Abstract
Antioxidant (AO) capacity of instant, espresso, filter and Turkish/Greek coffee brews, coffee substitutes (roasted chicory root, barley, pea, chickpea, carob and dried fig) and individual compounds (phenolic acids, flavonoids, methylxanthines, N-methyl pyridinium and HMW melanoidins) was assessed using DC polarographic assay based on decrease of anodic current originating from hydroxo-perhydroxo mercury complex formed in alkaline solutions of H2O2 at potential of mercury dissolution, as well as three spectrophotometric assays (DPPH, ABTS and FRAP). A large difference between applied assays ability to recognize various types of individual AOs was noticed. Only according to DC polarographic assay significant AO activity was ascribed to methylxanthines and N-methyl pyridinum. The total content of phenolics (TPC) present in complex samples was determined by FC assay. The highest TPC was ascribed to instant coffees and coffee substitutes while the lowest to decaffeinated filter coffee. Complex samples were grouped based on principal components analysis, phenolics AO coefficient, calculated as the ratio between AO capacity and TPC, and relative AO capacity index (RACI), calculated by assigning equal weight to all applied assays including FC. The highest values of RACI were ascribed to instant coffee brews, followed by substitutes while the lowest to the decaffeinated espresso coffee.
Keywords: Antioxidant, DC polarography, Hydrogen peroxide, Coffee, N-methyl pyridinium
Introduction
Coffee is the third most consumed beverage in the world (Wang and Ho 2009). Antioxidant (AO) capacity of coffee was investigated by mostly using spectrophotometric assays. In comparison to other polyphenolic beverages, coffee possesses superior AO activity (Fukushima et al. 2009; Carlsen et al. 2010). Origin, blending, roasting degree and grinding of coffee beans as well as the brewing influence AO capacity of coffee beverage (Ludwig et al. 2012). In order to imitate coffee and provide caffeine-free beverages without the adverse psychoactive effects of caffeine, certain grains and fruits are roasted and brewed in the same manner as coffee AO capacities of coffee substitutes and their ingredients such as carob (Sahin et al. 2009; Custodio et al. 2011), chicory (Jurgonski et al. 2011), chickpea (Segev et al. 2012), roasted barley (Omwamba and Hu 2010) and fig (Vinson et al. 2005) were reported.
Electrochemical assays were recently applied to determine AO capacity of coffee. Until now, methods based on the cyclic and square wave voltammetry at modified titanium electrodes (El Qouatli et al. 2011), adsorptive transfer stripping voltammetry at a boron-doped diamond electrode (Yardim, 2012), differential pulse voltammetry at multi-walled carbon nanotube-modified glassy carbon electrode (Ziyatdinova et al. 2013) and carbon paste electrode (Oliveira-Neto et al. 2016) were employed.
Here, dropping mercury electrode has been used for rapid measurement of AO activity of 24 different coffee brews (Turkish, instant, espresso and filter) and 6 coffee substitutes. A direct current (DC) polarographic assay based on the decrease of anodic current originating from hydroxo-perhydroxo mercury complex (HPMC) formed in alkaline solutions of H2O2 at potential of mercury dissolution has been applied in parallel with common spectrophotometric AO assays. The AO capacity of coffees and substitutes, as well as individual compounds present (phenolic acids, flavonoids, methylxanthines, N-methylpyridinium (NMP) and HMW melanoidins) has been compared to their scavenging activity against artificial radicals (ABTS and DPPH) and total reducing power (FRAP). Complex samples have been grouped based on relative AO capacity index (RACI) calculated by assigning equal weight to all applied assays, including FC, phenolics AO coefficient (PAC) calculated as the ratio between AO capacity determined by each AO assay and total phenolic content (TPC), and principal components analysis (PCA).
Materials and methods
Chemicals
Folin-Ciocalteu reagent, ammonium peroxodisulphate, sodium carbonate, sodium acetate trihydrate, acetic acid, hydrochloric acid, ferric chloride hexahydrate and ferric sulphate heptahydrateof analytical grade were supplied by Kemika (Zagreb, Croatia). DPPH (2.2-diphenyl-1-picrylhydrazyl) was supplied by Fluka (Buchs, Switzerland) and methanol (HPLC grade) was purchased from J.T.Baker (Deventer, Netherlands). Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), TPTZ (2,4,6- tripyridyl-S-triazine), ABTS (2.2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt), and gallic acid (GA) were obtained from Aldrich (Sigma-Aldrich Chemie, Steinheim, Germany).
Hydrogen peroxide, medical grade, 35% (v/v) solution and boric acid, potassium chloride and sodium hydroxide (analytical grade) of Merck were used.
Quercetin, rutin, chlorogenic and caffeic acid, caffeine, theophylline and theobromine were acquired from Sigma (St. Louis, MO, USA). Working standard solutions (2.0 mmol/L) were prepared daily in ethanol or water.
N-Me-Pyridinium iodide was prepared following literature procedure (Carlsson et al. 2012). 1.115 g of pure product was obtained (90%). M.p. 117–119 °C (Et2O). 1H NMR (200 MHz, DMSO-d 6) δ: 9.01 (2H, d, J = 5.62 Hz), 8.59 (1H, t, J = 7.86 Hz), 8.14 (2H, t, J = 6.74 Hz), 4.37 (3H, s). 13C (50 MHz, DMSO-d 6) δ: 145.56 (b), 145.13, 127.72, 48.03. NMR spectra were recorded in DMSO-d 6 on Varian Gemini 2000 (200/50 MHz) instrument. Chemical shifts were referenced with respect to solvent signal. Melting point was determined on Stuart SMPT-10 apparatus in an open capillary tube and was uncorrected. Concentration of working solution was 4 mmol/L.
HMW melanoidins were isolated from coffee brew by 48 h dialysis against distilled water in a cellulose dialysis tubing (cut off 2.4 kDa, Sigma). The contribution of HMW fraction to the total AO capacity of coffee was calculated based on a comparison between the activity of a whole (undialyzed) coffee sample and a dialyzed one.
Coffee and coffee surrogate samples
All coffee samples were commercially available and were purchased from local markets. The samples of Turkish, espresso and filter coffee were obtained in the form of medium roasted coffee beans, while instant coffee samples were in lyophilized form i.e. powder/granules. As for the coffee surrogates, carob and chicory were acquired in local bio-shops in roasted and ground form. Barley, chickpea and pea were purchased raw and roasted in a laboratory oven (DeLonghi EO 12001.W, Italy) at 200 °C for 15 min, while dried fig was further dried at 140 °C for 10 min.
Brews preparation
Turkish coffee samples were ground into a fine powder in an old traditional electric mill (Borac, Serbia) just before brewing. Brewing was conducted using a traditional Turkish coffee pot, prepared with 7 g of ground coffee and 50 mL of cold tap water. The brew was heated until it had foamed twice, allowed to settle for 5 min, and then decanted for analysis. Grinding of surrogate samples and preparation of beverages were conducted in the same manner as for Turkish coffee.
Roasted filter coffee beans were ground in the same mill as Turkish coffee, which was set to coarse grinding, and the samples were prepared by infusion method, employing filter coffee maker (Bartscher Regina, Germany). 7.5 g of ground coffee was used for every 125 mL of water. Extraction took about 5 min at 90 °C.
Roasted beans for espresso coffee were ground in an espresso grinder (LA Cimbali, model Cadet, Italy). Brews were made by pressure method in an espresso machine (LA Cimbali, M29 Select, Italy), using 6.5 g of ground coffee and hot water (T = 90 °C, p = 9 bar), for volume of 40 mL.
Instant coffee beverages were made by pouring 200 mL of hot water over 6 g of instant coffee and stirred until dissolved.
Antioxidant capacity by DC polarographic assay
Measurements were performed using Polarographic Analyzer PAR (Princeton Applied Research) model 174A coupled with X–Y recorder (Houston Instruments, Omnigraphic 2000). Three-electrode electrolytic cell was used. The cell volume was 30 mL. A dropping mercury electrode (DME) was the working electrode. Capillary constant of DME was m = 2.5 mg s−1 at mercury reservoir height of 75 cm. A programmed drop time of DME was 1 s. Current oscillations of DME were filtered with low pass filter of instrument positioned at 3 s. The saturated calomel electrode (SCE) and the platinum foil were used as the reference and the counter electrode, respectively.
The supporting electrolyte used was Clarc and Lubs (CL) buffer (pH 9.8), prepared by mixing 25 mL of 0.4 M H3BO3, 25 mL of 0.4 M KCl and 40.8 mL of 0.2 M NaOH. The volume of the supporting electrolyte in the cell was 19.9 mL. 0.100 mL of 1.00 M hydrogen peroxide was directly added in supporting electrolyte. The initial concentration of H2O2 was 5.0 mmol/L. The polarographic current–potential (i–E) curves with, or without, the analysed extracts were recorded starting from 0.1 V versus SCE towards negative potentials, with a sweep rate of 10 mV/s. In order to remove dissolved oxygen, the supporting electrolyte in the electrolytic cell was purged with pure nitrogen (>99.995%, Messer, Serbia) for 2 min before H2O2 addition, and 30 s after addition of each samples. T he atmosphere above the cell solution was kept inert during polarographic curve recording by a continuous flow of nitrogen.
Brews were gradually added into the cell solution in aliquots of 50 µL. Instant coffee brews were diluted 5 times. Gradual addition of brews into the buffered H2O2 solution caused uniform decrease of initial anodic limiting current, i p0. The relative decrease of i p0 upon each addition (∆i p) was calculated according to the following equation:
where: ∆i p (%) represents a relative decrease of i p0 upon addition of brews, while i p is the remaining part of i p0 after sample addition. Percentage of decrease was plotted versus volume of samples added. The slope of the linear part of obtained plots was used as a measure of AO capacity.
DPPH radical scavenging
The antioxidant capacity of the coffee and coffee substitute brews was determined using the DPPH radical scavenging assay (Brand-Williams et al. 1995), with some modifications. Antioxidant capacity was expressed as mmol Trolox equivalents (TE)/L, using the calibration curve of Trolox (0–1000 μM), a water soluble vitamin E analogue.
ABTS radical cation
The Trolox equivalent antioxidant capacity (TEAC) was estimated by the ABTS radical cation decolorization assay (Re et al. 1999). The results, obtained from triplicate analyses, were expressed as TE and derived from a calibration curve determined for this standard (100–1000 µM).
Ferric reducing/antioxidant power (FRAP)
The ferric reducing/antioxidant power (FRAP) assay was carried out according to standard procedure (Benzie and Strain 1996).All measurements were performed in triplicate. Aqueous solutions of FeSO4 × 7H2O (100–1000 μM) were used for the calibration and the results expressed as mmol Fe(II)/L, while results for individual samples as mmol TE/mol.
Relative antioxidant capacity index (RACI)
A standard score was calculated according to the following equation:
where: x was the raw data, μ was the mean, and σ was the standard deviation The standard scores of a sample for different assays when averaged gave a single unitless value, termed as RACI.
Comparison of results and statistical evaluation
Descriptive statistical analyses for calculating the means and the standard error of the mean were performed using Microsoft Excel 2007 software. The results were correlated separately for coffee brews and coffee substitute brews using regression analysis and statistically evaluated using analysis of variance (ANOVA), Brown–Forsythe test and PCA (Principal Components Analysis). Post-hoc Tukey’s HSD test was calculated to confirm statistically significant differences between different samples. Accuracy of DC polarographic assay in comparison with other assays applied in parallel was tested based on coefficients of variation.
Results and discussion
Antioxidant capacity of coffees and coffee substitutes’ brews
In order to obtain information regarding the AO activity of coffee brews (Turkish/Greek, instant, espresso and filter) and coffee substitutes (roasted chicory root, barley, pea, chickpea, carob and dried fig), multiple AO assays were performed. AO capacity of coffee and coffee substitute ingredients measured by the DC polarographic (HPMC) assay, DPPH and ABTS scavenging activity, FRAP and TPC are shown in Table 1. Results have been given following descending order of AO capacity determined by DC polarography.
Table 1.
Total phenolic content (TPC) and AO activity of coffees and coffee substitutes brews as determined using DC polarographic (HPMC), DPPH, FRAP and ABTS assays
| Sample no. | HPMC (%/mL) | TPC (g GAE/L) | DPPH (mM TE/L) | FRAP (mM Fe(II)/L) | ABTS (mM TE/L) |
|---|---|---|---|---|---|
| Coffees | |||||
| 1. Instant | 2391 ± 94f | 9.614 ± 0.205o | 12.66 ± 0.57n | 91.88 ± 0.70l | 44.73 ± 0.42o |
| 2. Instant | 2300 ± 100ef | 9.386 ± 0.114n | 13.24 ± 0.06o | 94.13 ± 0.61m | 47.41 ± 0.33q |
| 3. Instant | 2247 ± 114e | 9.159 ± 0.023m | 10.58 ± 0.47l | 92.26 ± 0.42lm | 46.50 ± 0.40p |
| 4. Instant* | 2229 ± 42e | 7.727 ± 0.000l | 10.75 ± 0.23l | 76.39 ± 0.28j | 37.51 ± 0.42m |
| 5. Instant* | 2197 ± 127e | 7.182 ± 0.227k | 11.57 ± 0.15m | 83.69 ± 0.56k | 40.56 ± 0.62n |
| 6. Turkish | 635 ± 36d | 3.523 ± 0.023ef | 6.40 ± 0.15j | 43.20 ± 0.33i | 21.40 ± 0.46kl |
| 7. Turkish | 618 ± 38cd | 3.795 ± 0.114g | 6.86 ± 0.54k | 44.51 ± 0.51i | 20.71 ± 0.26jk |
| 8. Espresso | 603 ± 22cd | 4.432 ± 0.205j | 7.03 ± 0.54k | 42.64 ± 0.80i | 20.12 ± 0.40j |
| 9. Turkish | 598 ± 19cd | 4.000 ± 0.182h | 6.99 ± 0.06k | 40.10 ± 0.27h | 22.15 ± 0.52l |
| 10. Espresso | 589 ± 30cd | 3.614 ± 0.023ef | 6.43 ± 0.32j | 36.18 ± 0.61g | 18.08 ± 0.33gh |
| 11. Turkish | 578 ± 20cd | 4.227 ± 0.045i | 5.60 ± 0.27gh | 32.81 ± 0.70f | 19.10 ± 0.52i |
| 12. Turkish | 571 ± 29cd | 3.795 ± 0.023g | 5.73 ± 0.26h | 38.05 ± 0.33gh | 17.66 ± 0.47fg |
| 13. Filter | 551 ± 26bcd | 3.500 ± 0.0 91ef | 4.91 ± 0.31d | 36.60 ± 2.34g | 15.62 ± 0.33c |
| 14. Turkish | 547 ± 14bcd | 3.591 ± 0.455ef | 5.44 ± 0.12fg | 29.96 ± 0.19e | 18.25 ± 0.20gh |
| 15. Turkish | 543 ± 17bcd | 3.295 ± 0.295cd | 6.01 ± 0.13i | 36.28 ± 0.70g | 16.69 ± 0.13de |
| 16. Filter | 542 ± 32bcd | 3.205 ± 0.295bc | 6.89 ± 0.15k | 31.36 ± 0.28ef | 18.35 ± 0.20ghi |
| 17. Turkish | 538 ± 25bcd | 3.455 ± 0.364de | 3.72 ± 0.46b | 24.12 ± 0.23c | 18.57 ± 0.67hi |
| 18. Filter | 527 ± 32bcd | 3.182 ± 0.182bc | 3.99 ± 0.11c | 27.12 ± 0.92d | 16.53 ± 0.26de |
| 19. Turkish | 525 ± 39bcd | 3.205 ± 0.068bc | 5.19 ± 0.39e | 30.27 ± 0.45e | 16.91 ± 0.20ef |
| 20. Turkish | 498 ± 29bcd | 3.636 ± 0.045fg | 2.58 ± 0.12a | 20.75 ± 0.49b | 8.56 ± 0.40a |
| 21. Turkish | 494 ± 16bc | 3.545 ± 0.182ef | 5.72 ± 0.23h | 37.17 ± 0.75g | 16.10 ± 0.72cd |
| 22. Turkish | 483 ± 13bc | 3.455 ± 0.045de | 5.32 ± 0.25ef | 33.00 ± 0.61f | 17.60 ± 0.20fg |
| 23. Turkish | 413 ± 22a | 3.068 ± 0.114b | 5.80 ± 0.13hi | 32.89 ± 0.27f | 15.73 ± 0.35c |
| 24. Filter* | 401 ± 16a | 2.295 ± 0.114a | 4.20 ± 0.25c | 16.15 ± 0.23a | 10.97 ± 0.27b |
| Ingredients for coffee substitutes | |||||
| 1. Carob | 517 ± 42f | 7.827 ± 0.091f | 44.82 ± 3.59g | 73.52 ± 3.67f | 37.58 ± 0.39g |
| 2. Barley | 457 ± 24e | 6.609 ± 0.036d | 36.96 ± 0.63f | 66.65 ± 0.28d | 29.36 ± 0.99e |
| 3. Chickpea | 437 ± 15d | 5.382 ± 0.039b | 34.14 ± 1.85d | 62.87 ± 6.89c | 25.77 ± 1.15c |
| 4. Chicory | 432 ± 8c | 5.309 ± 0.040a | 31.07 ± 1.21b | 51.58 ± 7.06b | 23.88 ± 2.26b |
| 5. Pea | 395 ± 15b | 5.091 ± 0.037c | 31.29 ± 0.73c | 50.24 ± 0.65a | 23.33 ± 0.12a |
| 6. Fig | 367 ± 10a | 5.291 ± 0.020a | 29.01 ± 0.40a | 50.05 ± 3.09a | 27.39 ± 1.07d |
| CV | 6.74 | 7.33 | 6.36 | 7.65 | 9.27 |
Data represent the means of a triplicate experiment ± standard deviation
CV coefficient of variation
* Decaffeinated samples
a–m, Values with the same letter in column, are not statistically different at the p < 0.05 level, 95% confidence limit, according to Tukey’s HSD test
No significant difference between the results of the DC polarographic (HPMC) assay and FC, FRAP, ABTS and DPPH assays were observed. Results of spectrophotometric assays have been found similar to DC polarographic ones at p < 0.01 significance level (FFC = 13741, FFRAP = 9012, FABTS = 6517, and FDPPH = 41113, with Fcrit = 2.059). FC has been found to be the most influential variable for the final AO result, while HPMC has been found more influential than FRAP, ABTS and DPPH assay. However, according to Brown–Forsythe test of homogeneity of variances, DC polarographic assay was more influential compared to spectrophotometric AO assays.
Considered coffees represent a rich source of phenolic compounds. The highest TPC was observed for instant coffees and coffee substitutes. Coffee substitutes were better source of phenolics than Turkish, espresso and filter coffees. The effect of the brewing method on TPC of differently prepared coffee brews has been found in accordance with previous results (Hečimović et al. 2011; Niseteo et al. 2012). Decaffeinated instant coffees exhibited lower TPC (up to 7.727 g GAE/L) than their regular coffee counterparts (up to 9.614 g GAE/L). The lowest TPC among 24 coffee samples has been ascribed to decaffeinated filter coffee.
Coffee brew AO capacity determined by DC polarography has been found superior in comparison to substitutes’ brews. Amongst the substitutes, the highest content of TPC has been found in carob. This substitute possesses superior AO activity according to all assays applied. The high AO activity of carob observed previously was explained by the presence of catechin and gallic acid (Custodio et al. 2011). Increasing of AO activity with roasting temperature and duration was reported earlier (Sahin et al. 2009). This increase suggested significant contribution of melanoidins. Roasted barley has been found to be the second substitute according to both TPC and AO activity determined by all four AO assays. HMW melanoidinic component was found to be a prevalent contributor to its AO activity (Papetti et al. 2006). Increase of AO activity of chickpea (Cicer arietinum L.) during roasting was also related to Maillard reaction products formation (Segev et al. 2012). The dried and roasted root of chicory (Cichorium intybus L.) contains both phenolic AOs and melanoidins (Jurgonski et al. 2011). Dried fig (Ficus carica L) has been considered a rich source of AOs (Vinson et al. 2005).
Relative antioxidant capacity index and phenolics antioxidant coefficients
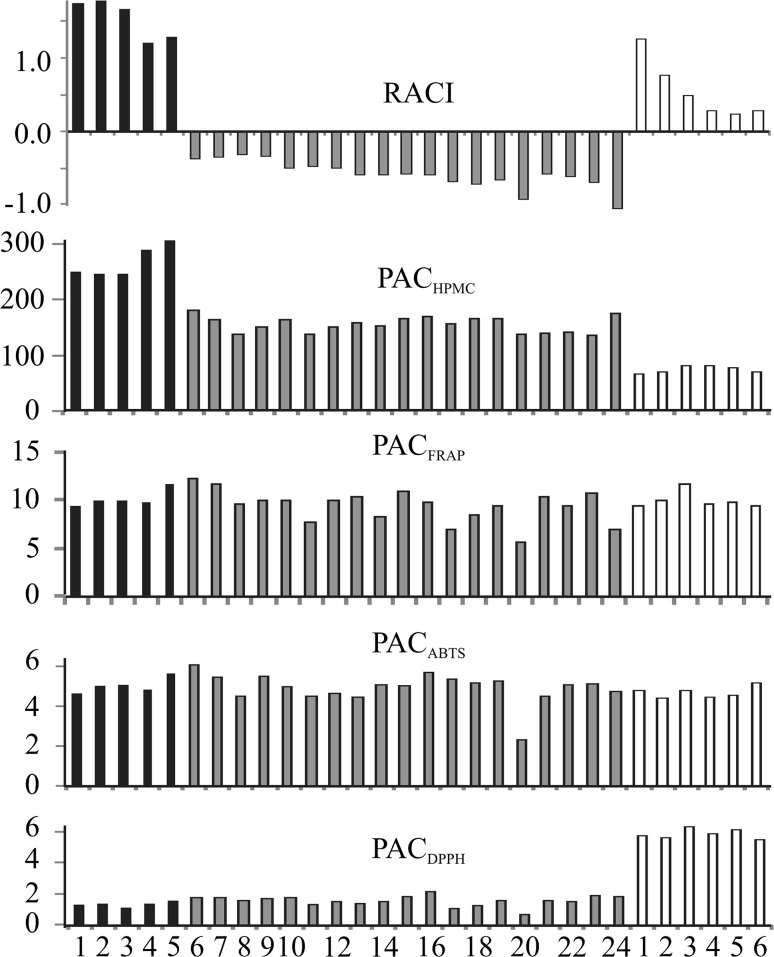
In order to get better insight into differences of samples’ AO capacity, relative antioxidant capacity index (RACI) has been calculated by assigning equal weight to AO assays applied, including FC as a measure of total reducing activity. Also, phenolic AO coefficients (PAC) have been calculated as the ratio between particular AO activity and total phenolic content (expressed in g GAE/L). As seen in Fig. 1, the highest values of RACI have been ascribed to instant coffee brews, followed by substitutes. The lowest values among instant coffees have been ascribed to decaffeinated samples. On the other side, decaffeinated instant and espresso coffee possess the highest PACHPMC in comparison to regular coffee. Positive values of RACI have been ascribed only to instant coffees and substitutes (1.27–1.73), while all other samples of coffees have negative RACI (from −1.03 to −0.36). The lowest value of RACI belongs to the decaffeinated espresso coffee (−1.03). RACI ascribed to substitutes have been found lower in comparison to instant coffees, while much higher than Turkish, espresso and filter coffee brews. Chickpea and chicory have the highest PACHPMC among coffee substitutes. Descending order of HPMC has been found in good corroboration with RACI for all three recognized groups of samples, particularly for substitutes.
Fig. 1.
Relative antioxidant capacity index (RACI) and phenolic antioxidant coefficients (PAC) for coffee and coffee substitute brews (black bar—instant coffees, grey bar—Turkish, filter and espresso coffees, white bar—substitutes)
PAC values calculated based on DC polarographic and spectrophotometric assays were found to be significantly different. Possibility to group samples based on PACABTS and PACFRAP has not been observed. According to PACFRAP and PACABTS similar level of phenolics efficiency for coffees and coffee substitutes has been noticed. According to PACHPMC, instant coffees can be easily recognized as a specific group of samples. According to PACHPMC, the efficiency of phenolics present in substitutes has been found the lowest while according to PACDPPH the highest amongst analysed samples. An almost equal value of RACI has been obtained for carob and decaffeinated instant coffee, while a large difference in their PACHPMC has been found.
Correlations between applied assays
Results of all applied assays and RACI have been correlated (Table 2). Coffees HPMC show good agreement with FC, FRAP, ABTS and DPPH. Low correlations have been obtained between HPMC and other AO assays and FC for coffee substitues.
Table 2.
Correlation coefficients between HPMC and FC GAE, FRAP, ABTS and DPPH, as well as with RACI for coffee and coffee surrogates brews (at 95% confidence limit)
| HPMC | FRAP | ABTS | DPPH | RACI | |
|---|---|---|---|---|---|
| Coffee brews | |||||
| FC GAE | 0.969 p < 0.01 |
0.969 p < 0.01 |
0.970 p < 0.01 |
0.920 p < 0.01 |
0.987 p < 0.01 |
| RACI | 0.984 p < 0.01 |
0.992 p < 0.01 |
0.991 p < 0.01 |
0.957 p < 0.01 |
|
| DPPH | 0.918 p < 0.01 |
0.965 p < 0.01 |
0.956 p < 0.01 |
||
| ABTS | 0.961 p < 0.01 |
0.982 p < 0.01 |
|||
| FRAP | 0.961 p < 0.01 |
||||
| Coffee substitutes brews | |||||
| FC GAE | 0.885 p = 0.02 |
0.896 p = 0.02 |
0.957 p < 0.01 |
0.957 p < 0.01 |
0.975 p < 0.01 |
| RACI | 0.922 p < 0.01 |
0.963 p < 0.01 |
0.938 p < 0.01 |
0.987 p < 0.01 |
|
| DPPH | 0.950 p < 0.01 |
0.949 p < 0.01 |
0.894 p = 0.02 |
||
| ABTS | 0.773 p = 0.08 |
0.834 p = 0.04 |
|||
| FRAP | 0.910 p < 0.01 |
||||
Principal component analysis
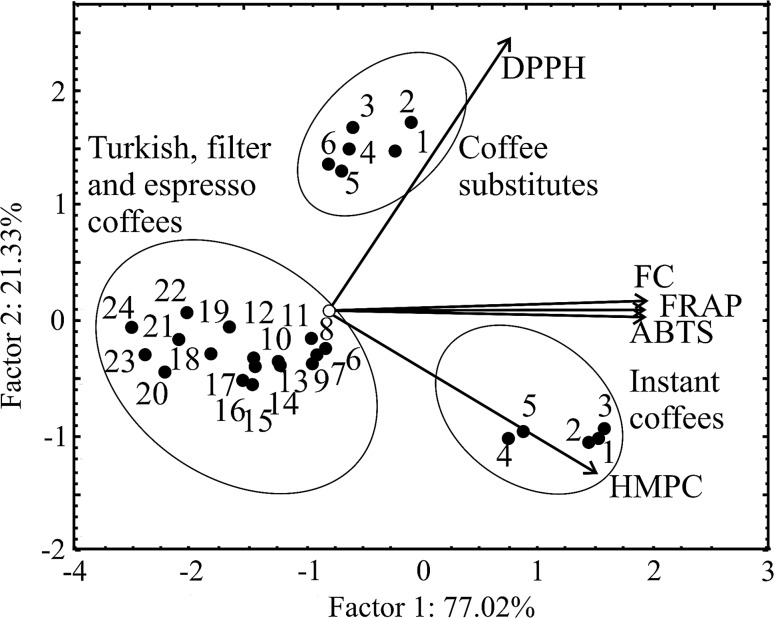
The PCA allows detection of structure in the relationship between measured parameters and different varieties of tested brews that give complimentary information. For visualizing the data trends and the discriminating efficiency of the used descriptors a scatter plot of samples using the first two principal components (PCs) issued from PCA of the data matrix is obtained (Fig. 2). FC (24.7%), FRAP (24.9%) and ABTS (24.8%) are the dominant variables in the first, while the DPPH (70.3%) and HPMC (29.5) in the second principle component. The first two principal components (98.27% of the total variability) are sufficient for data representation. Coffee substitutes are most pronounced in DPPH values, while instant coffees (samples 1–5) have the highest values of HPMC. Coffee samples no. 6 to 24 have correlated well with FC, FRAP and ABTS.
Fig. 2.
Biplot for AO activity of coffee and coffee substitutes brews
AO capacity of individual coffee constituents
In order to achieve a better understanding of variations in the total AO activity of brews, AO activity of individual compounds present in non roasted (NRC) (phenolic acids, flavonoids and methylxanthines) as well as melanoidins and N-methyl pyridinum present in roasted coffee (RC) has been determined (Table 3). A large difference between applied assays ability to recognize various types of AOs has been noticed. According to DC polarographic assay, significant AO activity has been ascribed to methylxanthines and N-methyl pyridinum, while according to spectrophotometric assays these compounds show no AO activity.
Table 3.
Individual compounds AO activity as determined by DC polarographic (HPMC), DPPH, ABST and FRAP assays
| DPPH (mM TE/mol) | FRAP (mM TE/mol) | ABTS (mM TE/mol) | HPMC (%/μmol) | |
|---|---|---|---|---|
| Chlorogenic acid | 1430 ± 5 | 1805 ± 50 | 3190 ± 31 | 14.96 ± 0.45 |
| Caffeic acid | 1070 ± 33 | 1180 ± 23 | 1515 ± 35 | 12.65 ± 0.75 |
| Caffeine | 12 ± 3 | 0 | 0 | 4.35 ± 0.23 |
| Theophylline | 17 ± 15 | 30 ± 10 | 8 ± 8 | 22.30 ± 0.95 |
| Theobromine | 27 ± 3 | 33 ± 11 | 12 ± 6 | 15.49 ± 0.68 |
| N-methylpyridinium | 50 ± 0 | 8 ± 3 | 28 ± 5 | 7.78 ± 0.50 |
| Catechin | 1402 ± 17 | 1903 ± 42 | 2169 ± 65 | 47.57 ± 3.28 |
| Quercetin | 3088 ± 36 | 4188 ± 72 | 5410 ± 59 | 37.37 ± 5.24 |
| Rutin | 2432 ± 99 | 2772 ± 102 | 4930 ± 46 | 24.65 ± 2.16 |
The data represents the means of a triplicate experiment ± standard deviation
In conclusion, high accuracy of the DC polarographic assay was clearly shown by correlation analysis, ANOVA and F-test, as well as Brown–Forsythe’s test. The accuracy of the assay was expressed by its low coefficient of variation. In comparison to spectrophotometric assays, DC polarographic assay enabled a specific insight into the AO activity of complex and individual samples analysed within the scope of this study. In contrast to negligible scavenging activity against DPPH and ABTS, as well as reducing power (FRAP), substantial AO capacity of physiologically active compounds (methylxanthines and N-methyl pyridinum) present in complex samples was established using the DC polarographic (HPMC) assay.
Acknowledgements
Authors sincerely express appreciation to Branko J. Drakulić for constructive suggestions as well assynthesis and characterization of N-methyl pyridinum.
Funding
This work was supported by the Ministry of Education and Science of Republic of Serbia, Grants 43010 and 31093.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
Contributor Information
Jovanka Laličić-Petronijević, Phone: +381 11 3283 185, Email: jovankal@agrif.bg.ac.rs.
Lato Pezo, Phone: +381 11 3283 185, Email: latopezo@yahoo.co.uk.
References
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. LWT. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Carlsen MH, Halvorsen BL, Holte K, Bohn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, Barikmo I, Berhe N, Willett WC, Phillips KM, Jacobs DR, Blomhoff R. The total antioxidant content of more than 3100 foods beverages spices herbs and supplements used worldwide. Nutr J. 2010;9:3. doi: 10.1186/1475-2891-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson ACC, Grafenstein J, Budnjo A, Laurila JL, Bergquist J, Karim A, Kleinmaier R, Brath U, Erdelyi M. Symmetric halogen bonding is preferred in solution. J Am Chem Soc. 2012;134:5706–5715. doi: 10.1021/ja301341h. [DOI] [PubMed] [Google Scholar]
- Custodio L, Fernandes E, Escapa AL, Fajardo A, Aligue R, Albericio F, Neng NR, Nogueira JMF, Romano A. Antioxidant and cytotoxic activities of carob tree fruit pulps are strongly influenced by gender and cultivar. J Agric Food Chem. 2011;59:7005–7012. doi: 10.1021/jf200838f. [DOI] [PubMed] [Google Scholar]
- El Qouatli SE, Rosie NT, Valery HG, Oubenamar H, Hissou H, Najih R, Chtaini A. Electrochemical analysis of the antioxidant capacity of coffee. Bulletin of the Catalysis Society of India. 2011;10(3):15–20. [Google Scholar]
- Fukushima Y, Ohie T, Yonekawa Y, Yonemoto K, Aizawa H, Mori Y, Watanabe M, Takeuchi M, Hasegawa M, Taguchi C, Kondo K. Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J Agric Food Chem. 2009;57:1253–1259. doi: 10.1021/jf802418j. [DOI] [PubMed] [Google Scholar]
- Hečimović I, Belščak-Cvitanović A, Horžić D, Komes D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011;129:991–1000. doi: 10.1016/j.foodchem.2011.05.059. [DOI] [PubMed] [Google Scholar]
- Jurgonski A, Milala J, Juskiewicz J, Zdunczyk Z, Krol B. Composition of chicory root peel seed and leaf ethanol extracts and biological properties of their non-inulin fractions. Food Technol Biotech. 2011;49:40–47. [Google Scholar]
- Ludwig IA, Sanchez L, Caemmerer B, Kroh LW, Paz de Pena M, Cid C. Extraction of coffee antioxidants: impact of brewing time and method. Food Res Int. 2012;48:57–64. doi: 10.1016/j.foodres.2012.02.023. [DOI] [Google Scholar]
- Niseteo T, Komes D, Belščak-Cvitanović A, Horžić D, Budeč M. Bioactive composition and antioxidant potential of different commonly consumed coffee brews affected by their preparation technique and milk addition. Food Chem. 2012;134:1870–1877. doi: 10.1016/j.foodchem.2012.03.095. [DOI] [PubMed] [Google Scholar]
- Oliveira-Neto JR, Garcia Rezende S, de Fatima Reis C, Benjamin SR, Rocha MV, de Souza Gil E. Electrochemical behavior and determination of major phenolic antioxidants in selected coffee samples. Food Chem. 2016;190:506–512. doi: 10.1016/j.foodchem.2015.05.104. [DOI] [PubMed] [Google Scholar]
- Omwamba M, Hu Q. Antioxidant activity in barley (Hordeum Vulgare L) grains roasted in a microwave oven under conditions optimized using response surface methodology. J Food Sci. 2010;75:C66–C73. doi: 10.1111/j.1750-3841.2009.01426.x. [DOI] [PubMed] [Google Scholar]
- Papetti A, Daglia M, Aceti C, Quaglia M, Gregotti C, Gazzani G. Isolation of an in vitro and ex vivo antiradical melanoidin from roasted barley. J Agric Food Chem. 2006;54:1209–1216. doi: 10.1021/jf058133x. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Bio Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sahin H, Topuz A, Pischetsrieder M, Ozdemir F. Effect of roasting process on phenolic antioxidant and browning properties of carob powder. Eur Food Res Technol. 2009;230:155–161. doi: 10.1007/s00217-009-1152-7. [DOI] [Google Scholar]
- Segev A, Badani H, Galili L, Hovav R, Kapulnik Y, Shomer I, Galili S. Effects of baking roasting and frying on total polyphenols and antioxidant activity in colored chickpea seeds. Food Nutr Sci. 2012;3:369–376. doi: 10.4236/fns.2012.33053. [DOI] [Google Scholar]
- Vinson JA, Zubik L, Bose P, Samman N, Proch J. Dried fruits: excellent in vitro and in vivo antioxidants. J Am Coll Nutr. 2005;24:44–50. doi: 10.1080/07315724.2005.10719442. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ho CT. Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem. 2009;57:8109–8114. doi: 10.1021/jf804025c. [DOI] [PubMed] [Google Scholar]
- Yardim Y. Electrochemical behavior of chlorogenic acid at a boron-doped diamond electrode and estimation of the antioxidant capacity in the coffee samples based on its oxidation peak. J Food Sci. 2012;77:C408–C413. doi: 10.1111/j.1750-3841.2011.02609.x. [DOI] [PubMed] [Google Scholar]
- Ziyatdinova G, Aytuganova I, Nizamova A, Budnikov H. Differential pulse voltammetric assay of coffee antioxidant capacity with MWNT-modified electrode food. Anal Method. 2013;6:1629–1638. doi: 10.1007/s12161-013-9591-y. [DOI] [Google Scholar]