Abstract
Although an increase in dietary lithium (Li) has been suggested as a possible method for mood stabilization and for decreasing violence and suicidal rates, no Li-enriched food has entered the market. Here we continue to explore the feasibility of mushrooms in this respect and have investigated the growth, accumulation and mineral content (Ca, K, Mg and Na) of Agrocybe cylidracea and Hericium erinaceus cultivated on substrates supplemented with 0.25–1.0 mM of Li as acetate or chloride. As demonstrated, supplementation with LiCl yielded more satisfactory results, did not alter mushroom biomass, appearance, shape or size regardless of Li concentration. It also had no significant effect on mineral composition and resulted in a concentration-dependent uptake of Li and its accumulation in fruiting bodies. More promising results were found for H. erinaceus. As calculated, consumption of 100 g dw of its fruiting bodies obtained from cultivation with 1.0 mM of Li (as acetate or chloride) would constitute 69% of the provisional recommended dietary daily intake of Li set at 1.0 mg. The study highlights that H. erinaceus could be selected for further studies on Li-enriched food that concern the bioavailability of Li from mushrooms, their safety and activity in animal experimental models and eventually, human studies.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2679-4) contains supplementary material, which is available to authorized users.
Keywords: Lithium, Biofortification, Hericium erinaceus, Agrocybe cylidracea, Functional food, Medicinal mushrooms
Introduction
While lithium (Li) is not considered officially as an essential element it has been theorized that it may influence or mediate some biochemical processes, e.g. those associated with magnesium (Diniz et al. 2013). Higher plants are known to complete their life cycle without Li, although in low concentrations it was shown to stimulate growth and development (Schäfer 2012). Furthermore, Li deficiency was potentially associated with decreased life-span, reduced lactation and reproduction in goats (Klemfuss and Schrauzer 1995; Schrauzer 2002).
A number of studies have also reported that Li can influence human behavior. For example, some studies conducted on different populations found a negative correlation between concentration of Li in tap water and violence and suicide rates (Ohgami et al. 2009; Koenig et al. 2015; Ishii et al. 2015; Marshall 2015; Hidvégi et al. 2016). In low doses (mainly un-ionized asparate and orotate) Li may act as a nutrient required for B12 vitamin and folate uptake and transport, neuromodulation and other biochemical processes. Li induces stem cell production and up-regulates neurotrophins (e.g. brain-derived neurotrophic factor, nerve-growth factor) (Marshall 2015). Moreover, some studies have demonstrated that Li reveals antioxidant, anti-inflammatory and anti-aging activity (Schäfer 2012; Terao 2015). Supplementary doses of Li may have potential in the prevention of neurological disorders (Diniz et al. 2013; Marshall 2015; Terao 2015). In high doses (mainly ionized carbonate and citrate) that exceed 50–300 times natural intake Li acts as a drug and has been used in psychiatry since the 1940s as a mood stabilizer in affective disorders and add-on therapy in depression (Müller-Oerlinghausen and Lewitzka 2010; Berghöfer et al. 2013; Malhi and Outhred 2016). In contrast to many psychiatric medicines, Li remains an indispensable element in contemporary psychopharmacology and has outlasted various pharmacotherapeutic “fashions” (Schulze et al. 2010).
Various medicinal properties of Li and particularly its neuroprotective and mood-stabilizing effects have created an interest in the production of food enriched with this element (Hajek and Weiner 2016). Mushrooms would be particularly suitable foodstuffs for such a purpose owing to their bioaccumulation capabilities (Niedzielski et al. 2015; Rzymski et al. 2016, 2017). Previous studies have shown that different mushroom species could be used in the production of Li-enriched food (de Assunção et al. 2012; Vieira et al. 2013; Nunes et al. 2014; Nunes et al. 2015; Mleczek et al. 2017). As demonstrated, Li concentration in P. ostreatus cultivated in a substratum fortified with lithium chloride (LiCl) was directly influenced by increasing concentrations of this salt in the subsoil. Furthermore, Li in enriched mushrooms showed greater accessibility than lithium carbonate (Li2CO3) used in psychiatric medicine (de Assunção et al. 2012). Research by Nunes et al. (2014, 2015) highlighted that different fungal species may differ in Li uptake, and that the effectiveness of this process depends on the form of Li. As found, lithium chloride and sulfate (Li2SO4) proved to be most promising. In turn, our previous study demonstrated the feasibility of using lithium acetate (CH3COOLi) for the bio-enrichment of Pleurotus mushrooms and Ganoderma lucidum (Mleczek et al. 2017).
Some studies have shown that the presence of Li may influence the nutritional composition of mushrooms but information in this regard is very scarce. As shown, substrate supplementation with 500 mg kg−1 LiCl did not alter crude protein content while content of potassium and calcium in fruiting bodies was slightly increased (de Assunção et al. 2012; Vieira et al. 2013). In turn, magnesium content was not significantly found to be altered by Li supplementation (de Assunção et al. 2012; Vieira et al. 2013).
The objective of this study was to further expand the potential of two other mushroom species that are valued for their bioactive properties, Agrocybe cylidracea (known as poplar mushroom, velver pioppin or Yanagi-matsutake) and Hericium erinaceus (traditionally called lion’s mane, bearded tooth, satyr’s beard, bearded hedgehog or pom pom), in production of food with increased Li content. For this purpose, mushrooms were grown on substrates enriched with Li in the form of chloride (LiCl) and acetate (CH3COOLi). The study evaluated the yielded biomass, levels of accumulated Li in fruiting bodies and the potential effect that Li could have on the uptake of important minerals–calcium, potassium, magnesium and sodium.
Materials and methods
Experimental design
The studied mushrooms were cultivated at conditions and on substrates optimal for their growth (Sokół et al. 2016). The substrate for A. cylindracea was prepared from a mixture of beech and alder sawdust (1:1 vol.) which was additionally supplemented with 20%, wheat bran, 10% wheat straw chaff, 5% corn flour, 3% soybean meal, 1% chalk and 1% gypsum in relation to the substrate weight. In the case of H. erinaceus a mixture of beech and alder sawdust was also used but the following supplements were added: 20% wheat bran, 5% corn flour, 3% soybean meal, 1% sucrose and 1% gypsum. The mixtures were moistened with distilled water to a moisture content of 45%. The substrate prepared as described above was placed in polypropylene bags and sterilized at a temperature of 121 °C for 1 h and was then cooled down to a temperature of 25 °C. Lithium salts were dissolved in deionized and sterilized water (Milli-Q Academic System (non-TOC)—Millipore, USA). The solution was added to the substrate and blended using an electric stirrer, POLYMIX PX-SR 90 D (KINEMATICA AG, Littau-Luzern, Switzerland), after which the substrate water content was 60%. The following concentrations of lithium salts (LiCl and CH3COOLi) were applied to the substrate: 0.25, 0.5, 0.75 and 1.0 mM. The substrates with lithium addition were mixed with grain spawn of the examined mushroom species (5% of substrate weight) and placed in polypropylene bottles of 1 dm3 volume. Each bottle was filled with 400 g of the substrate. After filling, the bottles were closed with covers. The covers had cellulose type 338 filters with a basic weight of 84 g/m2 and typical retention of 12–15 μm (Munktell, Bärenstein, Germany). Incubation was conducted at temperature of 25 °C and 80–85% air relative humidity until the substrate became completely covered with mycelium. Next, the bottles with removed covers were placed in the cultivation chamber. For fructification, air relative humidity was maintained at 85–90% and temperature at 17 ± 1 °C. The cultivation was additionally illuminated with fluorescent light of 500 lx intensity 12 h a day. The cultivation chamber was aerated in such a way as to maintain CO2 concentration below 1000 ppm. Fruiting bodies of A. cylindracea and H. erinaceus were harvested successively as they matured. Yield included whole fruiting bodies.
In order to determine Li content, substrate samples before and after cultivation as well as fruiting bodies were dried in an electric drier SLW 53 STD (Pol-Eko,Wodzisław Śląski, Poland) at a temperature of 50 °C for 48 h and weighed for dry weight analysis. They were then ground for 0.5 min in a Cutting Boll Mill 200 (Retsch GmbH, Haan, Germany). The powdered samples were treated in the extraction. The method described above was similar to the one which was presented by Niedzielski et al. (2015) with some minor changes.
Procedure
Accurately weighed 0.300 ± 0.001 g of a dry mushroom sample was extracted by phosphoric acid solution using an ultrasonic bath. Samples were then filtered and diluted with water to a total volume of 15.0 mL. Each of the samples was analysed in triplicate starting from weighing.
Analysis of lithium content
Instruments
The inductively coupled plasma optical emission spectrometer Agilent 5100 ICP-OES (Agilent, USA) was used for lithium determination in radial plasma observation mode. The following conditions were applied: wavelength 670.783 nm, Radio Frequency (RF) power 1.2 kW, nebulizer gas flow 0.7 L min−1, auxiliary gas flow 1.0 L min−1, plasma gas flow 12.0 L min−1, Charge Coupled Device (CCD) temperature −40 °C, viewing height for radial plasma observation 8 mm, accusation time 5 s, 3 replicates.
Reagents
All the reagents were of analytical purity. Deionized ultrapure water produced in a Milli-Q device (Millipore, Saint Luis, USA) was used. The ICP commercial analytical standard (Romil, England) was applied for ICP-OES analysis and phosphoric acid 1 mol l−1 solution obtained by dilution of concentrated acid was used for extraction (Honeywell, USA).
Analytical method validation
Detection limits were determined as 3-sigma criteria and were at the level of 0.01 mg kg−1 dry matter (DM). Uncertainty for the complete analytical process (including sample preparation) was at the level of 20%. Due to a lack of the proper certified standard materials, the standard addition technique with a recovery of 80–120% was applied.
Statistical analysis
The results were analysed using STATISTICA 12.0 software (StatSoft, USA). One-way analysis of variance (ANOVA) followed by the post hoc Tukey’s HSD test was applied to investigate the differences in Li accumulation and mushroom growth between the studied species. A value of p < 0.05 was considered as statistically significant.
Results
Morphometric analysis and biomass crop
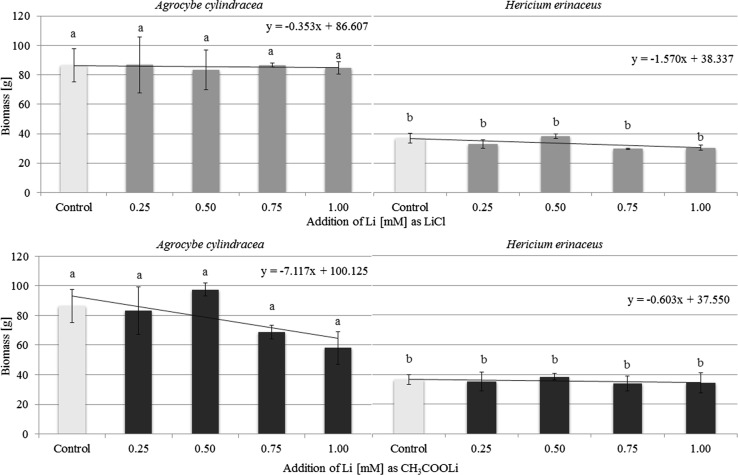
Supplementation of substrate with LiCl had no effect on the biomass of A. cylindracea regardless of the concentration of Li (Fig. 1). In this experimental model the mean biomass of fruiting bodies of this mushroom was 85.5 ± 1.3 g. Addition of CH3COOLi had a slight effect on A. cylindracea biomass which in this case amounted to 78.8 ± 13.9 g. Cultivation of H. erinaceus on substrates supplemented with LiCl or CH3COOLi yielded comparable biomass: 35.7 ± 1.6 and 33.6 ± 3.4 g, respectively. Generally, biomass of A. cylindracea fruit bodies was significantly higher than for H. erinaceus, independently of Li additions.
Fig. 1.
Characteristics of Agrocybe cylindracea (a) and Hericium erinaceus (b) biomass
Supplementation of both mushroom species was not related with any negative changes in the morphology of fruiting bodies’. Fruiting bodies collected from all combinations were of a similar size and shape (Fig. S1 of supplementary material).
Content of lithium in analysed mushroom species
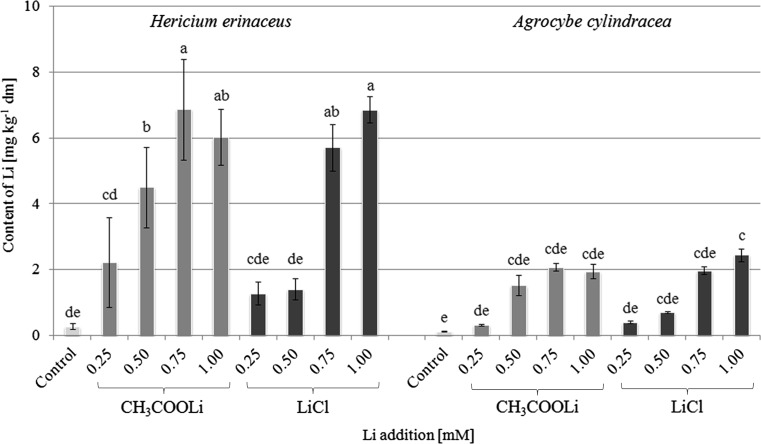
Content of Li in A. cylindracea and H. erinaceus fruit bodies was significantly diversified as presented in Fig. 2. Generally, the greatest accumulation for both Li salts and both mushroom species was found at a concentration of 0.75 and 1.0 mM. In the case of A. cylindracea, supplementation with 0.75 mM of LiCl and CH3COOLi resulted in 1.97 and 2.08 mg Li kg−1, respectively. In turn, supplementation with 1.0 mM of these salts resulted in 2.44 and 1.94 mg Li kg−1 , respectively. A significantly higher content of Li was accumulated in H. erinaceus. Once supplemented with 0.75 and 1.0 mM of Li, its levels in fruiting bodies reached a mean of 5.72 (LiCl) or 6.87 (CH3COOLi) and 6.86 (LiCl) or 6.03 (CH3COOLi) mg kg−1, respectively (Fig. 2).
Fig. 2.
Content of lithium (mg kg−1 dm) in mushroom fruiting bodies growing at substrates enriched with lithium chloride and lithium acetate
Effect of Li supplementation to macroelement accumulation
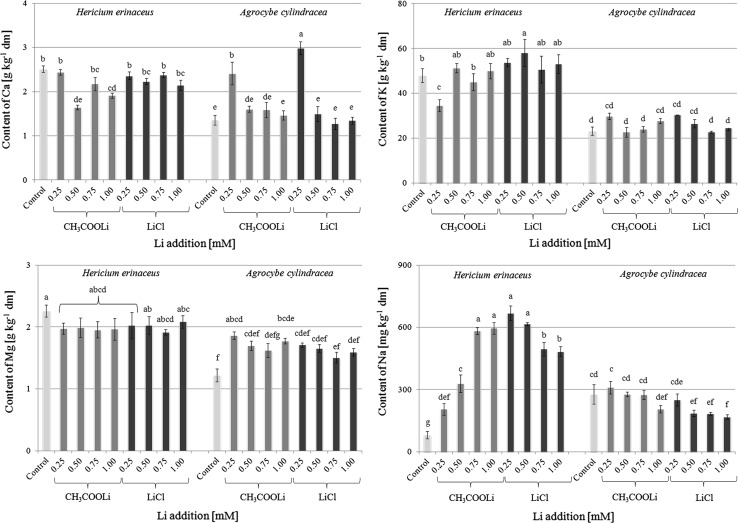
Generally, increase of Li content in both mushroom species was not related with significant changes in K and Mg content (Fig. 3). Content of Ca in H. erinaceus growing on substrate with LiCl generally decreased with increased Li addition in substrate, whereas fruit bodies of this species supplemented with CH3COOLi contained almost the same amount of Ca as in the control (2515 ± 71 mg kg−1 dm). It is worth noting that A. cylindracea was highest in fruit bodies growing on substrate with 0.25 mM of LiCl or CH3COOLi addition (2412 ± 256 and 2982 ± 148 mg kg−1 dm, respectively), while similar contents to the control value (1359 ± 112 mg kg−1 dm) were observed for other experimental models (0.50, 0.75 and 1.0 mM of salt addition). The greatest changes were stated for Na. Supplementation of H. erinaceus using LiCl was related to a significant increase in the content of this metal from 81 ± 18 to 597 ± 29 mg kg−1 dm, respectively for fruit bodies growing in control and in substrate with 1 mM addition of Li (LiCl). Simultaneously, a significant decrease of Na content was observed in H. erinaceus bodies after supplementation with CH3COOLi (all values were significantly higher than for the control). Addition of Li to substrate was also associated with a decrease of Na content in A. cylindracea fruit bodies but in this case the differences were not so great as those for H. erinaceus.
Fig. 3.
Characteristics of Ca, K, Mg and Na content (mg kg−1 dm) in mushroom species
Discussion
As demonstrated, A. cylindracea and H. erinaceus can be cultivated on substrates supplemented with Li for the potential production of Li-enriched food. The present study found that addition of Li to the substrate results in its uptake and accumulation in fruiting bodies without significantly altering mushroom growth or appearance. It mostly has no significant effect on the uptake of essential elements such as Ca, K, Mg or Na. The findings of the present study highlight that the investigated mushroom species, particularly H. erinaceus, could be selected for further research on potential health effects of consumption of food enriched in Li. However, before studies on humans (e.g. randomized clinical trials) could be launched, the safety of such mushroom products should be ensured by animal model research.
Both mushroom species investigated in the present study have been used in traditional folk medicine in different parts of Asia and have lately attracted increasing attention due to their bioactive properties evidenced in vitro, in vivo and partially, using clinical trials. Both are argued to be a valuable resource for development of functional food (Hu et al. 2011; Wang et al. 2014; Chien et al. 2015; Niedzielski et al. 2017). Once enriched in elemental Li, their potential applications could even be broadened. It has been recently postulated that fortification of cereal grain products with Li or addition of Li to food supplements may be beneficial in mood stabilization and in decreasing aggression rates (Goldstein and Mascitelli 2016). To date, no Li-fortified foodstuffs have been commercialized–a process that would need to be preceded by randomized clinical trials. Nevertheless, we are of the opinion that mushrooms bio-enriched in Li may represent a valuable method to increase intakes of Li on the population level. This is due to several reasons: (1) mushrooms are generally relatively easy and inexpensive to cultivate and may be grown on different substrates including waste (Sánchez 2010)–this is particularly important if Li supplementation was subjected to implementation in regions with lower income; (2) mushrooms are globally used as food, and in many cuisines they represent a delicacy (Siwulski et al. 2017); (3) bio-enrichment involves mycelia uptake of Li from the substrate and its incorporation in mushroom fruiting bodies–a strategy for food enrichment that could potentially gain wider consumer acceptance than traditional fortification as it mimics a natural process (and may be considered to be more “natural”); (4) the Li-enriched mushrooms displayed no alterations as regards their general appearance–shape, size or colour. This is also important in view of consumer acceptance of a novel product as changes in appearance may decrease the value of the marketed product (Nestel et al. 2006) (5) the addition of Li to substrates did not result in any significant decrease in yielded biomass of mushrooms. This is a particularly is relevant observation if commercial cultivation was to be run; (6) addition of Li to substrate could even increase the content of important minerals (Na) in fruiting bodies or at least not decrease the levels of some other elements (Ca, Mg, K) to any great extent.
It should be highlighted that biofortified mushrooms could not be used as an alternative to Li-based drugs used in the treatment of psychiatric disorders. Such therapies involve very high doses of Li within a daily range of 600–1200 mg. The only application that could be considered for such food products is a prophylactic mood stabilization in healthy subjects or former drug users or alcoholics as a moderate increase in Li intake has been shown to exert beneficial effects in these groups (Sartori 1986; Schrauzer and de Vroey 1994) Although Li is not considered as an essential element, its decreased serum levels have been linked to increased frequency of suicides, anxiety and homicides (Młyniec et al. 2014; Vita et al. 2015). This has led to a proposal that the provisional recommended dietary allowance for Li should be 1 mg for a 70-kg adult daily (Schrauzer 2002; Marshall 2015). A single consumption of 100 g of dried A. cylindracea cultivated with 0.75 mM of CH3COOLi and 1.0 mM of LiCl would account for 20.8 and 24.4% of the recommended level, respectively. More promising levels would be met by consumption of 100 g of H. erinaceus cultivated with 0.75 mM of CH3COOLi and 1.0 mM of LiCl–in these cases the recommended level would be met in 68.7% in both cases. This would predispose bio-enriched H. erinaceus as a potential increased dietary source of Li. In previous study some other cultivated mushroom species were found to be even more efficient in bioaccumulation of Li. To compare, 100 g of Ganoderma lucidum, Pleurotus ostreatus and Pleurotus eryngii grown on substrates supplemented with 1 mM would constitute 260, 80 and 50% of the provisional recommended dietary intake of Li (Mleczek et al. 2017). The feasibility of adopting P. ostreatus in the production of Li-enriched fruiting bodies was also previously evidenced by de Assunção et al. (2012).
Overall, this and previous studies (de Assunção et al. 2012; Mleczek et al. 2017) highlight that H. erinaceus, G. lucidum, P. ostreatus and P. eryngii should be considered in further application of Li-fortified products, including food supplements (containing mushrooms in the form of powder). Further studies should focus on (1) bioavailability of Li from bio-enriched mushrooms; (2) safety and effects of dietary consumption of bio-enriched mushrooms in animal models; (3) human randomized clinical trials testing potential health benefits of consumption of bio-enriched mushrooms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
Piotr Rzymski is supported by the Foundation for Polish Science within the “Start” Program (091.2016).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2679-4) contains supplementary material, which is available to authorized users.
References
- Berghöfer A, Alda M, Adli M, Baethge C, Bauer M, Bschor T, Grof P, Müller-Oerlinghausen B, Rybakowski JK, Suwalska A, Pfennig A. Stability of lithium treatment in bipolar disorder long-term follow-up of 346 patients. Int J Bipolar Disord. 2013;1:11. doi: 10.1186/2194-7511-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien RC, Tsai SY, Lai EY, Mau JL. Antiproliferative activities of hot water extracts from culinary-medicinal mushrooms, Ganoderma tsugae and Agrocybe cylindracea (Higher Basidiomycetes) on cancer cells. Int J Med Mushrooms. 2015;17:453–462. doi: 10.1615/IntJMedMushrooms.v17.i5.50. [DOI] [PubMed] [Google Scholar]
- de Assunção LS, da Luz JM, da Silva Mde C, Vieira PA, Bazzolli DM, Vanetti MC, Kasuya MC. Enrichment of mushrooms: an interesting strategy for the acquisition of lithium. Food Chem. 2012;134:1123–1127. doi: 10.1016/j.foodchem.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Machado-Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493–500. doi: 10.2147/NDT.S33086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MR, Mascitelli L. Is violence in part a lithium deficiency state? Med Hypotheses. 2016;89:40–42. doi: 10.1016/j.mehy.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Hajek T, Weiner MW. Neuroprotective effects of lithium in human brain? Food for thought. Curr Alzheimer Res. 2016;13:862–872. doi: 10.2174/1567205013666160219112712. [DOI] [PubMed] [Google Scholar]
- Hidvégi A, Rihmer Z, Döme P. Results of investigations on the association between lithium levels in drinking water and suicidal behaviour in the population: a narrative review. Psychiatria Hungarica. 2016;31:221–230. [PubMed] [Google Scholar]
- Hu DD, Zhang RY, Zhang GQ, Wang HX, Ng TB. A laccase with antiproliferative activity against tumor cells from an edible mushroom, white common Agrocybe cylindracea. Phytomedicine. 2011;15:374–379. doi: 10.1016/j.phymed.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Ishii N, Terao T, Araki Y, Kohno K, Mizokami Y, Shiotsuki I, Hatano K, Makino M, Kodama K, Iwata N. Low risk of male suicide and lithium in drinking water. J Clin Psychiatr. 2015;76:319–326. doi: 10.4088/JCP.14m09218. [DOI] [PubMed] [Google Scholar]
- Klemfuss H, Schrauzer GN. Effects of nutritional lithium deficiency on behavior in rats. Biol Trace Elem Res. 1995;48:131–139. doi: 10.1007/BF02789187. [DOI] [PubMed] [Google Scholar]
- Koenig D, Klein JM, Bluemi V, Kapusta ND. The anti-suicidal effect of lithium in drinking water: a short review. Suicidology. 2015;6:1–12. [Google Scholar]
- Malhi GS, Outhred T. Therapeutic mechanisms of lithium in bipolar disorder: recent advances and current understanding. CNS Drugs. 2016;30:931–949. doi: 10.1007/s40263-016-0380-1. [DOI] [PubMed] [Google Scholar]
- Marshall TM. Lithium as a nutrient. J Am Phys Surg. 2015;20:104–109. [Google Scholar]
- Mleczek M, Siwulski M, Rzymski P, Budzyńska S, Gąsecka M, Kalac P, Niedzielski P. Cultivation of mushrooms for production of food biofortified with lithium. Eur Food Res Technol. 2017 [Google Scholar]
- Młyniec K, Davies CL, de Agüero Sánchez IG, Pytka K, Budziszewska B, Nowak G. Essential elements in depression and anxiety. Pharmacol Rep. 2014;66:534–544. doi: 10.1016/j.pharep.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Müller-Oerlinghausen B, Lewitzka U. Lithium reduces pathological aggression and suicidality: a mini-review. Neuropsychobiol. 2010;62:43–49. doi: 10.1159/000314309. [DOI] [PubMed] [Google Scholar]
- Nestel P, Bouis HE, Meenakshi JV, Pfeiffer W. Biofortification of staple food crops. J Nutr. 2006;136:1064–1067. doi: 10.1093/jn/136.4.1064. [DOI] [PubMed] [Google Scholar]
- Niedzielski P, Mleczek M, Siwulski M, Rzymski P, Gąsecka M, Kozak L. Supplementation of cultivated mushroom species with selenium: bioaccumulation and speciation study. Eur Food Res Technol. 2015;241:419–426. doi: 10.1007/s00217-015-2474-2. [DOI] [Google Scholar]
- Niedzielski P, Mleczek M, Budka A, Rzymski P, Siwulski M, Jasińska A, Gąsecka M, Budzyńska S. A screening study of elemental composition in 12 marketable mushroom species accessible in Poland. Eur Food Res Technol. 2017 [Google Scholar]
- Nunes MD, Cardoso WL, Luz JMR, Kasuya MCM. Lithium chloride affects mycelia growth of white rot fungi: fungal screening for Li-enrichment. Afr J Microbiol Res. 2014;8:2111–2123. doi: 10.5897/AJMR2014.6619. [DOI] [Google Scholar]
- Nunes MD, Cardoso WL, Luz JMR, Kasuya MCM. Effects of lithium compounds on the growth of white rot fungi. Afr J Microbiol Res. 2015;34:1954–1959. [Google Scholar]
- Ohgami H, Terao T, Shiotsuki I, Ishii N, Iwata N. Lithium levels in drinking water and risk of suicide. Br J Psychiatr. 2009;194:464–465. doi: 10.1192/bjp.bp.108.055798. [DOI] [PubMed] [Google Scholar]
- Rzymski P, Mleczek M, Niedzielski P, Siwulski M, Gąsecka M. Potential of cultivated Ganoderma lucidum mushrooms for the production of supplements enriched with essential elements. J Food Sci. 2016;81:587–592. doi: 10.1111/1750-3841.13212. [DOI] [PubMed] [Google Scholar]
- Rzymski P, Mleczek M, Niedzielski P, Siwulski M, Gąsecka M. Cultivation of Agaricus bisporus enriched with selenium, zinc and copper. J Sci Food Agric. 2017;97:923–928. doi: 10.1002/jsfa.7816. [DOI] [PubMed] [Google Scholar]
- Sánchez C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol. 2010;85:1321–1337. doi: 10.1007/s00253-009-2343-7. [DOI] [PubMed] [Google Scholar]
- Sartori HE. Lithium orotate in the treatment of alcoholism and related conditions. Alcohol. 1986;3:97–100. doi: 10.1016/0741-8329(86)90018-2. [DOI] [PubMed] [Google Scholar]
- Schäfer U. Evaluation of beneficial and adverse affects on plants and animals following lithium deficiency and supplementation, and on humans following lithium treatment of mood disorder. Trace Elem Electrol. 2012;29:91–112. doi: 10.5414/TEX01222. [DOI] [Google Scholar]
- Schrauzer GN. Lithium: occurrence, dietary intakes, nutritional essentiality. J Am Coll Nutr. 2002;21:14–21. doi: 10.1080/07315724.2002.10719188. [DOI] [PubMed] [Google Scholar]
- Schrauzer GN, de Vroey E. Effects of nutritional lithium supplementation on mood. A placebo-controlled study with former drug users. Biol Trace Elem Res. 1994;40:89–101. doi: 10.1007/BF02916824. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Alda M, Adli M, Akula N, Ardau R, Bui ET, Chillotti C, Cichon S, Czerski P, Del Zompo M, Detera-Wadleigh SD, Grof P, Gruber O, Hashimoto R, Hauser J, Hoban R, Iwata N, Kassem L, Kato T, Kittel-Schneider S, Kliwicki S, Kelsoe JR, Kusumi I, Laje G, Leckband SG, Manchia M, Macqueen G, Masui T, Ozaki N, Perlis RH, Pfennig A, Piccardi P, Richardson S, Rouleau G, Reif A, Rybakowski JK, Sasse J, Schumacher J, Severino G, Smoller JW, Squassina A, Turecki G, Young LT, Yoshikawa T, Bauer M, McMahon FJ. The international consortium on lithium genetics (ConLiGen): an initiative by the NIMH and IGSLI to study the genetic basis of response to lithium treatment. Neuropsychobiology. 2010;62:72–78. doi: 10.1159/000314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwulski M, Mleczek M, Rzymski P, Budka A, Jasińska A, Niedzielski P, Kalač P, Gąsecka M, Budzyńska S, Mikołajczak P. Screening the multi-element content of Pleurotus mushroom species using inductively coupled plasma optical emission spectrometer (ICP-OES) Food Anal Method. 2017;10:487–496. doi: 10.1007/s12161-016-0608-1. [DOI] [Google Scholar]
- Sokół S, Golak-Siwulska I, Sobieralski K, Siwulski M, Górka K. Biology, cultivation, and medicinal functions of the mushroom Hericium erinaceum. Acta Mycol. 2016;50:1069. [Google Scholar]
- Terao T. Is lithium potentially a trace element? World J Psychiatr. 2015;5:1–3. doi: 10.5498/wjp.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira PAF, Gontijo DC, Vieira BC, Fontes EAF, de Assunção Leite JPV. Antioxidant activities, total phenolics and metal contents in Pleuotus ostreatus mushrooms enriched with iron, zinc or lithium. LWT—Food. Sci Technol. 2013;54:421–425. [Google Scholar]
- Vita A, Peri L, Sacchetti E. Lithium in drinking water and suicide prevention: a review of the evidence. Int Clin Psychopharmacol. 2015;30:1–5. doi: 10.1097/YIC.0000000000000048. [DOI] [PubMed] [Google Scholar]
- Wang M, Gao Y, Xu D, Konishi T, Gao Q. Hericium erinaceus (Yamabushitake): a unique resource for developing functional foods and medicines. Food Function. 2014;5:3055–3064. doi: 10.1039/C4FO00511B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.