Abstract
The aim of this work was to assess whether the characteristic polyphenol traits of cherry biotypes persisted in thermally processed cherry products, such as jam. Thus, the RP-HPLC-diode array detector profiles of both colorless polyphenols and anthocyanins from three cherry varieties (two sweet and one tart cherry) were compared with those of low-sugar jam sourced from the same cultivars. Individual components were characterized by mass spectrometry. The total phenolic and total anthocyanin content as well as the radical scavenging potential (residual 75–91, 88–91 and 73–75%, respectively) were only slightly reduced by deep thermal treatments. Apart from the interconversion among the isomers of chlorogenic acid, the profile of both colorless polyphenols and anthocyanins substantially survived the jam manufacturing under conventional temperature–time regimen (80 °C, 1 h). The species- and cultivar-specific polyphenol molecular asset, especially the anthocyanin pattern, has potential to be monitored for traceability purpose, aimed to the varietal assessment of cherry biotypes used for producing jam.
Keywords: Sweet cherry, Tart cherry, Jam, Polyphenols, Anthocyanins, HPLC-profiles
Introduction
The polyphenol composition of edible berries varies depending on numerous biotic and abiotic factors. However, in general the pattern of polyphenols and their sub-classes is characteristic and fluctuates quantitatively within narrow ranges. Thus, the anthocyanin profile is distinctive of colored berries (Chen et al. 2014) and can uphold a classification system of berry fruits on a molecular basis (Fang 2015). Colorless polyphenols exhibit species- and cultivar-specific traits as well, because of a differential gene-dependent regulation of the polyphenol metabolic pathways (Degu et al. 2014).
We have recently demonstrated that both anthocyanins and colorless polyphenols distinguish tart from sweet cherries and can support their varietal assessment (Picariello et al. 2016). In particular, the anthocyanin patterns of tart and sweet cherries, as well as those of the three sweet cherry cultivars that have been analyzed in the current study were strikingly different from each other both qualitatively and quantitatively, as demonstrated by RP-HPLC and mass spectrometry analysis. In consideration of the health promoting effects of some polyphenols, the identification of specific components is a key aspect to correlate a cherry biotype to its functional properties (Grafe and Schuster 2014). The characterization of the polyphenol patterns also provides opportune analytical targets to distinguish cherry cultivars, whose price considerable changes according to the variety. No more than 30–40% of the yearly produced cherries is eaten as fresh fruit. Indeed, the largest part, especially of tart cherries, is canned, processed to purees, juices and concentrates or to jam, marmalade and compotes to be used by confectionery in pastries or by dairy companies to flavor yogurts and related products. The information about the original cherry variety usually is omitted in (semi)-transformed products, also because their labeling is not currently regulated.
The HPLC analysis of phenolics has been already proposed to authenticate quince jam, in order to disclose fraudulent adulteration with pear puree (Silva et al. 2000). Similarly, García-Viguera et al. (1997) demonstrated that the thermal processing for jam manufacturing does not modify qualitatively the fruit-characteristic HPLC profiles of anthocyanins.
On the other hand, data about heat stability of polyphenols appear controversial, since it is strictly dependent on the fruit typology and on the composition of the entire phenolic fraction in addition to the severity of the thermal treatment, water activity and pH (Fischer et al. 2013; Patras et al. 2010). Thermal treatments degrade 10–80% of the anthocyanins, depending on the time/temperature regimen. Previous researches reported an increased stability of anthocyanins in concentrate solution with respect to diluted extracts, suggesting that the jam manufacturing conditions might contribute to preserve anthocyanins at least partly (Havlíková and Míková 1985). Colorless phenolics seem to be relatively heat-resistant, in that more than 70% of the original content and 65% of the antioxidant capacity are retained in cherry jams (Kim and Padilla-Zakour 2004). However, most of the studies have compared the antioxidant potential, the total phenolics and/or total anthocyanins of fresh fruit and jam (Rababah et al. 2011), while the modifications interesting individual polyphenols or the patterns of polyphenol subsets are still under-investigated (Levaj et al. 2010).
In this work, we aimed at establishing whether the species- and cultivar-specific traits of cherry persist in transformed products. The monitoring of metabolites could be more cost-effective than DNA-based techniques of biotype assessment. To the best of our knowledge, this is the first analysis specifically designed for tracing the fruit cultivar in jam. To this end, we compared the RP-HPLC-diode array detector (DAD) profiles of the polyphenol extracts from three cherry varieties (two sweet and one tart cherry) with those of jam produced under ordinary laboratory scale conditions using the same cherry varieties. Individual components were characterized by mass spectrometry. Due to the above described fluctuation of polyphenol components, we did not aim at assessing the quantitative losses of individual polyphenols during jam manufacturing. In contrast, we monitored the possible evolution of selected molecular probes and the patterns of polyphenol subsets.
Materials and methods
Solvents and chemicals were of the highest commercially available purity. Methanol, HPLC–MS grade acetonitrile (ACN) and concentrated HCl were provided by Carlo Erba (Milan, Italy). Trifluoroacetic acid (TFA), 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), Trolox, Folin–Ciocalteu’s phenol reagent, standard of cyanidin-3-O-glucoside, gallic acid, quercetin-3-O-rutinoside, quercetin-3-O-glucoside, quercetin-3-O-rhamnoside (quercitrin) and chlorogenic acid were from Sigma (St. Louis, MO, USA).
Cherries were sampled from three varieties, two of which Prunus avium (Napoleon and Bing cultivars) and one P. cerasus (Montmorency or ‘‘amarene’’), harvested at commercial maturity during spring/summer 2015 in an experimental orchard located in the Irpinia area (Campania Region, Italy). For each variety, about 3 kg of cherries were randomly harvested by several trees to mediate the effect on polyphenol content of different sunlight exposition. The pH was measured at the harvest with a solid-state pH probe SevenEasy S20 (Mettler-Toledo, Columbus, OH, USA). Polyphenols were extracted within 2 h from harvesting. Similarly, jam was prepared from fresh cherries. Soluble solid content (°Brix) of fresh cherry and jam was measured using a refractometer (Optech RM 65, Optical Technology, Munchen, Germany).
Laboratory scale jam preparation
For the preparation of low sugar jam, pitted cherries (500 g) were mixed with 25 g of a commercial low methoxyl pectin powder (purchased at a local market) in a stainless steel pot and warmed for 5 min at 80 °C. After incorporating sucrose (200 g) the mixture was processed at 80 °C for 1 h under continuous stirring. The temperature was raised at 100–102 °C during last 5 min. Hot jam was transferred into previously sterilized glass jars, capped and allowed to cool overnight at room temperature. Soluble solid content of jam was within the 62.5–65 °Brix range. Polyphenols were extracted from jam within 24 h after preparation.
Polyphenol extraction
Total polyphenols were extracted from pitted cherries or from jam according to Gonzàlez-Gòmez et al. (2010). Briefly, cherries (25 g) were homogenized with 100 mL of 70% aqueous methanol (v/v) containing 0.2% HCl, in an ice cold bath in the dark for 5 min. The suspension was then magnetically stirred for 24 h at −20 °C in the dark, centrifuged (2000 × g, 15 min, 4 °C), filtered on paper and finally stored at −20 °C until analysis.
Total polyphenols, total anthocyanins and antioxidant activity
The concentration of total phenols (TPC) in the extracts was determined by the Folin–Ciocalteu colorimetric method, according to the general procedures recommended by the European Pharmacopoeia for determination of total tannins (European Directorate for the Quality of Medicines 2007), monitoring the absorbance at 760 nm with a UV–vis spectrophotometer (Amersham Ultrospec 2100 Pro UV/Vis, GE Healthcare, Uppsala, Sweden). Gallic acid within the 100–500 mg/L concentration range was used as the standard. TPC was expressed as mg gallic acid equivalent/100 g of fresh weight fruit (mgGAE/100 g FW). Samples were assayed in triplicate and values were averaged.
The total monomeric anthocyanin (TAC) concentration of cherry and jam extracts was determined by the pH differential method using the procedure by Lee et al. (2005). TAC was expressed as mg cyanidin 3-O-glucoside equivalents (C3G)/100 g FW.
The radical scavenging capability of the cherry extracts was determined with the DPPH· method (Brand-Williams et al. 1995), using a methanol solution of anthocyanins (at test concentration) as the spectrophotometric blank, in order to minimize the interference of anthocyanins at the absorption wavelength λ = 517 nm (Ge and Ma 2013). The antiradical activity was expressed as percentage of DPPH· inhibition (% I) exerted by the sample extracts, according to the formula: % I = (ADPPH − Asample/ADPPH) × 100, where ADPPH and Asample are the absorbance of DPPH· before and after addition of the test sample solutions. Trolox was used as a standard. To test repeatability, samples were assayed in triplicate and results were averaged.
RP-HPLC analysis of cherry polyphenols
Polyphenols were separated using a modular chromatographer (HP 1100 Agilent Technologies, Paolo Alto, CA, USA) equipped with a 250 × 2.0 mm i.d. C18 reversed-phase column, 4 µm particle diameter (Jupiter Phenomenex, Torrance, CA, USA), at 37 °C using a thermostatic oven. Separations were carried out at a 0.2 mL/min constant flow rate, applying the following gradient of the solvent B (ACN/0.1%TFA): 0–4 min: 0% B; 4–14 min 0–14% B; 14–30 min 14–28% B; 30–34 min 28% B; 34–42 min 28–60% B; 42–45 min 60–80% B; 45–50 min 80–100% B. Solvent A was 0.1% TFA in HPLC-grade water. For each analysis 15 µL of the extracts were injected after a fivefold dilution with eluent A. Separations were monitored with a diode array detector (DAD) acquiring spectra every 2 s in the 190–650 nm range. Chromatograms were extracted at λ = 520, 360, 320 and 280 nm wavelengths and peaks were integrated using the HPLC ChemStation software vers. A.07.01 (Agilent) furnished with the chromatographer. HPLC peaks were manually collected for the subsequent MALDI-TOF MS analysis.
Mass spectrometry
HPLC isolated cherry and jam polyphenols were identified by off-line matrix assisted laser desorption ionization—time of flight (MALDI-TOF) MS using a Voyager DE-PRO (PerSeptive Biosystems, Framingham, MA, USA) instrument and by nanoflow-HPLC-electrospray ionization (ESI) tandem MS (MS/MS) using a nano-HPLC (Ultimate 3000 equipped with a Famos autosampler, Thermo/Dionex, Sunnydale, CA, USA) coupled to a quadrupole-TOF (Q-TOF) hybrid spectrometer (Q-Star Pulsar, Applied BioSystems, Foster City, CA, USA) equipped with a nano-ESI source (Protana, Odense, Denmark). MALDI-TOF MS analyses were carried out in both positive and negative ion modes, whilst nano-HPLC–ESI–MS/MS operated only in the positive ion mode, scanning the 250–1500 m/z range. This latter analysis was carried out in data dependent acquisition, selecting the most two intense ions for MS/MS fragmentation, applying a dynamic exclusion of 12 s. Mono-charged ions were selected for fragmentation provided that they had m/z >350 their MS1 intensity was at least 102 higher than noise ions (previously estimated with a blank run). MS/MS fragmentation spectra were generated at a collision energy of 25 units. Separation gradients and additional instrument parameters have been previously detailed (Picariello et al. 2016). Identification of the main compounds was confirmed by HPLC and MS comparison with authentic standards, when available.
Statistics
All data are the means of three replicate determinations. Data were subjected to analysis of variance (ANOVA) in order to compare the mean values of the investigated parameters at different statistical levels of significance. Statistical analysis were carried out with the Microsoft Excel 2013 software.
Results and discussion
TPC, TAC, radical scavenging properties
The pH, °Brix, TPC, TAC and DPPH· inhibition values of fresh cherries and jams are summarized in Table 1. Both TPC and TAC of fresh cherries were significantly higher than those obtained for the same varieties harvested two years before. Most likely, the increased polyphenol content was related to an exceptionally sunny spring-summer period during the vintage investigated. The values for jams are referred either to the jam weight or to the original fruit content. However, the effect of polyphenol concentration due to water evaporation during jam manufacturing has not been taken into account. Overall, 75–81% of TPC and 88–91% of TAC from fresh cherries (referred to the original fruit content) survived the heat processing of jam manufacturing. The DPPH radical scavenging values decreased at quite higher rates, probably due to the thermal degradation of non-polyphenol antioxidant compounds, such as ascorbic acid. As expected TPC, TAC and antiradical DPPH activity was higher for tart than for sweet cherries. This trend was also maintained in jam.
Table 1.
Chemical parameters (pH and °Brix), total phenolics components (TPC), total anthocyanin components (TAC) and in vitro anti-radical scavenging properties of cherry biotypes and corresponding jams
| Cultivar | pH | °Brix | TPC | TAC | DPPH (% I)c |
|---|---|---|---|---|---|
| mgGAE/100 g FWa | mgC3GE/100 g FWb | ||||
| Fresh cherries | |||||
| Napoleon | 4.74 | 18.0 | 64.3 | 10.5 | 65.7 |
| Bing | 4.20 | 16.5 | 116.5 | 51.2 | 72.3 |
| Montmorrency | 3.20 | 15.5 | 484.1 | 87.4 | 80.0 |
| mgGAE/100 g jam | mgC3GE/100 g jam | ||||
|---|---|---|---|---|---|
| (mgGAE/100 g fruit) | (mgC3GE/100 g fruit) | ||||
| Jam | |||||
| Napoleon | 3.57 | 65.0 | 34.7 (48.6) | 6.9 (9.6) | 48.0 |
| Bing | 3.60 | 63.0 | 67.6 (94.6) | 32.4 (45.3) | 54.2 |
| Montmorrency | 3.28 | 62.5 | 259.3 (363.3) | 55.2 (77.3) | 61.9 |
Values are average of triplicate determinations. Standard deviation was lower than 10% in all cases
a mgGAE, mg gallic acid equivalent
b mgC3GE, mg cyanidin-3-O-glucoside equivalent; FW, fresh weight (pitted cherries)
c %I, % inhibition of DPPH radicals
Identification of polyphenol compounds
In general, although the nano-HPLC ESI–MS/MS analysis enabled in most of cases the unambiguous identification of the polyphenol components, also included the minor ones, the RP-HPLC–DAD (narrow-bore column) analysis provided straightforwardly comparable chromatographic patterns, under both qualitative and quantitative standpoints. For this reason, the identification of polyphenol compounds and the comparison of chromatographic patterns rely on RP-HPLC–DAD coupled to off-line MALDI-TOF MS and nano-HPLC ESI–MS/MS, as complementary strategies. The off-line MALDI TOF MS analysis of isolated components allowed to correlate RP-HPLC and nano-HPLC–ESI–MS/MS chromatograms.
Colorless polyphenols
The definition of the polyphenol pattern has been proposed since years for assessing the authenticity of commercial jams from specific fruits, as reviewed by Fügel et al. (2005). However, in this sense the use of the patterns of colorless polyphenols can be affected by some drawbacks, due to: (1) the heterogeneity of the phenol fraction of many fruits; (2) the relatively high fluctuation of polyphenol components; (3) the hardly appreciable differences among strictly related fruits or among intra-species cultivars.
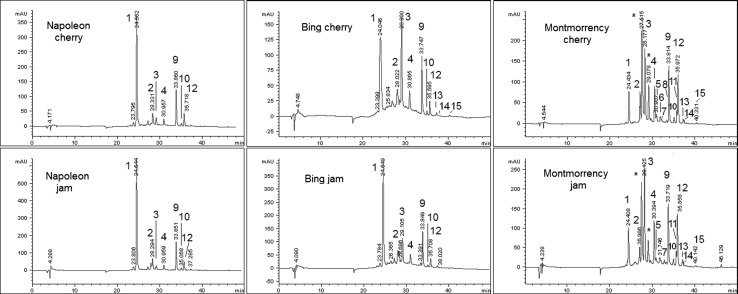
The RP-HPLC chromatograms (λ = 360 nm) of polyphenols from the three cherry varieties are compared to the corresponding jam extracts in Fig. 1. Main HPLC peaks are assigned in Table 2. The comparison among HPLC chromatograms confirmed that tart cherries have a more variegate metabolite asset than the sweet counterpart, including the occurrence of specie-specific metabolites such as kaempferol-3-O-rutinoside (Picariello et al. 2016). As expected, the dominant colorless polyphenols of both sweet and tart cherries are hydroxycinnamic acid derivatives, such as neochlorogenic and chlorogenic acids, and flavonols, such as quercetin glycosides. Differently from anthocyanins, hydroxycinnamic acid derivatives are major compounds of all the sweet and tart cherry cultivars, though their relative ratio varies greatly, depending on a number of environmental factors and post-harvest processing. In fact, the HPLC profiles of cherry varieties appeared significantly different in quantitative terms from those obtained from the same biotypes during different vintages (cfr. with the corresponding chromatograms in Picariello et al. 2016), the most evident discrepancies involving the hydroxycinnamic acids, primarily chlorogenic acid. In fresh and frozen cherries, chlorogenic acid undergoes natural fluctuation due to a partial conversion into the quinone form, mediated by endogenous polyphenoloxidase. This process is obviously not operating in thermal processed jam, due to enzyme inactivation. On the other hand, quinonic chlorogenic acid can be produced and regenerated by coupling reactions with anthocyanin pigments (Kader et al. 1998), also triggering the browning effects observed in jams. In addition, chlorogenic (3-O-caffeoylquinic) and neochlorogenic (5-O-caffeoylquinic) acids mutually interconvert and establish a thermodynamic equilibrium also with cryptochlorogenic (4-O-caffeoylquinic) acid even under mild conditions of temperature and pH (Li and Ho 2006). For these reasons, hydroxycinnamic acid derivatives are not good candidates as signature metabolites for the cherry varietal assessment. Thus, in general, while the HPLC profile of colorless polyphenols can enable a relatively straightforward discrimination between sweet and tart cherries, it can hardly support an intraspecific assessment of the cultivars.
Fig. 1.
HPLC comparison of polyphenol extracts from cherry varieties (upper) and corresponding jams (lower), with detection at 360 nm. Labelled peaks are assigned in Table 2. Asterisk anthocyanins
Table 2.
Tentative identification of the HPLC peaks of cherry and jam extracts based on spectrophotometric (DAD) and MS data (Picariello et al. 2016)
| HPLC peak | λmax | m/z | Anthocyanin |
|---|---|---|---|
| [M + H]+/M+ (aglycon) | |||
| 1 | 324 | 355 | Neochlorogenic acid |
| 2 | 310 | 339 n.d. | 3-p-coumaroylquinic acid |
| 3 | 324 | 355 | Chlorogenic acid |
| 4 | 351 | 773 (303) | Quercetin-3-O-glucosylrutenoside |
| 5 | 280 | 291 | Catechin |
| 6 | 310 | 339 n.d. | 4-p-coumaroylquinic acid |
| 7 | 280 | 291 | Epicatechin |
| 8 | 326 | 517 n.d. | 3,5-dicaffeoylquinic acid |
| 9 | 353 | 611 (303) | Quercetin-3-O-rutinoside |
| 10 | 354 | 465 (303) | Quercetin-3-O-glucoside |
| 11 | 345 | 595 (287) | Kaempferol-3-O-rutinoside |
| 12 | 352 | 625 (317) | Isorhamnetin-3-O-rutinoside |
| 13 | 346 | 449 (287) | Kaempferol-3-O-hexoside |
| 14 | 352 | 479 (317) | Isorhamnetin-3-O-hexoside |
| 15 | 353 | 449 (303) | Quercetin-3-O-rhamnoside |
| 16 | 518 | 611 (287) | Cyanidin-3-sophoroside (trace) |
| 17 | 516 | 757 (287) | Cyanidin-3-O-glucosylrutinoside |
| 18 | 521 | 611 (303) | Delphinidin-3-O-rutinoside |
| 19 | 521 | 727 (287) | Cyanidin -3-O-xylosylrutinoside |
| 20 | 519 | 595 (287) | Cyanidin-3-O-rutinoside |
| 21 | 517 | 609 (301) | Peonidin-3-O-rutinoside |
Except for the significant imbalance between the relative levels of chlorogenic acid isomers, no major differences were observed between the profiles of cherry extracts and the corresponding jams. Quercetin glycosides (flavonols) and minor flavan-3-ol components in the tart biotype, i.e. catechin and epicatechin, were easily distinguished within the chromatograms of the jam extracts as compared to the corresponding cherry ones. These findings confirm that colorless polyphenols are not drastically affected by severe heat treatments and modifications in the polyphenol composition of jam are substantially limited to moderate quantitative changes if compared to fresh cherries (Bonerz et al. 2007). Similar results have been reported when the phenolic compounds of quince fruit and jam have been compared (Silva et al. 2000). Based on the relevant peak area and intensity, some specific compounds, such as neochlorogenic acid, were more abundant in jams than in the corresponding cherry fruits (Fig. 1). This apparent incongruity is partly justified by the effect of concentration due to water evaporation during jam manufacturing. More importantly, polyphenols that are matrix-bound or entrapped in the cellular structures in fresh fruit can be released during pectin solubilization subsequent to the thermal processing (Dewanto et al. 2002). On the contrary, the possible thermal degradation of other components (e.g. simple phenolics) impacts the TPC content of jam that, in general, was lower than for fresh cherries.
Anthocyanins
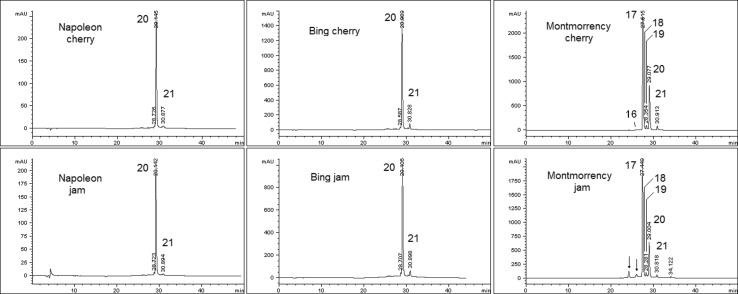
The HPLC profile of anthocyanins significantly differ between sweet and tart cherries. In addition, the anthocyanin profile of cherries is distinctive of the cultivar both qualitatively and quantitatively (Picariello et al. 2016; Dewanto et al. 2002). The RP-HPLC chromatograms of anthocyanins from cherry and jam extracts are compared in Fig. 2. The HPLC peaks are assigned in Table 2, based on spectrophotometric (DAD) and mass spectrometric data. In line with the TAC values, the most part of the anthocyanins endured the thermal processing. Indeed, 84.5, 76.0 and 79.6% of the anthocyanins in fresh Napoleon, Bing and Montmorency cherries, respectively, remained unmodified in jam, as assessed by comparing the total HPLC peak area values. The degradation process involved all the anthocyanin compounds at a comparable level, so that the relative ratio among anthocyanin peaks was well-conserved. The variability, estimated by the ratio of corresponding peaks in fresh cherry and jam pairs, ranged within 8% units. In line with these results, Zorić et al. (2014) demonstrated that the thermal degradation follows a first order decay for all the anthocyanins, so that the individual components decrease with a similar kinetics. Similarly, it has been established that anthocyanins are not unevenly lost during storage of jam and related products (Will et al. 2005). Thus, the HPLC patterns of jam anthocyanins closely reflected those of the respective cherry cultivar. Likewise, it is noteworthy that the anthocyanin patterns did not qualitatively change according to the vintage, as demonstrated by comparison with the analysis carried out previously (Picariello et al. 2016). Minor neo-formed components were detected by HPLC only in the jam of tart cherry (Fig. 2). These anthocyanin compounds were more hydrophilic than the original anthocyanins and probably are the results of thermo-induced coupling of anthocyanins to sugars. Nevertheless, by our analyses we did not achieve a definitive characterization of these compounds.
Fig. 2.
HPLC comparison of anthocyanin extracts from cherry varieties (upper) and corresponding jams (lower), with detection at 520 nm. Labelled peaks are assigned in Table 2. The low-abundance peaks of Montmorency jam indicated by arrows are probably neoformed anthocyanin components arising from thermally induced glycation
The stability of anthocyanins depends on a complex series of side factors, among which the concentration of colorless co-pigments, which can prevent degradation of anthocyanins during jam processing (Fischer et al. 2013; Shikov et al. 2008). Pectins from fruits or roots ordinarily used in jam preparation also can contribute to stabilize pigments and to reduce degradative browning, so justifying our findings about the relatively high stability of anthocyanins in cherry jams (Maier et al. 2009; Poiana et al. 2013). Our results conflict with previous ones from other authors that described a loss of more than 90% of anthocyanins subsequent to tart cherry jam making (Kim and Padilla-Zakour 2004), although they used process temperature significantly higher than ours (104–105 °C) and pH adjustment with citric acid. On the other hand, a large number of even very recent investigations agree to indicate that mild industrial or domestic jam processing retains most of the cherry polyphenols (Rababah et al. 2013) and, in particular, affects anthocyanin amounts at a low extent (Toydemir et al. 2013). Moreover, our results are in line with previous ones indicating a loss of individual anthocyanins ranging between 20 and 40%, depending also on storage conditions.
Conclusion
Most of the polyphenols survived the jam manufacturing under “ordinary” temperature–time regimen. The persistence of anthocyanins in processed fruits has relevant technological implications, related to the appearance of the final products, besides the potential antioxidant effect. Herein, we demonstrate that the anthocyanin patterns are substantially preserved in jam, thereby they can be monitored for the varietal assessment of cherries used for jam production. Due to clear qualitative and quantitative differences in the anthocyanins, the discrimination between jam from sweet and tart cherries is relatively straightforward. In particular, the relative amounts of cyanidin-3-O-xylosylrutinoside and kaempferol-3-O-rutinoside which were identified as exclusive of tart cherries (P. cerasus), did not change significantly. In addition, we demonstrated that in principle HPLC analysis of anthocyanins could enable the intra-specific identification of the cultivars. It is also expected that the anthocyanin profiling would allow to disclose the addition of pigments from foreign sources in jam and cherry-flavored products. Obviously, further investigations are required to explore the full potentiality of this strategy. In particular, the analysis should be extended to a broader number of varieties, including a proper statistical data clustering, considered that hundreds of cherry biotypes are currently cultivated worldwide. In perspective, our outcomes could promote the creation of a database containing HPLC profiles of polyphenols, especially anthocyanins, from reference cherry cultivars for traceability purposes.
Acknowledgements
This research was in part supported by the BenTeN project (Wellness from biotechnologies: New Processes and Products for Nutraceutical, Cosmeceutical and Human Nutrition), within the Biotechnology Network of Campania Region (Italy).
Author Contributions Statement
G.P. and M.G.V. conceived the experiments. F.D.C. and E.S. prepared the samples. G.P. performed HPLC and mass spectrometry analyses. G.P., M.G.V and P.F. analyzed reports and wrote the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
Contributor Information
Gianluca Picariello, Phone: +39 0825 299521, Email: picariello@isa.cnr.it.
Maria Grazia Volpe, Phone: +39 0825 299513, Email: mgvolpe@isa.cnr.it.
References
- Bonerz D, Würth K, Dietrich H, Will F. Analytical characterization and the impact of ageing on anthocyanin composition and degradation in juices from five sour cherry cultivars. Eur Food Res Technol. 2007;224:355–364. doi: 10.1007/s00217-006-0328-7. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chen L, Xin X, Yuan Q, Su D, Liu W. Phytochemical proprieties and antioxidant capacities of various colored berries. J Sci Food Agric. 2014;94:180–188. doi: 10.1002/jsfa.6216. [DOI] [PubMed] [Google Scholar]
- Degu A, Hochberg U, Sikron N, Venturini L, et al. Metabolite and transcript profiling of berry skin during fruit development elucidates differential regulation between Cabernet Sauvignon and Shiraz cultivars at branching points in the polyphenol pathway. BMC Plant Biol. 2014;26:188–208. doi: 10.1186/s12870-014-0188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activities. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- European Directorate for the Quality of Medicines. Council of Europe (2007) Determination of tannins in herbal drugs. In: European pharmacopoeia, 6th edn. Strasbourg, p A286
- Fang J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition. 2015;31:1301–1306. doi: 10.1016/j.nut.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Fischer UA, Carle R, Kammerer DR. Thermal stability of anthocyanins and colourless phenolics in pomegranate (Punica granatum L.) juices and model solutions. Food Chem. 2013;138:1800–1809. doi: 10.1016/j.foodchem.2012.10.072. [DOI] [PubMed] [Google Scholar]
- Fügel R, Carle R, Schieber A. Quality and authenticity control of fruit purées, fruit preparations and jams—a review. Trends Food Sci Technol. 2005;16:433–441. doi: 10.1016/j.tifs.2005.07.001. [DOI] [Google Scholar]
- García-Viguera C, Zafrilla P, Tomás-Barberán FA. Determination of authenticity of fruit jams by HPLC analysis of anthocyanins. J Sci Food Agric. 1997;73:207–213. doi: 10.1002/(SICI)1097-0010(199702)73:2<207::AID-JSFA703>3.0.CO;2-8. [DOI] [Google Scholar]
- Ge Q, Ma X. Composition and antioxidant activity of anthocyanins isolated from Yunnan edible rose (An ning) Food Sci Human Wellness. 2013;2:68–74. doi: 10.1016/j.fshw.2013.04.001. [DOI] [Google Scholar]
- Gonzàlez-Gòmez D, Lozano M, Fernàndez-Leòn M, Bernalte MJ, Ayuso AC, Rodrìguez AB. Sweet cherry phytochemicals: identification and characterization by HPLC-DAD/ESI-MS in six sweet-cherry cultivars grown in Valle del Jerte (Spain) J Food Compos Anal. 2010;23:533–539. doi: 10.1016/j.jfca.2009.02.008. [DOI] [Google Scholar]
- Grafe C, Schuster M. Physicochemical characterization of fruit quality traits in a German sour cherry collection. Sci Hortic. 2014;180:24–31. doi: 10.1016/j.scienta.2014.09.047. [DOI] [Google Scholar]
- Havlíková L, Míková K. Heat stability of anthocyanins. Z Lebensm Unters Forsch. 1985;181:427–432. doi: 10.1007/BF01027412. [DOI] [Google Scholar]
- Kader F, Haluk J-P, Nicolas J-P, Metche M. Degradation of cyanidin-3-glucoside by blueberry polyphenoloxidase: kinetic studies and mechanisms. J Agric Food Chem. 1998;46:3060–3065. doi: 10.1021/jf970926z. [DOI] [Google Scholar]
- Kim DO, Padilla-Zakour OI. Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: cherry, plum, and raspberry. J Food Sci. 2004;69:395–400. doi: 10.1111/j.1365-2621.2004.tb09956.x. [DOI] [Google Scholar]
- Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- Levaj B, Dragović-Uzelac V, Delonga K, Kovačević Ganić K, Banović M, Bursać Kovačević D. Polyphenols and volatiles in fruits of two sour cherry cultivars, some berry fruits and their jams. Food Technol Biotechnol. 2010;48:538–547. [Google Scholar]
- Li S, Ho CT. Stability and transformation of bioactive polyphenolic components of herbs in physiological pH. ACS Symp Ser. 2006;925:240–253. doi: 10.1021/bk-2006-0925.ch018. [DOI] [Google Scholar]
- Maier T, Fromm M, Schieber A, Kammerer DR, Carle R. Process and storage stability of anthocyanins and non-anthocyanin phenolics in pectin and gelatin gels enriched with grape pomace extracts. Eur Food Res Technol. 2009;229:949–960. doi: 10.1007/s00217-009-1134-9. [DOI] [Google Scholar]
- Patras A, Brunton NP, O’Donnell C, Tiwari BK. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci Tech. 2010;21:3–11. doi: 10.1016/j.tifs.2009.07.004. [DOI] [Google Scholar]
- Picariello G, De Vito V, Ferranti P, Paolucci M, Volpe MG. Species- and cultivar-depended traits of Prunus avium and Prunus cerasus polyphenols. J Food Compos Anal. 2016;45:50–57. doi: 10.1016/j.jfca.2015.10.002. [DOI] [Google Scholar]
- Poiana MA, Munteanu MF, Bordean DM, Gligor R, Alexa E. Assessing the effects of different pectins addition on color quality and antioxidant properties of blackberry jam. Chem Cent J. 2013;7:121–134. doi: 10.1186/1752-153X-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rababah TM, Al-Mahasneh MA, Kilani I, Yang W, Alhamad MN, Ereifej K, Al-U’datt M. Effect of jam processing and storage on total phenolics, antioxidant activity, and anthocyanins of different fruits. J Sci Food Agric. 2011;91:1096–1102. doi: 10.1002/jsfa.4289. [DOI] [PubMed] [Google Scholar]
- Rababah TM, Al-u’datt MH, Brewer S. Jam processing and impact on composition of active compounds. In: Preedy V, editor. Processing and impact on active components in food, Ch. 82. San Diego: Academic Press; 2013. pp. 681–687. [Google Scholar]
- Shikov V, Kammerer DR, Mihalev K, Mollov P, Carle R. Heat stability of strawberry anthocyanins in model solutions containing natural copigments extracted from rose (Rosa damascena Mill.) petals. J Agric Food Chem. 2008;56:8521–8526. doi: 10.1021/jf801946g. [DOI] [PubMed] [Google Scholar]
- Silva BM, Andrade PB, Mendes GC, Valentão P, Seabra RM, Ferreira MA. Analysis of phenolic compounds in the evaluation of commercial quince jam authenticity. J Agric Food Chem. 2000;48:2853–2857. doi: 10.1021/jf9911040. [DOI] [PubMed] [Google Scholar]
- Toydemir G, Capanoglu E, Gomez Roldan MV, de Vos RCH, Boyacioglu D, Hall RD, Beekwilder J. Industrial processing effects on phenolic compounds in sour cherry (Prunus cerasus L.) fruit. Food Res Int. 2013;53:218–225. doi: 10.1016/j.foodres.2013.04.009. [DOI] [Google Scholar]
- Will F, Hilsendegen P, Bonerz D, Patz C-D, Dietrich H. Analytical composition of fruit juices from different sour cherry cultivar. J Appl Bot Food Qual. 2005;79:12–16. [Google Scholar]
- Zorić Z, Dragović-Uzelac V, Pedisić S, Kurtanjek Ž, Garofulić IE. Kinetics of the degradation of anthocyanins, phenolic acids and flavonols during heat treatments of freeze-dried sour cherry Marasca Paste. Food Technol Biotechnol. 2014;52:101–108. [Google Scholar]