents and a good correlation exists among TPC and DPPH assays. Therefore, it can effectively be used in nutraceutical diet for treatment of diseases. Owing to its antioxidant properties, cardamom can remarkably increase levels of glutathione and antioxidant enzymes in the body by stimulating glutathione transferase and glutathione peroxidase activity (Amma et al. 2010). Ultimately, antioxidants interfere with free radicals production and inactivate them, thus protecting the biological systems against deleterious effects of oxidative processes. These substances are mostly natural products like ascorbates, carotenoids, tocopherols, polyphenols that subsidize the prevention and cure of diseases because of good antioxidant activity (Khalaf et al. 2008; Hossain et al. 2008).
As, free radicals are constantly formed in the human body which can cause damage to cellular bio-molecules. To encounter these radicals through antioxidants, there is a mounting interest in using of plant derived nutraceutical compounds. In this context, cardamom contains sufficient amount of antioxidants including phenolics and flavonoids that can inhibit lipid peroxidation. Owing to high antioxidant activity, the use of cardamom not only reduces the chances of oxidative rancidity in fatty foods but also minimize the risk of various diseases (Bhatti et al. 2010). In this regard, nutritionists are predominantly focusing to cure physiological dysfunctions through phytochemical enriched diets obtained from herbs rather than pharmaceuticals due to their safety, nutritional and therapeutic perspectives.
In addition to above mentioned facts, present study was performed to characterize and quantify the bioactive components through GC–MS and also to investigate the antimicrobial potential of green cardamom essential oil against food pathogens with special emphasis on quorum sensing inhibition of Chromobacterium violaceum.
Materials and methods
Procurement of raw materials
Green cardamom were purchased from a local market. Cardamom fruits were cleaned and then ground to fine powder. The resultant powder was packed in air tight glass jar and stored under ambient conditions for further analysis. In current study, all the reagents and standards used for analytical purposes were HPLC grade.
Proximate analysis of cardamom powder
Chemical composition of cardamom powder was carried out by using standard methods of (AOAC 2006). The analysis includes the estimation of moisture, crude protein, crude fat, crude fiber and ash on dry weight basis. Similarly, nitrogen free extract (NFE) was calculated according to the following expression:
Characterization and quantification of essential oil constituents through GC–MS
The cardamom essential oil samples were diluted in tert-butyl-methyl ether (10 µL/mL) and were analyzed by a gas chromatography equipped with mass spectrometer (GC–MS), Shimadzu GC 2010 Plus and GCMS-TQ8040. One micro liter (1 µL) of solution was injected with an auto sampler (AOC 6000), the inlet temperature was 220 °C, in a Shimadzu SH-Rxi-5Sil MS (30 m long, 0.25 mm ID, 0.25 µm Film) column. The temperature program was fixed as 35 °C 4 min, rate 20 °C/min and 250 °C 10 min while Helium constant flow was set at 1 mL/min. Individual identifications were made by matching their spectra with those from mass spectral libraries and the identity of each component was confirmed by comparison of its Kovat’s index with those from literature (Adams 2005). Each component was listed in order of elution from the SH-Rxi-5Sil MS (DB5 equivalent) column. Relative percentages were calculated using the MS detector by peak integration without calculating the detector response factor.
Bacterial strains and growth conditions
The bacterial sensor strains used were Listeria monocytogenes Scott A, Staphylococcus aureus ATCC 29213, Streptococcus mutans ATCC 33402, Escherichia coli O157:H7, Bacillus cereus, Salmonella enteric subsp. Typhimurium JSG 1748, Pseudomonas aeruginosa ATCC 14213 and Candida albicans ATCC 11006. The strains were cultured in Tryptic Soy Broth (BD Difco, USA) and were grown 18–24 h at 37 °C with shaking. Chromobacterium violaceum ATCC 12472 was used to determine the quorum sensing inhibition of cardamom essential oil. The strain was inoculated in Miller Luria–Bertani Broth (Acros Organics, Belgium) and was cultured for 24 h at 27 °C.
Disc diffusion assay
The disc diffusion test was conducted according to the (CLSI) Performance Standards for Antimicrobial Disk Susceptibility Tests with slight modifications (CLSI 2012). Bacterial strains grown overnight were diluted 1:100 with corresponding fresh media to yield approximately 106 CFU/mL. This was verified by the plate counting method. A sterile cotton tipped applicator (Pur-Wraps, USA) was saturated with bacterial suspention and dried by the wall of the tubes, then the agar was swiped in three different direction. A blank disc was saturated with antimicrobial compounds and placed on the agar surface. Finally, plates were incubated for 24 h at 37 °C aerobically. Radii of zones of inhibition were measured in millimeters (mm) with a digital caliper (Fischer Scientific, Pittsburg, PA, USA) from the edge of the disk to the edge of the inhibition zone.
Determination of minimum inhibitory concentrations using broth micro-dilution assay
Broth micro-dilution assay was performed according to the guidelines (CLSI 2007) with minor modifications. The frozen stocks of bacteria were inoculated into Tryptic Soy Broth (TSB) (Remel, USA) and incubated at 37 °C for 24 h to obtain ~109 CFU/mL. The number of CFU was confirmed by spot plate method and cell counting. The bacterial culture was diluted (1:1000, v:v) in fresh broth medium to achieve ~106 CFU/mL. On the day of experiment, the cardamom essential oil (Sigma-Aldrich, Germany) was dissolved at first into ethanol (95%) to obtain a concentration of 100 mg/mL and then further diluted into TSB. The serial dilutions of antimicrobial compounds were made ranging from 20 to 0.151 mg/mL in 96-wells micro-plate in order to determine the minimum inhibitory concentration (MIC) value. Then, 100 μL samples of diluted bacterial suspension were pipetted to a 100 μL of each dilution of antibacterial which has certain concentration of cardamom essential oil. Seventy microliters of mineral oil was added to each wells of 96 wells plate to avoid evaporation of mixture during the incubation. The mixtures were incubated for 24 h aerobically inside micro-plate reader (SmartSpecTM 3000). After the incubation time, MIC values of antimicrobials were evaluated by taking the kinetic growth curve reading at optical density (OD595). Tryptic Soy Agar (Remel, USA) was used for bacterial plating and enumeration. The kinetic growth curve was statistically calculated taking readings after 24 h.
Quorum sensing inhibitory assay
This assay was conducted according to Zhu et al. (2011) with minor modifications. C. violaceum was inoculated into Miller Luria–Bertani Broth (LB) and incubated overnight at 27 °C for 24 h. The overnight culture was diluted 1:1000 with LB to obtain a concentration of 106 CFU/mL. The green cardamom essential oil was dissolve in ethanol (95%) and diluted into LB to obtain a concentration of 10 mg/mL. The stock solution was serially diluted with LB using 48-wells microplate. Then 500 µL of bacterial dilution (106 CFU/mL) cells were separately added to each well of microplate containing 500 µL of certain concentration of essential oil and then plate was incubated 24–28 h at 27 °C.
After incubation, 1 mL of the each well was centrifuged at 8000×g for 5 min and the supernatant was discarded. But the pellet was resuspended into 1 mL Dimethyl sulfoxide (DMSO) and vortexed for 2 min. The sample was centrifuged again at 8000×g for 5 min. Aliquots, 200 µL of the supernatants were added into a 96-wells plate and the absorbance was measured at OD595 nm using the plate reader. In order to confirm that cardamom inhibit quorum sensing without influence on bacterial growth activity, the pellet containing bacteria cell was resuspended in 1 mL sterile water, vigorously vortexed and the optical density was measured at OD595 nm using a plate reader. The experiments were repeated two times in duplicate.
Results and discussion
Chemical analysis of cardamom powder
Results demonstrated that samples possessed moisture 8.22 ± 0.34, crude protein 12.17 ± 0.48, crude fat 1.62 ± 0.11, crude fiber 11.38 ± 1.95, ash 7.74 ± 0.94 and NFE 58.89 ± 3.22 percent on dry wt. basis.
The results of present study were comparable with the earlier findings which reported moisture 9%, crude protein 12.72%, crude fiber 8.5% and ash content 6.97% in cardamom seed powder samples (Amma et al. 2010). The compositional variations in cardamom samples relating proximate composition depend on climatic conditions, varietal differences and agronomic practices. Moreover, the maturity and the stage of production are also very important factors that affect its compositional parameters.
Characterization and quantification of essential oil constituents through GC–MS
Twenty-six bioactive compounds were identified through GC–MS analysis listed in Table 1, representing 99.01% of the cardamom essential oil. The major bioactive components were found to be α-terpinyl acetate (38.4%), 1,8-cineole (28.71%), linalool acetate (8.42%), sabinene (5.21%), and linalool (3.97%).
Table 1.
Chemical composition of the green cardamom essential oil (E. cardamomum)
| Sr. no. | Retention time | Retention index | Name of compounds | Composition (%) |
|---|---|---|---|---|
| 1 | 7.619 | 931 | a-Thujene | 0.18 |
| 2 | 7.718 | 939 | a-Pinene | 1.65 |
| 3 | 8.176 | 978 | Sabinene | 5.21 |
| 4 | 8.243 | 983 | b-Pinene | 0.32 |
| 5 | 8.327 | 990 | Myrcene | 1.61 |
| 6 | 8.719 | 1029 | p-Cymene | 0.13 |
| 7 | 8.815 | 1040 | 1, 8 Cineole | 28.71 |
| 8 | 9.047 | 1064 | g-Terpinene | 0.13 |
| 9 | 9.168 | 1076 | Linalool oxide | 0.35 |
| 10 | 9.313 | 1091 | Terpinolene | 0.05 |
| 11 | 9.396 | 1099 | Linalool | 3.97 |
| 12 | 9.458 | 1107 | Tetrahydro Linalool | 0.15 |
| 13 | 10.258 | 1202 | a-terpineol | 2.62 |
| 14 | 10.404 | 1222 | cis-sabinene hydrate acetate | 0.95 |
| 15 | 10.556 | 1243 | Geraniol | 0.2 |
| 16 | 10.606 | 1249 | Linalool acetate | 8.42 |
| 17 | 10.771 | 1271 | Geranial | 0.41 |
| 18 | 11.142 | 1321 | Acetate | 0.12 |
| 19 | 11.384 | 1356 | a-terpinyl acetate | 38.4 |
| 20 | 11.525 | 1376 | Geranyl acetate | 0.94 |
| 21 | 11.966 | 1441 | g-Elemene | 0.08 |
| 22 | 12.448 | 1514 | a-farnesene | 0.46 |
| 23 | 12.763 | 1566 | (E) Nerolidol | 1.43 |
| 24 | 14.941 | 2035 | n-Hexadecanoic acid | 0.35 |
| 25 | 15.996 | 2452 | Fatty acids (C18) | 1.95 |
| 26 | 16.146 | 2509 | Fatty acids (C18) | 0.22 |
| Total | 99.01 |
Results of present study relating quantification of cardamom bioactive constituents were consistent with some earlier findings that reported that 1,8-cineole, α-terpinyl acetate and linalool as major compounds. A chemical characterization study of cardamom essential oil reported 1,8-cineole (28.4%), α-terpinyl acetate (21.3%) as main constituents of essential oil (Menon et al. 1999). Similarly, another study documented that 1,8-cineole (10.7%), α-terpinyl acetate (44.3%), α-terpineol (9.8%), linalool (8.6%) were the major compounds of essential oil (Singh et al. 2008).
Hamzaa and Osman (2012) characterized the green cardamom essential oil using GC–MS and found α-terpenyl acetate (60.65%), 1,8-cineol (13.63%), and limonene (7.12%) as major components. Recently, hydro-distilled cardamom essential oil obtained through Clevenger-type apparatus was analyzed by GC–MS and the results are in line with the current study as they reported the principle components were α-terpinyl acetate (40.7%), 1,8-cineole (25.6%), and linalool (6.3%) (Savan and Kucukbay 2013).
Moreover, the variations in essential oil composition were also depend on several factors viz. extraction method, experimental conditions, analytical chromatography conditions, particularly that of stationary phase using polar column. Furthermore, the essential oil is sensitive to heating as temperature about 110 °C produces citronellol, linalyl acetate, limonene-1,2-epoxide, linalool, thujyl alcohol, trans-pinocarveol and nerol that effect quality. Specifically, generation of linalyl acetate (17.8%) and borneol (12.1%) increased when oil was exposed to sunlight (Sultana et al. 2009).
Disc diffusion assay against some food and health pathogens
The results explicated a variable degree of antimicrobial activity of essential oil against different bacterial sensor strains. In current study, C. albicans and S. mutans were most sensitive microorganisms having maximum zones of inhibition 11.6 ± 0.56 mm and 11.4 ± 0.55 mm followed by S. aureus, L. monocytogenes, B. cereus and S. typhimurium sensor strains with 9.8 ± 0.20, 9.8 ± 0.17, 8.1 ± 0.15 and 5.7 ± 0.20 mm zones of inhibition, respectively. However, P. aeruginosa was the most resistant strain as cardamom essential oil did not revealed any inhibitory effect and unable to suppress its growth.
The outcomes of present research exhibited the inhibitory effects of cardamom essential oil against most pathogens causing food spoilage and S. mutans which is causal microorganism for various illnesses in human beings. Conclusively, cardamom essential oil possessed antimicrobial effects and assayed interesting results so it can effectively be used as an alternative in the development of novel antimicrobial drugs as body is producing resistance against conventional antibiotics used.
Minimum inhibitory concentrations (MIC) of green cardamom essential oil (GCEO)
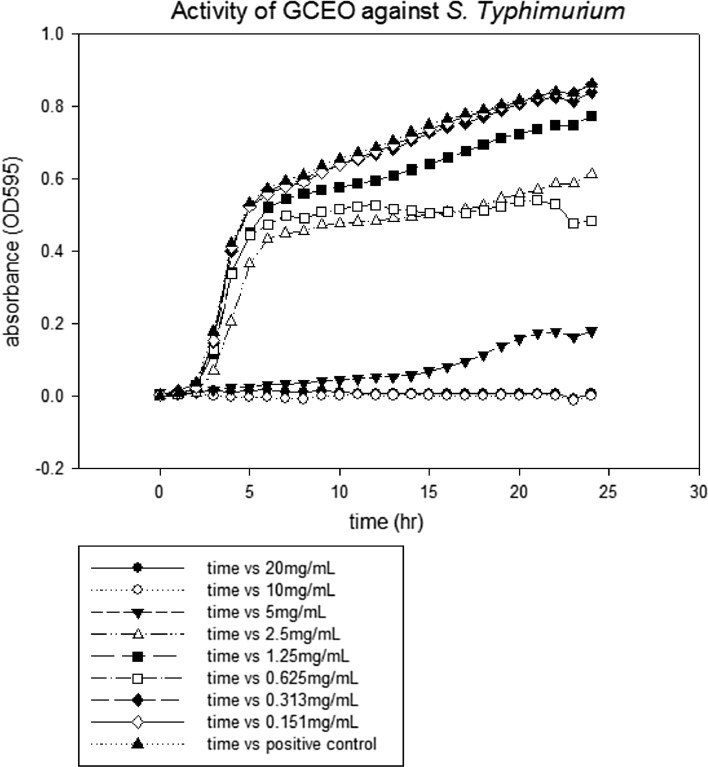
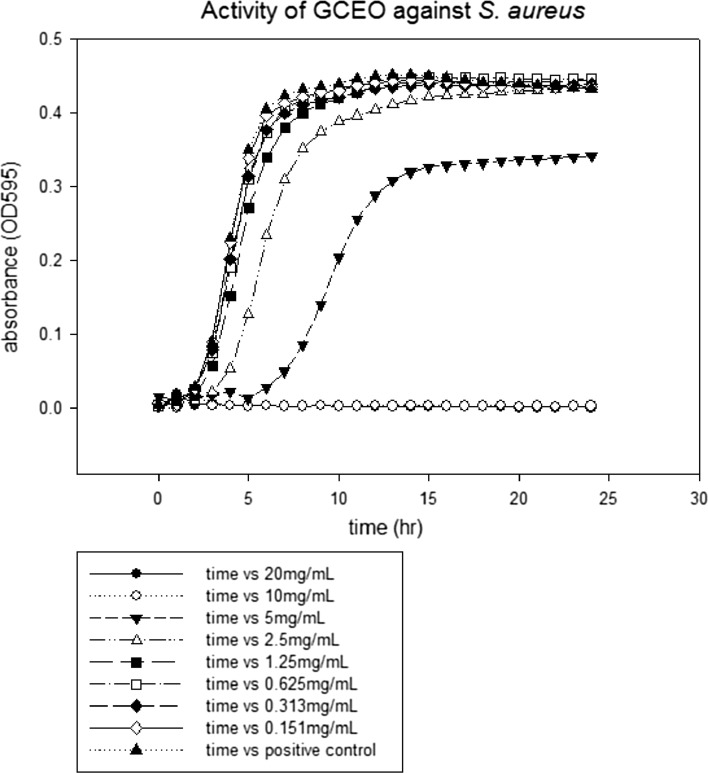
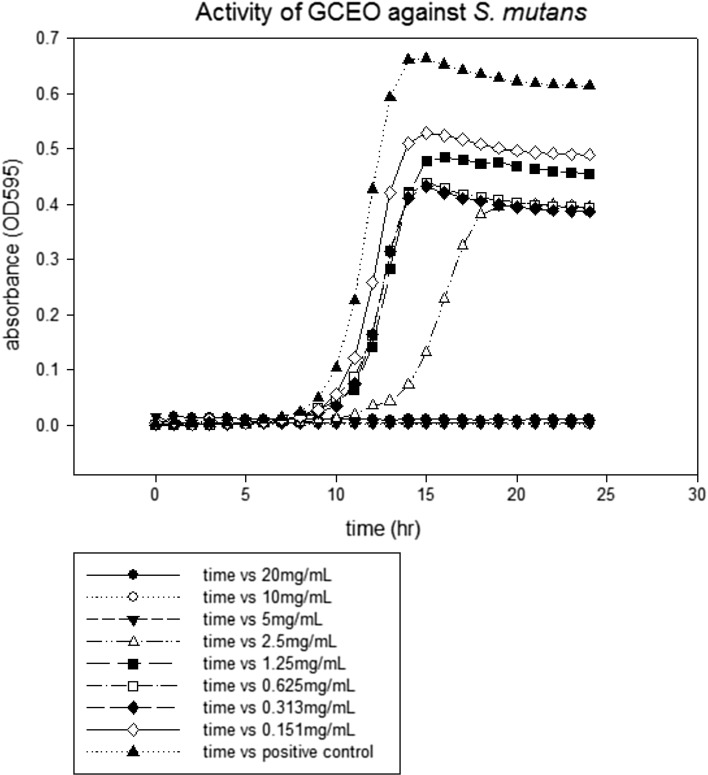
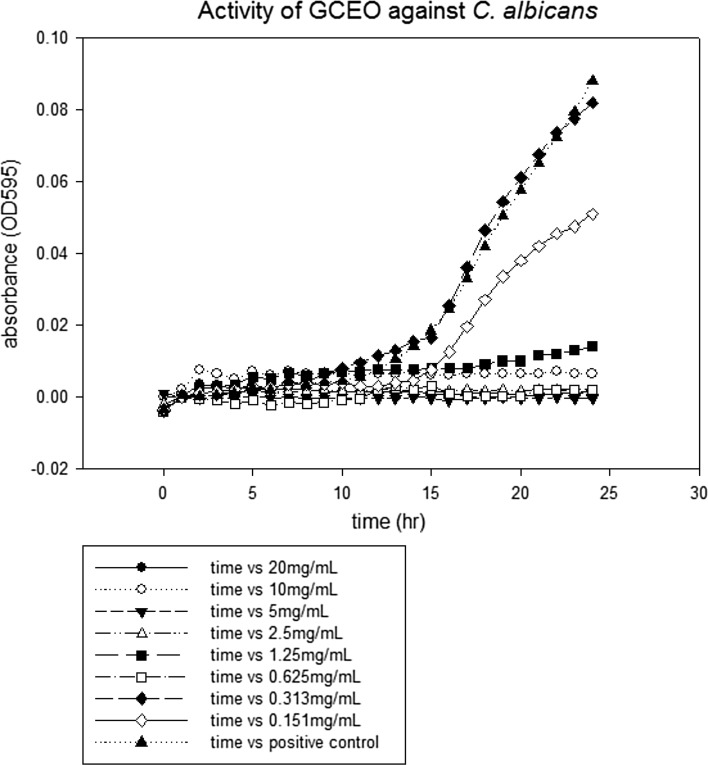
The lowest concentration of antimicrobial agent that inhibits the visible growth of microbial cells after overnight incubation is known as minimum inhibitory concentration (CLSI 2007). MIC values of GCEO were determined against a fungal and certain bacterial strains. All the tested strains showed growth inhibition at various MICs values as depicted in figures. The MICs of GCEO were 10 ± 0.00 mg/mL against S. typhimurium and S. aureus (Figs. 1, 3) whilst 5 ± 0.00 mg/mL against S. mutans and C. albicans strains, respectively (Figs. 2, 4).
Fig. 1.
The minimal inhibitory concentration of GCEO against Salmonella enteric subsp. Typhimurium JSG 1748
Fig. 3.
The minimal inhibitory concentration of GCEO against Staphylococcus aureus ATCC 29213
Fig. 2.
The minimal inhibitory concentration of GCEO against Streptococcus mutans ATCC 33402
Fig. 4.
The minimal inhibitory concentration of GCEO against Candida albicans ATCC 11006
Present study proved that cardamom essential oil is effective against Staphylococcus aureus, Salmonella typhi, Candida albicans and streptococci mutans. Hence, it may play imperative role in the discovery of novel antibiotics due to its significant antibacterial properties. It can also be used as preventive agent against dental caries as it stimulates salivary flow. Similarly, it exhibited antifungal activity and inhibit the growth of Aspergillus terreus (Singh et al. 2008; Aneja and Radhika 2009; Kaushik et al. 2010). In a efficacy study, cardamom has shown significant results in curing the physiological disorders as effectively reduced blood pressure (antihypertensive), enhanced fibrinolysis, antiplatelet aggregation and improved antioxidant status without altering blood lipid profile and fibrinogen levels significantly, in stage 1 hypertensive individuals (Verma et al. 2009).
Quorum sensing (QS) inhibition by green cardamom essential oil (GCEO)
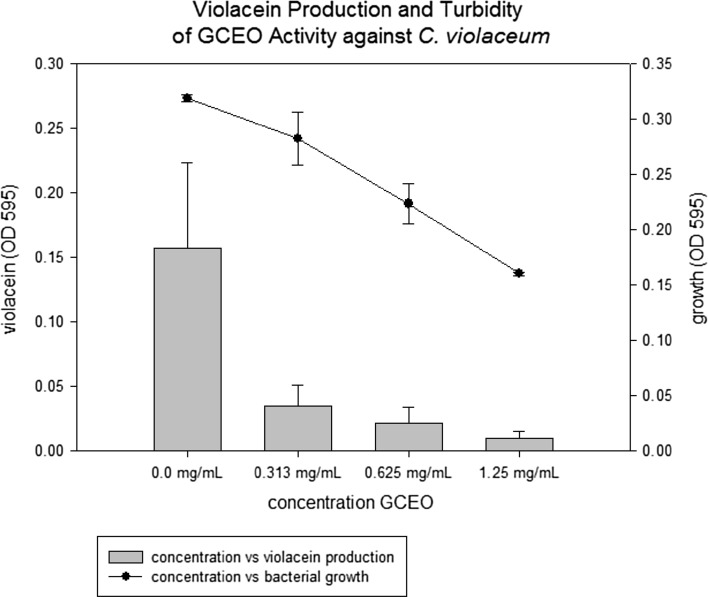
The effect of different concentrations of GCEO on violacein production was evaluated and results elicited moderate to good inhibition. C. violaceum, was used as biological indicator to determine quorum sensing inhibition of Gram-negative bacteria. The results were represented as QS inhibition (positive) when C. violaceum strain is not able to produce violet pigment (violacein) and if violacein is produced then we say no QS inhibition (negative).
Due to diverse nature of cardamom essential oil certain bioative components that were found both as major or minor constituent caused QS inhibition. The results showed quorum sensing inhibition with concentrations which were lower than MIC revealed values. The tested concentrations 0.625 and 0.313 mg/mL inhibited violacein production with very little effect on growth of C. violaceum (Fig. 5). However, 1.25 mg/mL showed more than 90% QS inhibition but with bacterial growth inhibition compared with positive control (bacterial cells without GCEO).
Fig. 5.
Production of violacein by C. violaceum in comparison with bacterial growth in presence of GCEO
QS inhibition is important to reduce the production of virulence factors released by pathogenic microorganism. It was evidenced that certain plant-derived compounds from many plant essential oils can interrupt quorum expression through inhibition of peptidoglycan synthesis, changing microbial membrane structures or regulating QS, which could influence biofilm formation (Niu and Gilbert 2004). Quite a number of mechanisms have been described to inhibit QS including inhibition of signal detection, blocking the production of signaling molecules using quorum sensing inhibitors (QSI). First QSI described was halogenated furanones, derived from marine alga Delisea pulchra, which inhibited QS by proteolytic degradation (Belapurkar et al. 2014). However, it has been shown that the effective dose of halogenated furanones is toxic (Torres et al. 2016). In addition, the disruption of the binding of signaling molecules to their receptor by competitive inhibition or decreasing the DNA-binding activity of receptor is influencing downstream gene expression (Zhao et al. 2015). Quorum quenching of signaling molecules could inactivate or degrade QS signals by many ways like, chemically (changing pH), physically (High-low temperature), enzymatically (degrading enzymes) and by microorganism (metabolizing signaling molecules) (Torres et al. 2016).
Conclusion
GC–MS characterization of cardamom essential oil (CEO) resulted in identification of twenty-six compounds with α-terpinyl acetate (38.4%), 1,8-cineole (28.71%), linalool acetate (8.42%), sabinene (5.21), and linalool (3.97%) as major components. Study results also demonstrated that CEO was effective against Staphylococcus aureus, Salmonella typhi, Escherichia coli, streptococci mutans and Candida albicans. Furthermore, among tested concentrations of CEO, 0.625 and 0.313 mg/mL revealed effective in bacterial quorum sensing inhibition by checking violacein production with very little effect on growth of C. violaceum. In conclusion, CEO may play imperative role in developing apparently safe and novel antibiotics against conventional antibiotics being used.
Acknowledgements
We are thankful to Shimadzu Scientific Instruments (19 Schoolhouse Road, Suite 107, Somerset, NJ, 08873) for providing the GC–MS instrument in support of the research work of the Center for Sensory Sciences and Innovation at Rutgers University. We also acknowledged the support of Department of Biochemistry and Microbiology, Rutgers, The State University of New Jersey. We also wish to thank Higher Education Commission (HEC), Pakistan for providing financial assistance to support the Ph.D. research work.
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. J Am Soc Mass Spectrom. 2005;16:1902–1903. doi: 10.1016/j.jasms.2005.07.008. [DOI] [Google Scholar]
- Amma KPAP, Rani MP, Sasidharan I, Nisha VNP. Chemical composition, flavonoid—phenolic contents and radical scavenging activity of four major varieties of cardamom. Int J Biol Med Res. 2010;1:20–24. [Google Scholar]
- Aneja K, Radhika J. Antimicrobial activity of Amomum subulatum and Elettaria cardamomum against dental caries causing microorganisms. Ethnobotan Leafl. 2009;13:849. [Google Scholar]
- AOAC (2006). Official methods of analysis, 18th edn. Washington DC
- Belapurkar R, Tale VS, Madkaikar R. Exploiting quorum sensing to inhibit the bacterial pathogens. Int J Curr Microbiol App Sci. 2014;3:453–458. [Google Scholar]
- Bhatti HN, Zafar F, Jamal MA. Evaluation of phenolic contents and antioxidant potential of methanolic extracts of green cardamom (Elettaria cardamomum) Asian J Chem. 2010;22:4787–4794. [Google Scholar]
- CLSI (2007) Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard—Seventh Edition, M11-A7
- CLSI (2012) Performance standards for antimicrobial disk susceptibility tests; approved standard—Eleventh Edition, M02-A11
- El-Yamani MAS. Cinnamon, cardamom and ginger impacts as evaluated on hyperglycemic rats. Res J Specif Educ. 2011;20:667–676. [Google Scholar]
- Hamzaa RG, Osman NN. Using of coffee and cardamom mixture to ameliorate oxidative stress induced in γ-irradiated rats. Biochem Anal Biochem. 2012;1:113–119. [Google Scholar]
- Hossain MB, Bruntonb NB, Barry-Ryana C, Martin-Dianaa AB, Wilkinson M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J Chem. 2008;1:751–756. [Google Scholar]
- Jamal A, Siddiqui A, Aslam M, Javed K, Jafri M. Antiulcerogenic activity of Elettaria cardamomum Maton. and Amomum subulatum Roxb. seeds. Indian J Trade Knowl. 2005;4:298–302. [Google Scholar]
- Kapoor IPS, Singh B, Singh G, Isidorov V, Szczepaniak L. Chemistry, antifungal and antioxidant activities of cardamom (Amomum subulatum) essential oil and oleoresins. Int J Essent Oil Ther. 2008;2:29–40. [Google Scholar]
- Kaushik P, Goyal P, Chauhan A, Chauhan G. In vitro evaluation of antibacterial potential of dry fruitextracts of Elettaria cardamomum Maton (Chhoti Elaichi) Iran J Pharm Res. 2010;9:287. [PMC free article] [PubMed] [Google Scholar]
- Khalaf NA, Shakya AK, Al-Othman A, El-Agbar Z, Farah H. Antioxidant activity of some common plants. Turk J Biol. 2008;32:51–55. [Google Scholar]
- Menon AN, Chacko S, Narayanan CS. Free and glycosidically bound volatiles of cardamom (Elettaria cardamomum Maton var. miniscula Burkill) Flav Fragr J. 1999;14:65–68. doi: 10.1002/(SICI)1099-1026(199901/02)14:1<65::AID-FFJ789>3.0.CO;2-A. [DOI] [Google Scholar]
- Niu C, Gilbert ES. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl Environ Microbiol. 2004;70:6951–6956. doi: 10.1128/AEM.70.12.6951-6956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmatullah M, Noman A, Hossan MS, Rashid M, Rahman T, Chowdhury MH, Jahan R. A survey of medicinal plants in two areas of Dinajpur district, Bangladesh including plants which can be used as functional foods. Am Eur J Sustain Agric. 2009;3:862–876. [Google Scholar]
- Savan EK, Kucukbay FZ. Essential oil composition of Elettaria cardamomum Maton. J Appl Biol Sci. 2013;7:42–45. [Google Scholar]
- Sharma S, Sharma J, Kaur G. Therapeutic uses of Elettaria cardamomum. Int J Drug Formul Res. 2011;2:102–108. [Google Scholar]
- Shukla S, Mistry H, Patel V, Jogi B. Pharmacognostical, preliminary phytochemical studies and analgesic activity of Amomum subulatum Roxb. Pharm Sci Monit. 2010;1:90–102. [Google Scholar]
- Singh G, Kiran S, Marimuthu P, Isidorov V, Vinogorova V. Antioxidant and antimicrobial activities of essential oil and various oleoresins of Elettaria cardamomum (seeds and pods) J Sci Food Agric. 2008;88:280–289. doi: 10.1002/jsfa.3087. [DOI] [Google Scholar]
- Sultana S, Ali M, Ansari SH, Bagri P. Effect of physical factors on the volatile constituents of Elettaria cardamomum fruits. J Essent Oil Bear Plants. 2009;12:287–292. doi: 10.1080/0972060X.2009.10643722. [DOI] [Google Scholar]
- Torres M, Rubio-Portillo E, Anton J, Ramos-Espla AA, Quesada E, Llamas I. Selection of the N-acylhomoserine lactone-degrading bacterium alteromonasstellipolaris PQQ-42 and of Its potential for biocontrol in aquaculture. Front Microbiol. 2016;7:646. doi: 10.3389/fmicb.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SK, Jain V, Katewa SS. Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of cardamom (Elettaria cardamomum) Indian J Biochem Biophys. 2009;46:503–506. [PubMed] [Google Scholar]
- Zhao J, Chen M, Quan CS, Fan SD. Mechanisms of quorum sensing and strategies for quorum sensing disruption in aquaculture pathogens. J Fish Dis. 2015;38:771–786. doi: 10.1111/jfd.12299. [DOI] [PubMed] [Google Scholar]
- Zhu H, He CC, Chu QH. Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular. Lett Appl Microbiol. 2011;52:269–274. doi: 10.1111/j.1472-765X.2010.02993.x. [DOI] [PubMed] [Google Scholar]