Abstract
The odor-active compounds of the conventional yellow passion fruit influence the aroma during ripeness and the acceptance of the juice. HS–GC–MS and GC–OSME analysis and sensory acceptance of the conventional passion fruit from different stages of ripeness were studied to characterize the aroma of the fruit and, aroma and flavor of the juice. Ethyl butanoate, ethyl hexanoate and propyl acetate showed high odoriferous importance in the passion fruit from the 1/3 yellow skin color. Cis-3-hexen-1-ol and diethyl carbonate plus the odor-active compounds from the 1/3 yellow skin color showed high odoriferous importance in the 2/3 yellow skin color, and butyl acetate and alpha-terpineol plus the same odor-active compounds from 2/3 were the most important for the 3/3 yellow skin color. There was difference in the aroma and flavor of the juices, with higher acceptance means for the passion fruit from the 3/3 yellow skin color. The passion fruit volatile compounds peak area, odoriferous intensity and sensory acceptance of the juices increased during ripeness, indicating that the conventional passion fruit characteristic aroma is completely expressed when the fruit reaches the whole maturation, at the 3/3 yellow skin color.
Keywords: HS–GC–MS–OSME, Passion fruit (Passiflora edulis Sims f. flavicarpa Deg.), Stage of ripeness, Olfactometry, Sensory acceptance
Introduction
Brazil is the greatest producer and consumer of passion fruit in the world. 838 thousand ton of passion fruit were produced in 2013, mostly Passiflora edulis Sims f. flavicarpa Deg., which accounts for 95% of the cultivation area (IBGE 2014). Yellow passion fruit is used as in natura fruit, mainly to produce industrialized juice.
Yellow passion fruit should be harvested when still connected to the plant to improve shelf life and commercial value. In Brazil, passion fruit is regularly harvested after falling to the ground favoring microbial contamination and dehydration. The skin color is a simple criterion to identify the ideal point of harvest, making it possible to obtain ripe, more uniform fruits, with better nutritional and commercial quality, and phytosanitary conditions. Although the entire yellow skin color of ripe fruits, fruits with 2/3 yellow skin color that are not completely ripe may show physicochemical and sensorial characteristics close to the ripe fruits (De Marchi et al. 2000; Amaro and Monteiro 2001; Macoris et al. 2012).
The passion fruit aroma is described as predominantly fruity, floral and slightly sulfurous (Winterhalter 1991; Pino 1997). The esters ethyl butanoate, hexyl butanoate, ethyl hexanoate and hexyl hexanoate are the major contributing to the characteristic fruity and sweety aroma of passion fruit, while the terpenes limonene, myrcene, trans-ocimene, terpinolene, linalool and alpha-terpineol contribute to the floral and fruity aroma, the aldehydes hexanal, octanal and benzaldehyde contribute to the green and citrus aroma, and the alcohol hexanol is important for green aroma of passion fruit (Pino 1997; Werkhoff et al. 1998). There is little information about the aroma of passion fruit at different stages of ripeness (Casimir et al. 1981; Janzantti and Monteiro 2014). The volatile composition of yellow passion fruit depends on the cultivation system, cultivar and edaphoclimatic conditions, among others factors. The stage of ripeness could influence the aroma profile of passion fruit and consequently its acceptance. Organic yellow passion fruit (Feltrin selection) showed the same odoriferous profile at the 2/3 and 3/3 yellow skin color, pointing out its consumption in earlier stages of ripeness (Janzantti and Monteiro 2014).
The aim of this work was to study the odor-active compounds of the conventional yellow passion fruit from different stages of ripeness corresponding to the skin color to identify which of them characterize the aroma during ripeness and influence acceptance of aroma and flavor of the juice.
Materials and methods
Chemicals
The volatile compounds standards (purity >99.0%) were from Sigma-Aldrich (St. Louis, USA) and Fluka (Steinheim, Germany), all listed in Table 1. Dichloromethane was from J. T. Baker (Philipsburg, USA) and n-alkanes (C8–C30) from Supelco (Bellefonte, USA), both GC grade. NaCl analytical grade was from Merck (Darmstadt, Germany).
Table 1.
Volatile and odor-active compounds of the conventional passion fruit in the 1/3, 2/3 and 3/3 yellow skin color stages of ripeness
| Peak | RI1 | RI2 | Compound3 | Aroma description | GC–FID4 | GC–O5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1/3 | 2/3 | 3/3 | 1/3 | 2/3 | 3/3 | |||||
| 1 | 922 | <800 | ethyl propanoatea,b,c | Not detected by OSME | 0.92 | 1.73 | 3.32 | – | – | – |
| 2 | 932 | <800 | propyl acetatea,b,c | Skin, passion fruit | nd–0.20 | 0.26 | 6.40 | 4.62 | 6.65 | 7.15 |
| 4 | 939 | <800 | methyl butanoatea,b,c | Passion fruit/fruity | 12.47 | 8.31 | 2.34 | 3.40 | 3.47 | 2.11 |
| 6 | 974 | <800 | 2-methylpropyl acetatea,b,c | Passion fruit | 9.69 | 16.12 | 11.41 | – | 0.93 | 0.93 |
| 9 | 1019 | 803 | ethyl butanoatea,b,c | Sweet/strawberry | 435.71 | 621.08 | 1047.15 | 8.77 | 8.85 | 8.49 |
| 10 | 1027 | <800 | 2-methyl-3-buten-2-olb,c | Not detected by OSME | 11.24 | 18.23 | 18.01 | – | – | – |
| 12 | 1066 | 815 | butyl acetatea,b,c | Green, sweet | 5.98 | 11.60 | 15.44 | – | 2.77 | 4.72 |
| 14 | 1073 | <800 | hexanala,b,c | Not detected by OSME | 51.93 | 19.60 | 13.70 | – | – | – |
| 15 | 1096 | <800 | diethyl carbonateb,c | Plastic | nd–0.32 | 0.72 | 0.79 | 3.37 | 5.23 | 6.60 |
| 17 | 1125 | 886 | o-xyleneb,c | Plastic | tr–0.15 | nd–0.72 | 0.94 | – | – | – |
| 18 | 1131 | <800 | methyl 2-methylbutanoateb,c | Not detected by OSME | 1.19 | 4.31 | 3.40 | – | – | – |
| 19 | 1155 | <800 | 1-butanola,b,c | Sweet/fruity | 1.20 | 2.72 | 6.02 | 2.32 | 2.08 | 3.26 |
| 20 | 1172 | 993 | beta-myrcenea,b,c | Citric/fruity | 13.99 | 38.03 | 28.49 | – | – | 1.57 |
| 22 | 1175 | 934 | methyl hexanoatea,b,c | Strawberry, earthy | 0.84 | 1.52 | 5.87 | – | 0.91 | 1.55 |
| 23 | 1184 | 1029 | D-limonenea,b,c | Not detected by OSME | 22.60 | 41.87 | 22.56 | – | – | – |
| 24 | 1210 | 972 | butyl butanoatea,b,c | Sweet | 1.55 | 2.02 | 4.33 | – | – | 1.15 |
| 25 | 1228 | 1001 | ethyl hexanoatea,b,c | Syrup, guarana | 54.38 | 70.88 | 160.33 | 6.42 | 6.13 | 6.61 |
| 26 | 1244 | 1052 | cis-beta-ocimeneb,c | Not detected by OSME | 8.75 | 15.31 | 17.18 | – | – | – |
| 28 | 1268 | 1019 | hexyl acetatea,b,c | Floral | 15.03 | 38.10 | 62.22 | – | – | 0.94 |
| 29 | 1274 | 1006 | octanala,b,c | Sweet, acid | 1.09 | 2.09 | 2.05 | – | 1.54 | 1.61 |
| 30 | 1275 | 990 | ethyl cis-3-hexenoateb,c | Fruity/floral | nd–0.45 | nd–tr | tr–0.35 | 2.00 | 2.06 | 1.52 |
| 32 | 1300 | 1009 | trans-3-hexenyl acetateb,c | Fruity, citrus | 0.51 | 1.42 | 2.17 | – | – | 1.24 |
| 33 | 1309 | 991 | cis-3-hexenyl acetateb,c | Fruity, green | 15.37 | 34.02 | 42.77 | 2.33 | 2.53 | 3.04 |
| 34 | 1328 | 1021 | ethyl trans-2-hexenoateb,c | Floral | 1.18 | 1.55 | 1.32 | – | – | – |
| 36 | 1355 | 870 | 1-hexanola,b,c | Passion fruit, skin | 13.41 | 25.66 | 52.21 | – | – | 2.58 |
| 37 | 1362 | 1039 | trans-3-hexen-1-olb,c | Not detected by OSME | 1.18 | 1.84 | 3.40 | – | – | – |
| 38 | 1382 | 855 | cis-3-hexen-1-ola,b,c | Passion fruit, grass | 4.77 | 9.39 | 11.95 | 2.31 | 4.26 | 4.35 |
| 39 | 1402 | 1193 | butyl hexanoatea,b,c | Unripe fruit | 0.51 | 2.38 | 4.25 | – | – | 1.72 |
| 40 | 1406 | 1193 | hexyl butanoatea,b,c | Not detected by OSME | 5.38 | 18.12 | 43.99 | – | – | – |
| 42 | 1449 | 1198 | ethyl octanoatea,b,c | Earthy | 0.23 | 0.34 | 1.25 | 3.88 | 3.43 | 3.26 |
| 43 | 1452 | 1185 | cis-3-hexenyl butanoateb,c | Not detected by OSME | 2.25 | 5.84 | 10.94 | – | – | – |
| 46 | 1495 | 1385 | alpha-copaeneb,c | Green, fruity | tr–0.18 | tr–0.45 | 0.97 | 1.52 | 1.12 | 0.98 |
| 47 | 1515 | 971 | benzaldehydeb,c | Lavander | 0.77 | 1.29 | 1.38 | – | 1.14 | 0.87 |
| A | 1537 | – | ni | Citric | nd | nd | nd | 1.96 | 2.09 | 1.32 |
| B | 1570 | – | ni | Citric, lavander | nd | nd | nd | – | – | 1.59 |
| 48 | 1575 | 1100 | beta-linaloola,b,c | Sweet, citrus | 0.73 | 2.27 | 2.36 | 1.63 | 2.02 | 2.42 |
| 51 | 1598 | 1387 | hexyl hexanoatea,b,c | Synthetic, rubber | 1.95 | 7.22 | 18.11 | 1.11 | 1.12 | 1.11 |
| 52 | 1646 | 1381 | cis-3-hexenyl hexanoateb,c | Green, citric | 0.70 | 2.31 | 4.55 | – | 1.44 | 1.40 |
| 53 | 1689 | 1487 | germacrene Db,c | Passion fruit | 0.36 | 0.23 | 0.28 | 0.94 | 1.75 | 1.85 |
| C | 1705 | – | ni | Passion fruit | nd | nd | nd | 1.17 | 1.12 | 0.50 |
| 54 | 1715 | 1195 | alpha-terpineola,b,c | Passion fruit/leafy | nd -0.92 | 0.72 | 2.59 | 1.44 | 2.87 | 4.48 |
| 56 | 1753 | – | ni | Green, passion fruit | nd–0.30 | nd | nd | – | 1.80 | 2.54 |
| 57 | 1771 | – | ni | Green, leafy | nd | nd–0.52 | nd | – | 0.98 | 2.11 |
| D | 1785 | – | ni | Green, sweet | nd | nd | nd | – | 1.09 | 1.57 |
| E | 1813 | – | ni | Passion fruit | nd | nd | nd | 1.73 | 1.51 | 1.31 |
| 58 | 1840 | – | ni | Sweet, molasses | nd | nd–0.34 | nd | 4.27 | 3.83 | 3.57 |
| F | 1913 | ni | Rubber, passion fruit | nd | nd | nd | – | 0.87 | 1.92 | |
| 59 | 1941 | – | ni | Sweet | 0.65 | 1.11 | 0.90 | – | – | – |
| G | 1960 | – | ni | Fruity, peach | nd | nd | nd | 2.44 | 2.15 | 2.31 |
| 60 | 1962 | – | ni | Passion fruit, sweet | nd | 0.33 | nd | – | – | 0.10 |
| 61 | 1963 | 1487 | dodecanolb,c | Sweet | 0.93 | 1.31 | 1.07 | 1.36 | 1.33 | 0.78 |
| 63 | 2072 | – | ni | Candy floss | nd–0.21 | 0.30 | 0.54 | 2.33 | 4.88 | 4.49 |
| 64 | 2166 | – | ni | Candy floss/caramel | nd | nd | tr | – | – | 2.22 |
| 65 | 2188 | – | methyl hexadecanoateb,c | Metalic/solvent | nd–0.22 | nd–0.25 | 0.38 | – | – | – |
| 66 | 2217 | – | ni | Solvent | nd | nd | tr | 4.72 | 1.89 | 3.74 |
1RI = retention index of peak in the DB-Wax column
2RI = retention index of peak in the DB-5 column; - not calculated
3Compounds have identical number in the chromatogram and aromagram. Letters were attributed to the odor active compounds not correlated to the volatile compounds detected by GC-FID
4GC-FID: relative area of peak (n = 3) in the GC-FID, multiplied by 100, nd compound not detected, ni compound not identified, tr trace, relative area of peak < 0.1
5GC-O: maximum odoriferous intensity, ≥ 4.0: between moderate and strong, 3.0 to 3.9: moderate, 0.1 to 2.9: weak, – none (not detected by OSME)
acompound identified by pure standards
bcompound identified by mass spectrometry
ccompound identified by calculating the retention index
Yellow passion fruit samples
Conventional yellow passion fruits (Passiflora edulis Sims f. flavicarpa Deg.) from Feltrin selection were cultivated in Sumaré, SP, Brazil (22°49′19″ S and 47°16′01″ W, 625 m altitude). Fruits were harvested in the stages of ripeness corresponding to the 1/3, 2/3 and 3/3 yellow skin color (De Marchi et al. 2000; Amaro and Monteiro 2001). Passion fruits (15 kg) of each stage of ripeness were harvested at random, choosing fruits of similar size. After harvesting fruits were selected, classified according to the skin color, and washed. The pulp was separated from the seeds and peel, packed into hermetically closed glass flasks and analyzed.
Isolation of volatile compounds from pulp
In each stage of ripeness volatile compounds were isolated in triplicate using dynamic headspace (HS) by vacuum suction (79.99 mm Hg) at room temperature (25 °C) and a Porapak Q trap (100 mg, 150–180 μm, Waters Associates, Milford, U.S.A). Passion fruit pulp (300 g) and NaCl (30/100 g) were put in the volatiles capture system flask, isolated for 2 h and eluted with 300 μL dichloromethane (Macoris et al. 2011).
High resolution gas chromatography (HSGC–FID)
A 2010 Shimadzu (Kyoto, Japan) gas chromatograph with a flame ionization detector (GC-FID) and a DB-Wax (30 m × 0.25 mm × 0.25 μm) column (J&W, Folsom, USA) at 40 °C for 10 min, then 200 °C at 3 °C/min, held for 10 min and hydrogen at 1.3 mL/min were used. Injection (2 μL) was in splitless mode. Injector temperature was 230 °C and detector 250 °C. A pentadecane internal standard solution (10 μL, 4 μL/mL) was added into the isolate and the volatile compounds were quantified. The relative peak area was obtained.
Gas chromatography–mass spectrometry (GC–MS)
A 5975C Agilent (Wilimington, USA) gas chromatograph–mass spectrometer, with electron impact ionization source (70 eV) in scan mode and mass range of 35–350 m/z, and a DB-Wax column (30 m × 0.25 mm × 0.25 μm) at 40 °C for 10 min, then to 200 °C at 3 °C/min, held for 10 min and helium at 1.3 mL/min were used. Injector temperature was 230 °C and detector 240 °C. The DB-5 (J&W, Folsom, USA) column (60 m × 0.25 mm × 0.25 μm) at 50 °C, then to 250 °C at 3 °C/min, held for 10 min, and helium at 1.0 mL/min were also used. Injector and detector temperatures were 250 °C.
The volatile compounds were identified according to the criteria reported by Janzantti and Monteiro (2014). A solution of alkanes in dichloromethane was injected in the DB-Wax and DB-5 columns, under the same chromatographic conditions, to calculate the retention indices.
Gas chromatography–Olfactometry (GC–O)
The odor-active compounds were analyzed by OSME (Da Silva et al. 1994) using the SCDTI (time-intensity data collection system) data collection program (Da Silva 1999). The GC–FID was modified for the GC–O–OSME analysis according to Janzantti et al. (2012). Four trained and selected judges, 23–37 years old, were requested to appoint the intensity using the SCDTI scale and describe the odor of the passion fruit pulp from each stage of ripeness in triplicate. Data collected from the SCDTI software for each GC–O analysis session of each judge and each stage of ripeness were used to construct the individual aromagram, considering that peaks should be detected in at least two of three repetitions. Then, a consensual aromagram was constructed based on individual aromagrams, in which peaks should be detected by at least two of four judges. Description of each odor was established combining descriptions of all judges, and checked against literature (Acree and Arn 2004; Jordán et al. 2002; Janzantti et al. 2012; Janzantti and Monteiro 2014). Retention indices in the consensus aromagram were calculated to confirm that the chromatographic data and compounds identity were correctly related to the olfactometric data. The odoriferous intensity 4.0 or more, between terms “moderate” and “strong”, was of high importance, and those between 3.0 and 3.9, of moderate importance and between 0.1 and 2.9 of weak importance.
Sensory evaluation
102 consumers (students and servers of UNESP) recruited from both gender, between 18 and 50 years old, who liked at least slightly and consumed passion fruit pulp and juice at least once in 15 days, participated in the acceptance test. Pulps from the 1/3, 2/3 and 3/3 yellow skin color stages of ripeness were used to prepare juices in a proportion of pulp:water of 1:4 (v/v). Juices were served (30 mL) at 12 °C in plastic glasses, coded with random three-digit numbers in monadic and randomized order (Macfie et al. 1989). Consumers evaluated appearance and aroma of the juices in standardized sensory booths, then they sweetened the juice with sugar or sweetener according to their taste, and evaluated overall impression, flavor, characteristic passion fruit flavor and sourness. A nine-point hedonic scale (9 = like extremely, 5 = neither like nor dislike, 1 = dislike extremely) was applied.
Statistical analysis
The olfactometric data were analyzed using Excel (Microsoft Office, 2007). Sensory data were submitted to ANOVA and Tukey test (P ≤ 0.05). The principal component analysis (PCA) was performed based on the correlation matrix, using relative peak area of volatile compounds and odoriferous intensity, as well as acceptance means. The volatile compounds were represented by name and/or chemical class and aroma description of passion fruit in each stage of ripeness. PCA was carried out using the Statistica 7.0 software (StatSoft, Tulsa, USA).
Results and discussion
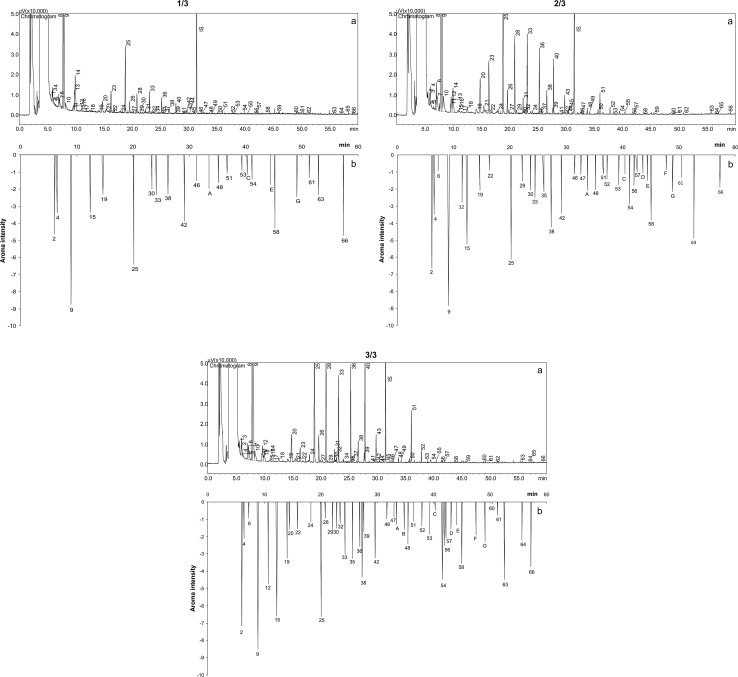
Sixty volatile compounds were detected in the conventional yellow passion fruit at the 1/3 yellow skin color stage of ripeness, 62 at 2/3 and 60 at 3/3 (Table 1 and Fig. 1a). Esters (26) was the main chemical class, plus alcohols (7), terpenes (6), aldehydes (3), acid (1) and hydrocarbon (1). Ethyl butanoate, ethyl hexanoate, hexanal, D-limonene and a non-identified compound (peak 8, RI 1016) were major in the 1/3 yellow skin color. Exception of hexanal, the 2/3 yellow skin color had the same compounds as the 1/3, plus hexyl acetate, beta-myrcene, cis-3-hexenyl acetate and 1-hexanol. The 3/3 yellow skin color showed the same compounds as the 2/3, plus hexyl butanoate (Table 1). All the compounds had relative peak area increased during maturation, exception of methyl butanoate and hexanal. Hexanal was major only in the 1/3 yellow skin color and had peak area reduced during ripeness, probably because its conversion into hexanol (Damodaran et al. 2007; El Hadi et al. 2013; Janzantti and Monteiro 2014). Passion fruit volatile compounds are formed from chemical and enzymatic reactions of non-volatile precursors, degradation products of carotenoids, free fatty acids and sulphur-containing components (Engel and Tressl 1991; Winterhalter 1991; Pino 1997). No sulphur compounds were identified in this work (Table 1), as reported in other studies (Jordán et al. 2002; Pontes et al. 2009).
Fig. 1.
Chromatogram (a) and consensual aromagram (b) of the volatile compounds from the conventional passion fruit in the stages of ripeness corresponding to the 1/3, 2/3 and 3/3 yellow skin color
In the organic passion fruit (Feltrin selection) from the same stages of ripeness, ethyl butanoate, ethyl hexanoate and cis-beta-ocimene were major, exception of ethyl hexanoate at the 1/3 yellow skin color (Janzantti and Monteiro 2014). In the conventional passion fruit from Afruvec material, ethyl butanoate and hexanoate, hexyl acetate, butanoate and hexanoate, and hexanol were also major compounds in the 3/3 yellow skin color (Macoris et al. 2011; Janzantti et al. 2012), differently from the present work. Hexyl hexanoate, 1-hexanol, hexadecanoic acid, linalool and alpha-terpineol were reported as major compounds in the ripe passion fruit from Golden Yellow variety (Pino 1997), different from the present work. Differences in passion fruit volatile composition are probably due to the material/cultivar, edaphoclimatic conditions and extraction technique.
Twenty-three odor-active compounds were perceived in the conventional passion fruit at the 1/3 yellow skin color, and 34 and 42 in 2/3 and 3/3, respectively (Table 1 and Fig. 1b). Ethyl butanoate (sweet, strawberry) and hexanoate (guarana syrup), and propyl acetate (skin, passion fruit) showed the highest odoriferous importance for the passion fruit in the 1/3 yellow skin color. Cis-3-hexen-1-ol (passion fruit, grass) and diethyl carbonate (plastic) plus the compounds from the 1/3 yellow skin color showed high odoriferous importance in the 2/3 yellow skin color, and butyl acetate (sweet, green) and alpha-terpineol (passion fruit/leafy) plus the same compounds from 2/3 were the most important for the 3/3 yellow skin color.
The volatile compounds improved aroma intensity during ripeness. Butanol (sweet/fruity), cis-3-hexenyl acetate (fruity, green), and alpha-terpineol (passion fruit/leafy) showed weak odoriferous intensity in the 1/3 and 2/3 yellow skin color and moderate in 3/3. Butyl acetate (green, sweet) and a non-identified compound (RI 1339, plastic, sweet) without odoriferous importance in the 1/3, showed weak odoriferous importance in 2/3 and moderate in 3/3. Beta-myrcene (citric/fruity), butyl butanoate (sweet), hexyl acetate (floral), trans-3-hexenyl acetate (fruity, citrus), 1-hexanol (passion fruit, skin), butyl hexanoate (unripe fruit), and two non-identified compounds (RI 1570, citric, lavender and IR 2166, candy floss/caramel) were weakly perceived only in the 3/3.
Ethyl butanoate and hexanoate had high odoriferous importance in the organic passion fruit (Feltrin selection) in the 1/3 and 2/3 yellow skin color, plus propyl acetate in the last one (Janzantti and Monteiro 2014). Ethyl hexanoate, diethyl carbonate and propyl acetate showed high odoriferous importance in the conventional and organic passion fruit from the Afruvec material, with a more expressive intensity in the organic fruit (Janzantti et al. 2012).
Acceptance analysis
Acceptance of the juices increased during maturation. Means of appearance ranged from 7.1 to 7.4, aroma 6.2 to 7.4, overall impression 5.6 to 6.9, flavor 5.3 to 6.9, characteristic passion fruit flavor 5.4 to 7.0 and sourness 5.0 to 6.0, from the 1/3 to 3/3 yellow skin color. No difference (P > 0.05) was found between the 2/3 and 3/3 yellow skin color juice appearance and 1/3 and 2/3 for overall impression, flavor, passion fruit characteristic flavor and sourness. Juices from all stages of ripeness differed (P ≤ 0.05) for aroma.
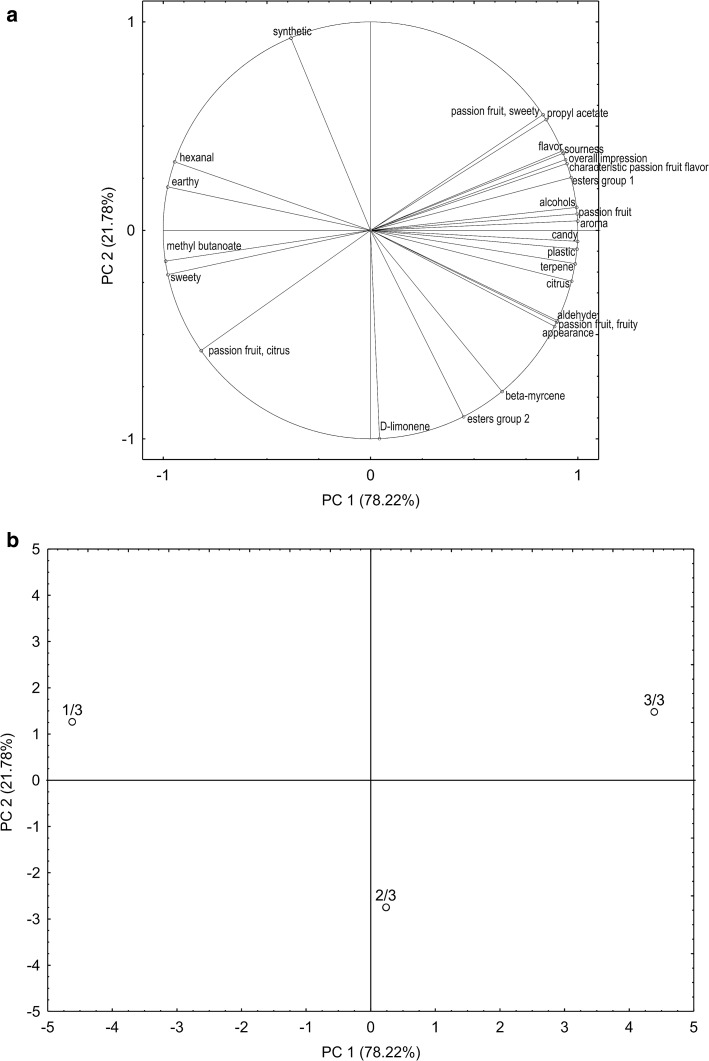
PCA analysis
The 1/3 yellow skin color, loaded negatively on PC 1 and positively on PC 2, was characterized by hexanal and methyl butanoate, and aroma “earthy” and “sweety”. They are important for the unripe passion fruit aroma. The 2/3 yellow skin color, loaded positively on PC 1 and negatively on PC 2, was characterized mainly by D-limonene, beta-myrcene and esters group 2 (2-methylpropyl acetate, methyl 2-methylbutanoate and ethyl trans-2-hexenoate). The 3/3 yellow skin color, loaded positively on PC 1 and PC 2, was characterized by propyl acetate, esters group 1 (ethyl propanoate; diethyl carbonate; amyl isobutanoate; butyl, 3-methyl-2-butenyl, hexyl, trans-3-hexenyl and cis-3-hexenyl acetate; ethyl, butyl, hexyl, trans-3-hexenyl and cis-3-hexenyl butanoate; methyl, ethyl, butyl, hexyl and cis-3-hexenyl hexanoate; ethyl cis-3-hexenoate; ethyl octanoate and methyl hexadecanoate), terpenes, aldehydes and alcohols, and aroma “passion fruit”, “passion fruit, fruity”, “passion fruit, sweety”, “candy”, “citrus” and “plastic”, important for the ripe passion fruit aroma (Fig. 2). Sensory acceptance in the ripe fruit was highlighted. Characteristic and natural aroma of passion fruit were completely developed in the ripe fruit as perceived by the judges using GC–O–OSME and acceptance test.
Fig. 2.
PCA of volatiles and odor-active compounds from the conventional passion fruit in the 1/3, 2/3 and 3/3 yellow skin color stage of ripeness and sensory acceptance of the juices. 1/3 = conventional passion fruit from the 1/3 yellow skin color stage of ripeness, 2/3 = conventional passion fruit from the 2/3 yellow skin color stage of ripeness, 3/3 = conventional passion fruit from the 3/3 yellow skin color stage of ripeness
Conclusion
The CG–O–MS discriminated quite well the conventional passion fruit during maturation. The passion fruit volatile compounds peak area, odoriferous intensity and sensory acceptance of the juices increased during ripeness, indicating that the conventional passion fruit characteristic aroma is completely expressed when the fruit reaches the whole maturation, at the 3/3 yellow skin color. These outcomes will guide passion fruit producers to harvest ripe fruit in order to yield juice of better quality and flavor to meet consumer demand.
Acknowledgements
The authors thank to Mariana Macoris for the GC–O analysis, the Brazilian Federal Agency for Support and Evaluation of Graduate Education-CAPES (Prodoc No. 06/58967-0), Cia das Ervas and Fazenda Gralha Branca-Sumaré for the financial support.
References
- Acree TE, Arn H (2004) Flavornet. http://www.flavornet.org/flavornet.html. Accessed 26 June 2016
- Amaro AP, Monteiro M. Rendimento de extração da polpa e características físico-químicas do maracujá amarelo (Passiflora edulis f. Flavicarpa Sims. Deg.) produzido por cultivo orgânico e convencional em relação à cor da casca. Alim Nutr. 2001;12:171–184. [Google Scholar]
- Casimir DJ, Kefford JF, Whitfield FB. Technology and flavour chemistry of passion fruit juice and concentrate. Adv Food Res. 1981;27:243–293. doi: 10.1016/S0065-2628(08)60300-6. [DOI] [Google Scholar]
- Da Silva MAAP. Avaliação de atributos sensoriais por técnicas tempo-intensidade. In: Almeida TCA, Hough G, Damásio MH, Da Silva MAAP, editors. Avanços em Análise Sensorial. São Paulo: Livraria Varela; 1999. pp. 49–61. [Google Scholar]
- Da Silva MAAP, Lundhal DS, McDaniel MR. The capability and psychophysics of Osme: a new GC-olfactometry technique. In: Maarse H, Van Der Heij DG, editors. Trends in Flavour Research. Amsterdam: Elsevier; 1994. pp. 191–209. [Google Scholar]
- Damodaran S, Parkin KL, Fennema OR. Fennema’s food chemistry. Boca Raton: CRC Press; 2007. [Google Scholar]
- De Marchi R, Monteiro M, Benato EA, Silva CAR. Uso da cor da casca como indicador de qualidade do maracujá amarelo (Passiflora edulis Sims. f. flavicarpa Deg.) destinado à industrialização. Food Sci Technol. 2000;20:381–387. [Google Scholar]
- El Hadi MAM, Zhang F-J, Wu F-F, Zhou C-H, Tao J. Advances in fruit aroma volatile research. Molecules. 2013;18:8200–8229. doi: 10.3390/molecules18078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel KH, Tressl R. Identification of new sulfur-containing volatiles in yellow passion fruits (Passiflora edulis F. flavicarpa) J Agric Food Chem. 1991;39:2249–2252. doi: 10.1021/jf00012a030. [DOI] [Google Scholar]
- IBGE. Instituto Brasileiro de Geografia e Estatística. Banco de dados agregados. (2014) Quantidade produzida, valor da produção, área plantada e área colhida da lavoura permanente. ftp://ftp.ibge.gov.br/Producao_Agricola/Producao_Agricola_Municipal_%5Banual%5D/2013/tabelas_pdf/tabela03.pdf. Accessed 26 June 2016
- Janzantti NS, Monteiro M. Changes in the aroma of organic passion fruit (Passiflora edulis Sims f. flavicarpa Deg.) during ripeness. Food Sci Technol LEB. 2014;59:612–620. doi: 10.1016/j.lwt.2014.07.044. [DOI] [Google Scholar]
- Janzantti NS, Macoris MS, Garruti DS, Monteiro M. Influence of the cultivation system in the aroma of the volatile compounds and total antioxidant activity of passion fruit. Food Sci Technol LEB. 2012;46:511–518. doi: 10.1016/j.lwt.2011.11.016. [DOI] [Google Scholar]
- Jordán MJ, Goodner KL, Shaw PE. Characterization of the aromatic profile in aqueous essence and fruit juice of yellow passion fruit (Passiflora edulis Sims F. Flavicarpa degner) by GC-MS and GC/O. J Agr Food Chem. 2002;50:1523–1528. doi: 10.1021/jf011077p. [DOI] [PubMed] [Google Scholar]
- Macfie HJ, Bratchell N, Greenhoff K, Vallis CV. Designs to balance the effect of order of presentation and first-order carry-over in hall tests. J Sens Stud. 1989;4:129–148. doi: 10.1111/j.1745-459X.1989.tb00463.x. [DOI] [Google Scholar]
- Macoris MS, Janzantti NS, Garruti DS, Monteiro M. Volatile compounds from organic and conventional passion fruit (Passiflora edulis F. flavicarpa) pulp. Food Sci Technol. 2011;31:430–435. [Google Scholar]
- Macoris MS, De Marchi R, Janzantti NJ, Monteiro M. The influence of ripening stage and cultivation system on the total antioxidant activity and total phenolic compounds of yellow passion fruit pulp. J Sci Food Agr. 2012;92:1886–1891. doi: 10.1002/jsfa.5556. [DOI] [PubMed] [Google Scholar]
- Pino JA. Los constituyentes volatiles de la fruta de la passion. Alimentaria. 1997;280:73–81. [Google Scholar]
- Pontes M, Marques JC, Câmara JS. Headspace solid-phase microextraction-gas chromatography-quadrupole mass spectrometric methodology for the establishment of the volatile composition of Passiflora fruit species. Microchem J. 2009;93:1–11. doi: 10.1016/j.microc.2009.03.010. [DOI] [Google Scholar]
- Werkhoff P, Guentert M, Krammer G, Sommer H, Kaulen J. Vacuum headspace method in aroma research: flavor chemistry of yellow passion fruits. J Agric Food Chem. 1998;46:1076–1093. doi: 10.1021/jf970655s. [DOI] [Google Scholar]
- Winterhalter P. Fruits IV. In: Maarse H, editor. Volatile compounds in foods and beverages. New York: Marcel Dekker Inc.; 1991. pp. 389–409. [Google Scholar]