Abstract
In recent years, there has been an ever growing interest in finding new natural sources of food antioxidants. As a main fruit crop, olive is also valued due to its phenolic-containing leaves. Mathematically based optimization methods are used as powerful tools to extract different antioxidant compounds. The present study is aimed to provide an efficient extraction method for total phenol content (TPC), total flavonoid content (TFC) and antioxidant ability (DPPH scavenging assay and FRAP). The effects of ultrasonic temperature (35–65 °C), ultrasonic time (5–15 min), and ethanol to water ratio (Et: W) (25–75%) were evaluated. Second-order polynomial models were used through a rotatable Box-Behnken design (BBD) consisting of 15 experimental runs with three replicates at the center point. Interactional effects of the studied factors were significant in most cases for all responses. The highest extraction efficiency was found to be fifty-one percent of ethanol (65 °C, 15 min) to water ratio. Under optimal conditions, values for TPC, TFC, DPPHsc and FRAP assay were 183.4 (mg GAE. g−1 DW), 696.77 (mg Quercetin. g−1 DW), 78.98 (DPPHsc %) and 1942 µmol Fe+2/g DW, respectively. R 2 values (R 2 > 0.92) showed that RSM models could efficiently predict the yield of all responses. In the LC–ESI–MS–DAD profiling of the optimized extract, 27 compounds were identified with oleuropein as the main compound. In the present study, olive leaf is introduced as a promising source of natural antioxidant and can be used in food industries following further studies.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2676-7) contains supplementary material, which is available to authorized users.
Keywords: RSM: Response surface methodology, TPC: Total phenol content, TFC: Total flavonoid content
Introduction
Phenolic compounds are ubiquitous secondary metabolites and are important determinants of the sensory and nutritional quality of plants (Ignat et al. 2011). Moreover, phenolic compounds can play an important role in virtually any interaction a plant can have with its environment, biotic or abiotic (Niknam and Ebrahimzadeh 2002). Recently, plant origin by-products with abundant sources of these compounds are attracting worldwide interest. Olive (Olea europaea) is one of the valuable fruits not only widely considered for its alimentary use as fruit but also is important for phenolic-containing leaves (Özcan and Matthäus 2017). Olives are consumed as a natural source for extracting compounds having functional values such as bio-phenolic compounds (Ghanbari et al. 2012). In recent decades, olive leaves have been used in medicine, cosmetics, and in pharmaceutical products (Erbay and Icier 2010). The multifunctional capacity of the leaves allows the extraction process to be more effective with regard to the antioxidant properties.
Because of containing natural antioxidants and broad-spectrum of antimicrobial oleuropein and its derivatives, olive leaves are also used in jam-packed with plant antioxidants (Jemai et al. 2008). The extraction process has a dramatic effect on final concentrations of extracted compounds. Therefore, optimization of its extraction procedure is very important. Optimization of various extraction parameters such as time, temperature, solvent composition, solid/liquid ratio and their interactions are important in recovering phenolic compounds (Ilaiyaraja et al. 2015).
In optimization related works, conventional methods can be very time-consuming. When interactions exist among the variables, it is less likely to find a true optimum condition. RSM has been used to optimize many phytochemicals, which was first introduced by Box and Wilson (Box and Wilson 1951). RSM can be effectively employed to evaluate the effects of multiple factors and their interactions on one or more responses (Myers and Montgomery 2003). The advantage of RSM is the reduced number of experimental trials. Thus, it is widely used in the optimization of parameters in the extraction of some compounds, such as polysaccharides (Chen et al. 2015a), phenolic compounds (Fattahi and Rahimi 2016; Ilaiyaraja et al. 2015), and carotenoids from different plant materials.
Ultrasonic treatment has been employed to extract phenolic compounds from different plant materials in recent years (Chen et al. 2015b; Lee et al. 2013). High-efficiency responses by ultrasonic treatment are mainly attributed to its mechanical effects, which greatly facilitate mass transfer between immiscible phases through a super agitation (Vinatoru et al. 1997). The advantages of ultrasonic treatments are a shear wave, micro-jetting, and micro-streaming (Chandrapala and Leong 2015). Optimization of ultrasound-assisted extraction of olive leaf has been studied by extraction parameters including solid/solvent ratio, time and ethanol concentration (Şahin and Şamlı 2013). However, temperature was not included in their study. In the present study, we decided to optimize another extraction method in order to shorten the time and reduce the consumption of energy which are the most important restricting factors in industrial procedures. In all extraction processes, shorter extraction times reduced organic solvent consumption, and energy and cost saving are the main tasks pursued (Chemat et al. 2017). Moreover, the aim of this study was to determine the optimum conditions for individual and simultaneous determination of TPC, TFC and antioxidants from olive leaves. We tried to optimize the conditions for obtaining maximum yield of polyphenols, total flavonoids and antioxidants using RSM.
Materials and methods
Plant material
Olive leaves (Olea europaea cv. Koroneiki) were harvested from the cultivars grown at Tarom Olive Research Station located in Tarom, Zanjan, Iran. To obtain uniform material for phenolic extraction, all the leaf samples were obtained from one cultivar and dried at the room temperature in the dark.
Chemical materials
Folin-Ciocalteu’s phenol reagent, gallic acid, 2, 2-dipheynl-1-picrylhydrazy (DPPHsc), methanol, acetonitrile, acetic acid, and quercetin were purchased from Sigma (St. Louis, MO, USA). Water was purified by using a Milli-Q system (Millipore Lab, Bedford, MA, USA).
Preparation of leaf extracts
Dried leaf material was ground to a fine powder. Then, 300 mg of the leaf samples were placed in separate vials with different ethanol to water ratio (25–75%) and treated in different ultrasonic temperatures (35–65 °C) for 5–15 min. Data were taken using an ultrasonic water bath (750 W, 50/60 Hz, E 120 H Elmasonic) supplied by Elma (Singem, Germany). The extracts were filtered (HPLC 0.45 μm porosity) into clean vials and stored in well-closed vials at 4 °C in the dark before measuring the parameters (Fattahi and Rahimi 2016).
Measurement of TPC
Total phenol content (TPC) of the extracts were determined colorimetrically, following Slinkard and Singleton (1977) using Folin-Ciocalteu reagent with some modifications. Ten µl of the extracts with 600 µl of Folin-Ciocalteu reagent (10%) was mixed with 90 µl of distilled water and kept for 10 min. Then, 480 µl of sodium carbonate was added to the solution and stored in the dark for 2 h. The absorbance of the solution was determined colorimetrically at the wavelength of 765 nm using UV-2100 spectrophotometer (UNICO, China). Gallic acid (GAE) was used as standard phenol and the results were expressed as mg gallic acid equivalents per g dry matter basis.
Measurement of TFC
Total flavonoid content (TFC) was determined by a colorimetric assay (Shin et al. 2007). 30 µl of above-mentioned extracts was added to a 15-ml tube containing 2 ml of deionized water. 150 µl of 5% sodium nitrite was added and the mixture was stored at room temperature for 5 min. Then, 300 µl of 10% aluminum chloride (AlCl3. 6 H2O) was added. The solution was kept for 6 min, and then 1 ml of 1 mol. l−1 sodium hydroxide was added. Final volume of mixture was reached to 5 ml by adding distilled water. The absorbance of the mixture was measured immediately at 380 nm. The results were expressed as quercetin equivalents using a standard curve prepared from pure quercetin.
Antioxidants assay
DPPH scavenging assay
Free radical scavenging activity (DPPHsc) of the extracts was measured according to the method described by Nakajima et al. (2004) with some modifications reported by (Chiou et al. 2007). Five microliters of the extracts were added to 950 µl of 6 × 10−5 mol. l−1 (free radical, 95%) in methanol. The mixtures were shaken and were stored at room temperature for 30 min. Then, the absorbance was measured at 517 nm using a UV–VIS spectrophotometer. The percent of reduction of DPPH was calculated by using the following formula:
| 1 |
Abs sample indicates the absorbance at 517 nm of the DPPHsc in the presence of the sample and Abs control shows the absorbance of DPPHsc solution in methanol without extracts.
FRAP assay
Antioxidant activity of the extracts was also measured by using the ferric reducing ability of plasma (FRAP) assay described by Benzie and Strain (1996). In this method, antioxidant ability can reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+), producing the blue-colored Fe2+ tripyridyltriazine compound which increases absorbance at 593 nm. The FRAP reagent was prepared by mixing acetate buffer (300 mmol/l), 2, 4, 6-tripyridyl-S-triazine (TPTZ) (10 mmol/l), and FeCl3.6H2O (20 mmol/l) (10:1:1). Freshly prepared reagent (3 ml) was placed in the test tubes and incubated at 37 °C (T0). 300 μl of sample (285 μl of reagent and 15 μl of plant extraction) and 500 μl of deionized water were added to each test tube. After mixing thoroughly, the samples were incubated at 37 °C for 4 min, and then absorbance was measured at 593 nm. The change in absorbance between T0 and after 4-min condition was used to calculate FRAP values. Linear calibration curves were established by six assays of FeSO4·7H2O. Then, a regression equation was calculated according to y = ax + b, where y and x represent the absorbance at 593 nm and concentration, respectively. The results were expressed as μmol Fe+2/g dry weight.
RSM
Ultrasonic temperature (X1), Ultrasonic time (X2), and ethanol to water ratio (X3) were regarded as independent variables. A Box–Behnken design (BBD) with three center points was used to investigate the effects of independent variables on six dependent responses (TPC, TFC, DPPHsc %, FRAP). Three levels of the independent variables were transformed into three codes (−1, 0, 1), with a complete design consisting of 15 experimental runs with three replications of the center points (all factors at level 0) (Table 1). STATISTICA release 8.0 was used to carry out analysis of variance and drawing of surface plots.
Table 1.
The BBD matrix and the RSM’s experimental data for the responses, independent data; X 1 (Ultrasonic temperature (°C)), X 2 (Ultrasonic time (min.)), and X 3 (Ethanol-to-water (%)) and the responses are TPC (total phenol content), TFC (total flavonoid content), antioxidant (DPPHsc (%)), antioxidant (RRAP)
| Run | Factors | Observed | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | TPC | TFC | %DPPHsc | FRAP | |
| 1 | 0 | 0 | 0 | 180.0 | 550.0 | 60.0 | 1762 |
| 2 | 0 | 0 | 0 | 181.0 | 560.8 | 59.7 | 1760 |
| 3 | 0 | 0 | 0 | 181.5 | 546.7 | 59.0 | 1763 |
| 4 | 0 | −1 | −1 | 91.4 | 298.3 | 41.2 | 1669 |
| 5 | 0 | −1 | 1 | 137.1 | 595.8 | 78.0 | 1691 |
| 6 | 0 | 1 | 1 | 150.3 | 553.3 | 59.0 | 1709 |
| 7 | 1 | 0 | 1 | 196.4 | 726.7 | 77.7 | 1762 |
| 8 | 1 | 1 | 0 | 178.2 | 695.8 | 78.5 | 1971 |
| 9 | −1 | 0 | 1 | 99.6 | 338.3 | 45.0 | 1744 |
| 10 | −1 | 1 | 0 | 124.0 | 369.2 | 48.6 | 1789 |
| 11 | −1 | 0 | −1 | 102.3 | 360.8 | 42.3 | 1704 |
| 12 | 0 | 1 | −1 | 158.9 | 490.8 | 76.9 | 1744 |
| 13 | 1 | 0 | −1 | 127.3 | 506.7 | 66.1 | 1851 |
| 14 | 1 | −1 | 0 | 185.7 | 624.2 | 70.2 | 1731 |
| 15 | −1 | −1 | 0 | 97.8 | 357.5 | 43.8 | 1727 |
| Symbols | Coded levels | |||
|---|---|---|---|---|
| Variables | −1 | 0 | 1 | |
| Ultra sonic temp. | X 1 | 35 | 50 | 65 |
| Ultra sonic time | X 2 | 5 | 10 | 15 |
| Ethanol to water (%) | X 3 | 25 | 50 | 75 |
The quadratic polynomial models were fit to the predicted responses and obtained regression coefficients with changing factors including ultrasonic temperature (35–65 °C), ultrasonic time (5–15 min), and ethanol to water ratio (25–75%) in the extracted samples. The generalized second-order polynomial model used in the response surface analysis is illustrated in Eq. (2):
| 2 |
Yn indicates the responses function of the independent variables (X1 − X3), b 0 represents constant coefficient, b 1, b 2, and b 3 show the linear coefficients; b 11, b 22, and b 33 represent the quadratic coefficients; and b 12, b 13 and b 23 illustrate the cross-coefficients. The analysis of ANOVA was carried out to evaluate the accuracy of the estimated coefficient using the F-test at 1 and 5%, indicating coefficient R 2.
ESI–MS and HPLC–DAD conditions
A Shimadzu LCMS-2010 System equipped with an SPD-M10A vp diode array detection (DAD) detector and an LC-10AD binary pump coupled on line with an MS-2010 mass spectrometer was used in the present study (Shimadzu, Kyoto, Japan). The analysis was carried out using negative and positive ion modes with spectra acquired within the range of m/z 100-950. Electrospray ionization (ESI–MS) was performed at a fragmentation voltage of 215 V (positive) and—175 V (negative). Drying gas temperature and drying gas (N2) flow were 190 °C and 9.0L/min, respectively. Capillary voltages were 3.5 kV (negative) and 4 kV (positive). Calibration was performed with internal reference masses of m/z 112.9855 (purine) and m/z 980.0164 (HP-0921) in the negative mode, and m/z 121.050873 (purine) and m/z 922.009798 (HP-0921) in the positive mode. Chromatographic separation was performed on a C18 column (4.6 × 150 mm, 5 μm), the mobile phase conducted for 62 min, consisting of acidified water A (0.5% acetic acid, v/v) and acetonitrile B, respectively. The gradient was programmed as follows: 0 min, 0% B; 20 min, 20% B; 30 min, 30% B; 40 min, 50% B; 50 min, 75% B; 60 min, 100% B; 62 min 0% B. The flow from the HPLC system into the ESI–MS detector was 0.2 mL/min. The injection volume was 10 μL and the column temperature was set at 25 °C. The DAD was set at 243 nm to provide real time chromatograms and UV spectra from 200 to 390 nm were recorded for plant component identification. UV and MS data were acquired and processed using Shimadzu LCMS Solution Software.
Results and discussion
In recent decades, olive leaves have been considered as important sources of phenolic antioxidants (García-Villalba et al. 2014). Hence, it seems necessary to develop a simple, rapid, fast, and accurate method for extraction of oil components including phenolics and antioxidants. To this end, a Box-Behnken design (BBD) with three center points was employed to discover an efficient ultrasound-assisted extraction method from olive leaf samples. Since ethanol and water are the two most common safe solvents in the food industrial process, they were selected as extraction solvents (Khiari et al. 2009). Table 1 illustrates the experimental design, recorded values for the experiment, and the levels of the independent variables (X1-X3) based on BBD and RSM.
Fitting the RSM models
The regression models (R 2 and R 2-adj) for all of the responses (TPC, TFC and DPPHsc %, FRAP) with satisfactory coefficients of multiple determinations (R 2 and R 2-adj >0.92 and 0.78) suggests the fitness of the RSM used in this study (Supplementary material 1). A good fit was obtained when there was a high R 2, indicating that a great variation shared among samples can be attributed to the factors selected for the model (Puértolas et al. 2011). The calculated regression coefficients of intercept, linear, and quadratic models and interactions of the models based on least square method of analysis of variance (ANOVA) are summarized in supplementary material 1. The coefficients of responses’ P-values indicated that all of linear terms of TPC, TFC, and DPPHsc % and X1 and X2 in the case of FRAP were highly significant (p < 0.05). Interaction effects of the independent variables (except X1X2 in the case of TPC, TFC and DPPHsc %) were also shown to be highly significant. Quadratic term of X3X3 was not shown to be significant (p > 0.05) for DPPHsc % contrary to the other responses which were shown to be statistically significant (p < 0.05). The significant of other responses are illustrated in supplementary material 1. For all the terms in the models, a lower p value suggests a higher significant effect on the respective response variables.
The RSM models based on the experimental data for each of the responses are presented based on the following equations:
| 3 |
| 4 |
| 5 |
| 6 |
Y indicates the predicting responses and X 1, X 2, and X 3 represent ultrasonic temperature, ultrasonic time, and ethanol to water ratio %, respectively.
Effects of extraction conditions on TPC based on RSM
Figure 1a–c shows that ultrasonic extraction time plays a determining role in achieving higher extraction yield with an optimum value of 12 min. The surface plots’ shapes indicate the nature and the extent of different factors’ interactions (Prakash et al. 2008). Ultrasound and the increased effect of vibrating homogenization and cavitation may accelerate the dissolution of TPC (Abbas et al. 2013). In the case of interaction between the ratio of the ultrasonic time (X2) and ethanol-to-water, extraction efficiency for TPC was initially enhanced by increasing the ratio of ethanol-to-water, but it consequently went down. In the optimum ratio of ethanol to water (~60%) cell pores can be increased because of suitable tumescence of leaves (Fattahi and Rahimi 2016). Since polyphenols have varied rates of solubility, a mixture of ethanol and water may be more effective than any single solvents to achieve a better extraction procedure (Ilaiyaraja et al. 2015).
Fig. 1.
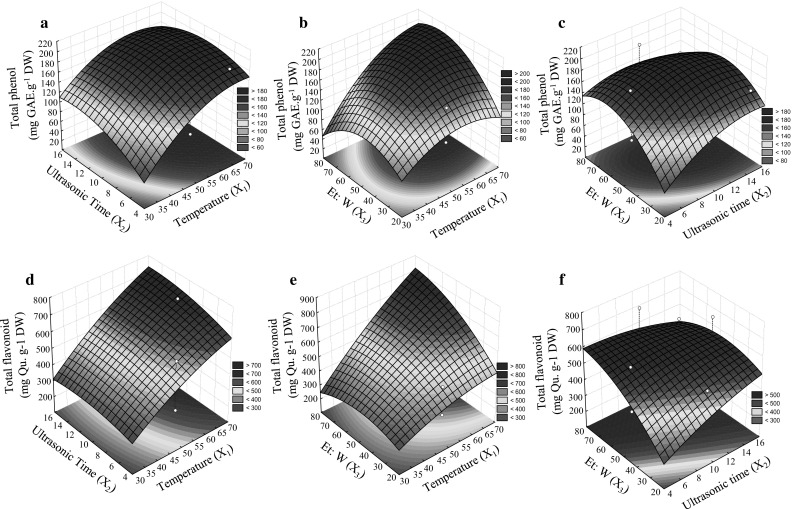
The surface describing the effect of the three independent variables on response variables; TPC based on the interaction between variables X1 to X3 (a–c), TFC based on interaction between variables X1 to X3 (d–f), independent variables; X 1 (Ultrasonic temperature (°C)), X 2 (Ultrasonic time (min.)), and X 3 (Ethanol to water (%))
Effects of extraction conditions on TFC based on RSM
TFC of olive leaves was influenced by X1 − X3 (Supplementary material 1) and similar results with TPC were obtained. The data for three-dimensional surface plots for TFC based on interaction between factors are shown in Fig. 1d–f. The rate of extraction recovery was increased at higher temperatures (65–70 °C) and sonication times >10 min. The corresponding values under optimal conditions of time and temperature were 730–740 (mg Quercetin/g−1 DW). Time and temperature are depending on to each other in ultrasonic assist extractions. Temperature plays an important role in extraction process by affecting the softening rate of tissues, increasing solubility and diffusion coefficient of the substances (Shi et al. 2003). Extraction time is another important factor. Shortening of extraction time with higher amounts of compounds is economical for industrial aims (Chemat et al. 2017). Based on Fig. 1d–f, Ethanol to water ratio was the other influential factor in the recovery of TFC. Higher total flavonoid (~600 mg Quercetin/g−1 DW) achieved by increasing the ethanol to water ratio until 60% and then was decreased. Slightly polar ethanol in a mixture with more polar water effectively can extract flavonoids and their glycosides over a limited compositional range (Radojkovića et al. 2012). Temperatures between 40 to 55 °C have been previously reported by other researchers to be suitable during extraction of flavonoids (Liyana-Pathirana and Shahidi 2005; Silva et al. 2007). In the case of ultrasonic assisted extraction, some authors have reported a beneficial effect of temperature rise from 20 to 70 °C compared to non-sonicated extractions (Chemat et al. 2017). It seems that shortening of time in ultrasonic assisted extraction compared to non-sonicated extraction methods allows temperature enhancement (65–70 °C) before the destruction of phenolic compounds.
Effect of extraction conditions on total antioxidant capacity
DPPH scavenging assay
Antioxidant assay (2, 2-Diphenyl-1-picrylhydrazyl scavenging assay (DPPHsc)) based on the interaction between the independent variables lent support to the effective role of temperature and time (Fig. 2a-c). Maximum Free radical scavenging ability was obtained at the higher temperatures (65–70 °C) with the higher extraction durations (14–16 min). Under optimum conditions, the DPPHsc ability reached to 95%. Lower DPPH scavenging value in the increased extraction time and temperature may be attributed to the thermal degradation of other antioxidants at a high temperature (Prommuak et al. 2008). Higher efficiency of DPPHsc assay at the higher concentration of ethanol to water indicates that ethanol is an effective solvent in the short time periods. The findings suggest that water needs more time than ethanol to extract the components. In food industries, extraction of antioxidants is of paramount importance (Chiou et al. 2007; Khiari et al. 2009). Acting as free radical scavengers, antioxidants bind with free radicals and protect the cells from many diseases (Valko et al. 2007). Antioxidants may exert influence on biological systems through a number of mechanisms including electron donation, co-antioxidants, or gene expression regulation (Lobo et al. 2010). Flavonoids are the major active nutraceutical ingredients in plants which are responsible for antioxidant activity. As the typical for the phenolic compounds, they can act as potent antioxidants and metal chelators (Lobo et al. 2010).
Fig. 2.
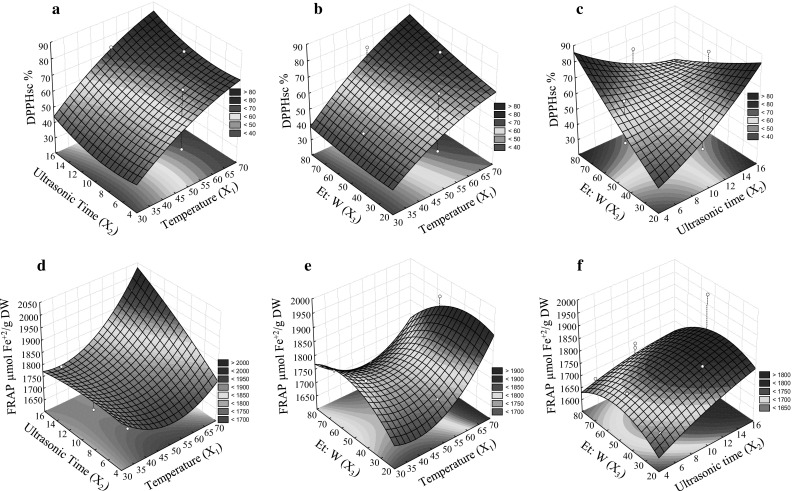
The surface describing the effect of the three independent variables on response variables; DPPHsc (%) based on the interaction between variables X1 to X3 (a–c), FRAP-based on interaction between variables X1 to X3 (d–f), independent variables; X 1 (Ultrasonic temperature (°C)), X 2 (Ultrasonic time (min.)), and X 3 (Ethanol to water (%))
FRAP
The results relevant to the three-dimensional surface plots for FRAP in the interaction of X1 to X3 are illustrated in Fig. 2d–f. First, the extraction recovery rate was increased by enhancing the Et: W%, but it was subsequently decreased. The highest FRAP was observed at middle temperatures ranging between 50 to 52 °C. Additionally, the higher amounts of FRAP were obtained in the higher ranges of sonication temperature and time. Under optimal conditions (time >14 and temperature 65), the FRAP amount was reached to 2050 µmol Fe+2/g DW. In the present study, DPPHsc results were highly correlated to polyphenols than those of FRAP, indicating that DPPH scavenging assay is more effective than FRAP method. The action of TP, TF, and others constituents (chlorophyll, enzymes) may be responsible for the extracts’ observed antioxidant activity. Therefore, the extract’s antioxidant activity highly varies according to the plant material (Meziant et al. 2014).
Simultaneous optimization of variables for responses
In order to obtain an extraction condition for phenolic antioxidant, simultaneous optimization of four responses, namely, TPC, TFC, DPPHsc %, and FRAP was carried out based on the desirability values. Optimal extraction parameters for obtaining simultaneous highest responses were 51% ethanol; at 65 °C for 15 min. Under optimum conditions, the corresponding desirability values were more than 0.92. In addition, under optimum conditions TPC, TFC, DPPHsc %, and FRAP were 183.4 (mg GAE. g−1 DW), 696.77 (mg Quercetin/g−1 DW), 78.98 (DPPHsc %), and 1942 (FRAP µmol Fe+2/g DW), respectively. In Figs. 3a–c surface plots for desirability (all responses) based on the interaction between the variables X1 to X3 are shown in response surface plots. The interaction effect of X1 and X2 on desirability amount, when the X3 was kept at middle range, demonstrates that the desirability values were highest when X1 and X2 were 14 min and 6 °C in the ultrasonic set. Additionally, the high desirability value was reported in 55% (v/v) of ethanol-to-water ratio. The highest desirability values were found in the mid-range of ethanol concentrations in the interaction of X2X3. In the present study at higher ratios of ethanol-to-water, the amount of chlorophyll and carotenoid increased (data not shown), and consequently, solvent capacity was occupied partially by chlorophyll instead of phenolic compounds.
Fig. 3.
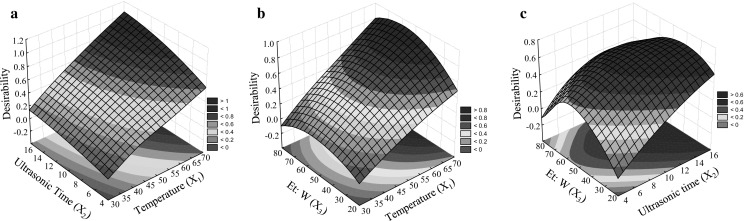
Desirability amount of the three responses based on the interactions of independent variables (X1 to X3), Independent variables; X 1 (Ultrasonic temperature (°C)), X 2 (Ultrasonic time (min.)), and X 3 (Ethanol to water (%)) and the responses are TPC (total phenol content), TFC (total flavonoid content), and (DPPHsc (%)), and FRAP are responses included in the desirability indices
Different polarities of ethanol and water lead to the extraction of different amounts of chlorophyll (Macías-Sánchez et al. 2009). Ethanol is a molecule with both a polar and a non-polar end. Thus, enhancing the ratio of ethanol-to-water in the extract results in the decrease of the less polar compounds like chlorophyll due to readable solubility (Fattahi and Rahimi 2016).
Accuracy of RSM models
In order to evaluate the accuracy of the model’s predictions, the R 2 values are reported. Accordingly, the R 2 values were recorded between experimental data and the data obtained from RSM models. High R 2 values of models (>0.92) indicate a satisfactory model fit for the relationship between RSM models with the experimental data.
Identification of optimized extract by LC–DAD–ESI–MS
Compound’s name, mass patterns in negative and positive ESI mode, UV spectrum, and possible molecular formula are presented in Table 2. In both ESI modes, two replications were injected and similar compounds were identified with the same mass in the range of m/z 100–970. Samples were more sensitively detected in negative as opposed to the positive ion mode. As shown in Table 2 and Fig. 4, 30 compounds were detected and 27 of them were identified in this study. Major compounds consisted of oleuropein diglucoside (isomer 2), retention time (TR), 44.5, [M − H] ion at m/z 701, [M + Na] ion at m/z 725, UV/Vis absorption maxima (λ max 222, 276, 322), apigenin rutinoside (TR, 47.7, [M − H] ion at m/z 577 and [M + H] ion at m/z 579, λ max 244, 279, 322, Oleuropein (TR, 50.6, [M − H] ion at m/z 539, [M + H] 541, [M + Na] 563, [M + K] 579 (M + K), λ max 244, 279, 322), Lucidumoside C (TR, 51.8, [M − H] ion at m/z 583, [M + K] 625 λ max 231, 280; Oleuropein isomer (TR, 52.8, [M − H] ion at m/z 539, [M + Na] 563, λ max 227, 279. Other major compounds in the optimized extraction consisted of elenolic acid derivative, quinic acid, gluconic acid, 4-Syringin, hydroxyferulic acid hexoside, and ligstroside. Other compounds’ characteristics and detailed information are described in Table 2. Oleuropein as the main compound has several pharmacological properties including antioxidant, anti-inflammatory, anti-atherogenic, anti-cancer, antimicrobial, antiviral, cardioprotective, anti-ischemic, and hypolipidemic (Omar 2010). Therefore, oleuropein and its derivatives in the optimized extract is highly recommended for pharmacological and nutraceutical purposes.
Table 2.
Compounds’ name, molecular weight, maximum UV absorption, and molecular formula of the identified compounds
| Peak no. | Compounds name | [M − H]− | [M − H]+ [M + X] | UV (nm) | Molecular formula |
|---|---|---|---|---|---|
| 1 | Sugar | 181 | 204 (M + Na) | – | C6H12O6 |
| 2 | Quinic acid | 191 | 193 | 218, 221, 322 | C7H12O6 |
| 3 | Gluconic acid | 195 | 221 (M + Na + 2H) | 218, 280, 322 | C6H12O7 |
| 4 | 4-Syringin | 317 | – | 215, 279, 322 | C17H24O9 |
| 5 | Hydroxyferulic acid hexoside | 371 | 206 (M/2 + H + K) | 217, 223, 322 | – |
| 6 | Secologanoside | 389 | 391 | 207, 211, 260 | C16H22O11 |
| 7 | Unknown | 343 | 345 | – | C18H16O7 |
| 8 | Hirsutidin 3-O-glucoside | 505 | – | – | |
| 9 | 7-Epiloganin | 389 | 391 | 260 | C17H26O10 |
| 10 | Oleuropeinaglycone derivative | 377 | 379 | – | – |
| 11 | Oleoside methyl ester | 403 | 427 (M + Na) | 202, 270, 317 | C17H24O11 |
| 12 | 10-Elenolic acid glucoside | 403 | 427 (M + Na) | 217, 279 | C17H24O11 |
| 13 | Unknown | 525 | 527 | 202, 281 | – |
| 14 | 2-(2-ethyl-3-hydroxy-6-propionylcyclohexyl)Ac Ac glucoside | 403 | 427 (M + Na) | 202, 279, 317 | C17H24O11 |
| 15 | 2′′-Methoxyoleuropein isomer 1 | 569 | 589 (M + NH4 + H) | 221, 322, 366 | C26H34O13 |
| 16 | Elenolic acid derivative | 601 | 620 (M + NH4 + H), 647 (M + 2Na − H) | 202, 279, 322 | |
| 17 | Oleuropein diglucoside (isomer 1) | 701 | 725 (M + Na) | 202, 280, 317 | C31H42O18 |
| 18 | Hydroxyoleuropein | 555 | – | 204, 279 | C25H33O14 |
| 19 | Oleuropein diglucoside (isomer 2) | 701 | 725 (M + Na), 741 (M + K) | 222, 276, 322 | C31H42O18 |
| 20 | Luteolin-7-glucoside | 447 | 449 | 222, 346 | C21H20O11 |
| 21 | Apigenin rutinoside | 577 | 579 | 244, 279, 322 | C27H30O14 |
| 22 | Oleuropein diglucoside (isomer 3) | 701 | 725 (M + Na) | 227, 279, 322 | C31H42O18 |
| 23 | Oleuropein | 539 | 541, 563 (M + Na) 579 (M + K) | 244, 279, 322 | C25H32O13 |
| 24 | Lucidumoside C | 583 | 625 (M + K) | 231, 280 | C27H36O14 |
| 25 | Oleuropein isomer | 539 | 563 (M + Na) | 227, 279 | C25H32O13 |
| 26 | Ligstroside | 523 | 547 (M + Na) | 227, 276 | C25H32O12 |
| 27 | Luteolin | 285 | 287 | 268 | C15H10O6 |
| 28 | Quercetin | 301 | 303 | 256, 273 | C15H10O7 |
| 29 | Diosmetin | 299 | 301 | – | C16H12O6 |
| 30 | Unknown | 343 | 389 (M + 2Na − H) | – | C18H17O7 |
Fig. 4.
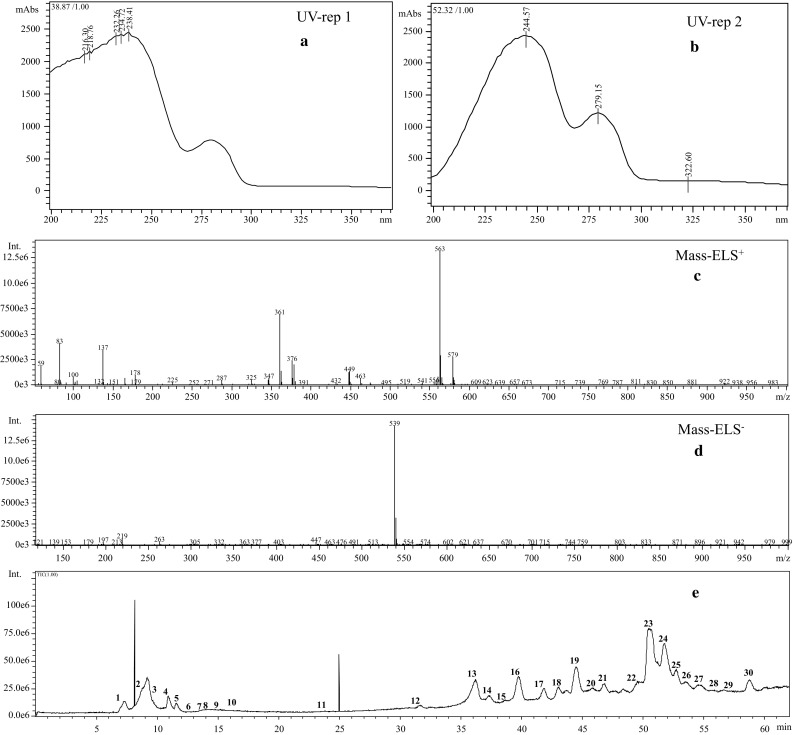
UV absorption pattern and mass spectra peaks of oleuropein (a–d). Chromatograms of HPLC–DAD peaks corresponding to optimized extract (E)
Conclusion
The present study investigated the impact of extraction conditions on TPC, TFC, and antioxidant assay on olive leaves through RSM. The findings demonstrated that in the ultrasonic-assisted method, extraction time was dramatically decreased in comparison with other extraction methods. Extraction at high temperature (i.e., 65 °C) was deemed to be an effective factor in decreasing the extraction time. The confirmatory experimental optimum conditions for the simultaneous recovery of TPC, TFC, and antioxidant were as follows: 51% of ethanol; at 65 °C for 15 min. Bing highly close to the experimental yield values, under optimal condition, TPC, TFC, DPPHsc %, and FRAP were 183.4 (mg GAE. g−1 DW), 696.77 (mg Quercetin/g−1 DW), 78.98 (DPPHsc %), and 1942 (FRAP µmol Fe+2/g DW), respectively. The higher R 2 values suggest that models could efficiently predict the responses’ yield. In addition, ESI-DAD-MS analysis identified 27 compounds with a higher rate of oleuropein and its derivatives.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Analysis of variance (ANOVA) and the estimated regression coefficients for the total phenolic content (TPC), total flavonoid content (TFC), and DPPHsc (%), FRAP. values to determine the suitability of second-order polynomial model fitting in the experimental data (DOC 63 kb)
Acknowledgements
Work performed in Department of Department of Plant Biology, College of Science, University of Tehran. The authors are very grateful to the members of “Soil and Water Research Department of Zanjan Agricultural and Natural Resources Research and Education Center” and “Department of Horticulture of Urmia University” for their scientific support in some parts of the present study.
Funding
This study was funded by University of Tehran.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest in this study.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Publication has been approved by all individual participants.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2676-7) contains supplementary material, which is available to authorized users.
Contributor Information
Habib Shirzad, Email: hshirzad1354@yahoo.com.
Vahid Niknam, Phone: +982161113637, Email: vniknam@khayam.ut.ac.ir.
References
- Abbas S, Hayat K, Karangwa E, Bashari M, Zhang X. An overview of ultrasound-assisted food-grade nanoemulsions. Food Eng Rev. 2013;5:139–157. doi: 10.1007/s12393-013-9066-3. [DOI] [Google Scholar]
- Benzie IF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Box GE, Wilson K. On the experimental attainment of optimum conditions. J R Stat Soc Ser B (Methodol) 1951;13:1–45. [Google Scholar]
- Chandrapala J, Leong T. Ultrasonic processing for dairy applications: recent advances. Food Eng Rev. 2015;7:143–158. doi: 10.1007/s12393-014-9105-8. [DOI] [Google Scholar]
- Chemat F, Rombaut N, Sicaire A-G, Meullemiestre A, Fabiano-Tixier A-S, Abert-Vian M. Ultrasound-assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Chen C, Shao Y, Tao Y, Wen H. Optimization of dynamic microwave-assisted extraction of Armillaria polysaccharides using RSM, and their biological activity. Food Sci Technol-LEB. 2015;64:1263–1269. doi: 10.1016/j.lwt.2015.07.009. [DOI] [Google Scholar]
- Chen M, Zhao Y, Yu S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015;172:543–550. doi: 10.1016/j.foodchem.2014.09.110. [DOI] [PubMed] [Google Scholar]
- Chiou A, Karathanos VT, Mylona A, Salta FN, Preventi F, Andrikopoulos NK. Currants (Vitis vinifera L.) content of simple phenolics and antioxidant activity. Food Chem. 2007;102:516–522. doi: 10.1016/j.foodchem.2006.06.009. [DOI] [Google Scholar]
- Erbay Z, Icier F. The importance and potential uses of olive leaves. Food Rev Int. 2010;26:319–334. doi: 10.1080/87559129.2010.496021. [DOI] [Google Scholar]
- Fattahi M, Rahimi R. Optimization of extraction parameters of phenolic antioxidants from leaves of Capparis spinosa using response surface methodology. Food Anal Method. 2016;9:2321–2334. doi: 10.1007/s12161-016-0414-9. [DOI] [Google Scholar]
- García-Villalba R, Larrosa M, Possemiers S, Tomás-Barberán F, Espín J. Bioavailability of phenolics from an oleuropein-rich olive (Olea europaea) leaf extract and its acute effect on plasma antioxidant status: comparison between pre-and postmenopausal women. Eur J Nutr. 2014;53:1015–1027. doi: 10.1007/s00394-013-0604-9. [DOI] [PubMed] [Google Scholar]
- Ghanbari R, Anwar F, Alkharfy KM, Gilani A-H, Saari N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)-a review. Int J Mol Sci. 2012;13:3291–3340. doi: 10.3390/ijms13033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Ilaiyaraja N, Likhith K, Babu GS, Khanum F. Optimisation of extraction of bioactive compounds from Feronia limonia (wood apple) fruit using response surface methodology (RSM) Food Chem. 2015;173:348–354. doi: 10.1016/j.foodchem.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Jemai H, Bouaziz M, Fki I, El Feki A, Sayadi S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem-Biol Interact. 2008;176:88–98. doi: 10.1016/j.cbi.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Khiari Z, Makris DP, Kefalas P. An investigation on the recovery of antioxidant phenolics from onion solid wastes employing water/ethanol-based solvent systems. Food Bioprocess Tech. 2009;2:337–343. doi: 10.1007/s11947-007-0044-8. [DOI] [Google Scholar]
- Lee L-S, Lee N, Kim YH, Lee C-H, Hong SP, Jeon Y-W, Kim Y-E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules. 2013;18:13530–13545. doi: 10.3390/molecules181113530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyana-Pathirana C, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93:47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macías-Sánchez M, Mantell C, Rodriguez M, de la Ossa EM, Lubián L, Montero O. Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta. 2009;77:948–952. doi: 10.1016/j.talanta.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Meziant L, Benchikh Y, Louaileche H. Deployment of response surface methodology to optimize recovery of dark fresh fig (Ficus carica L., var. Azenjar) total phenolic compounds and antioxidant activity. Food Chem. 2014;162:277–282. doi: 10.1016/j.foodchem.2014.04.054. [DOI] [PubMed] [Google Scholar]
- Myers WR, Montgomery DC. Response surface methodology. Encycl Biopharm Stat. 2003;1:858–869. [Google Scholar]
- Nakajima J-i, Tanaka I, Seo S, Yamazaki M, Saito K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries . J BioMed Biotech. 2004;2004:241–247. doi: 10.1155/S1110724304404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknam V, Ebrahimzadeh H. Phenolics contents in Astragalus species. Pak J Bot. 2002;34:283–289. [Google Scholar]
- Omar SH. Oleuropein in olive and its pharmacological effects. Sci Pharm. 2010;78:133–154. doi: 10.3797/scipharm.0912-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan MM, Matthäus B. A review: benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur Food Res Technol. 2017;243:89–99. doi: 10.1007/s00217-016-2726-9. [DOI] [Google Scholar]
- Prakash O, Talat M, Hasan S, Pandey RK. Factorial design for the optimization of enzymatic detection of cadmium in aqueous solution using immobilized urease from vegetable waste. Bioresource Technol. 2008;99:7565–7572. doi: 10.1016/j.biortech.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Prommuak C, De-Eknamkul W, Shotipruk A. Extraction of flavonoids and carotenoids from Thai silk waste and antioxidant activity of extracts. Sep Purif Technol. 2008;62:444–448. doi: 10.1016/j.seppur.2008.02.020. [DOI] [Google Scholar]
- Puértolas E, Saldana G, Álvarez I, Raso J. Experimental design approach for the evaluation of anthocyanin content of rose wines obtained by pulsed electric fields. Influence of temperature and time of maceration. Food Chem. 2011;126:1482–1487. doi: 10.1016/j.foodchem.2010.11.164. [DOI] [Google Scholar]
- Radojkovića M, Zekovića Z, Jokićb S, Vidovića S. Determination of optimal extraction parameters of mulberry leaves using Response Surface Methodology (RSM) Rom Biotech Lett. 2012;17:7295–7308. [Google Scholar]
- Şahin S, Şamlı R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason sonochem. 2013;20:595–602. doi: 10.1016/j.ultsonch.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Shi J, Yu J, Pohorly J, Young JC, Bryan M, Wu Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J Food Agric Environ. 2003;1:42–47. [Google Scholar]
- Shin Y, Liu RH, Nock JF, Holliday D, Watkins CB. Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biol Tec. 2007;45:349–357. doi: 10.1016/j.postharvbio.2007.03.007. [DOI] [Google Scholar]
- Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55:381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Viticult. 1977;28:49–55. [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vinatoru M, Toma M, Radu O, Filip P, Lazurca D, Mason T. The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrason Sonochem. 1997;4:135–139. doi: 10.1016/S1350-4177(97)83207-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of variance (ANOVA) and the estimated regression coefficients for the total phenolic content (TPC), total flavonoid content (TFC), and DPPHsc (%), FRAP. values to determine the suitability of second-order polynomial model fitting in the experimental data (DOC 63 kb)


