Abstract
Repeated heating of cooking oils is known to cause their degradation and generation of toxins. Dietary Advanced glycation end products (dAGEs) are formed when the foods are cooked in dry heat at very high temperatures. dAGEs are believed to contribute significantly to total pool of AGEs in body. In this study, cooking oil samples used for frying snacks were collected from 102 shops. AGEs were extracted using Aqueous-TCA–chloroform method. Fluorescent AGE levels were determined using a fluorescence spectrophotometer and compared with AGEs in corresponding fresh oil samples collected from same shops. Palm oil was most commonly (62.5%) used for cooking. Most of the samples were subjected to several rounds of heating (1–6). AGE specific fluorescence (ASF) in used oil (range = 8.5–745.11) samples was found to be significantly higher in 88/102 as compared to the corresponding fresh oil samples. Treatment with inhibitors like lime concentrate and vitamin C decreased ASF (10/14 and 10/11 samples respectively) of the used oils. The results suggest that cooking oil subjected to repeated heating can contribute to increase in fluorescent AGEs in diet. Simple practices like liberal use of common household substances like lime concentrate may help to reduce these in fried food.
Keywords: Advanced glycation end products, Cooking oil, Fluorescence, Frying, Diet
Introduction
Vegetable based cooking oils are commonly used to prepare different fried snacks all over the world. In many countries the cooking oil is subjected to repeated heating and the temperature involved in each bout of frying is as high as 170–220 °C. Such procedures are known to lead to considerable physico-chemical deterioration of cooking oils rendering them hazardous to health (Muhizi 2014). One of the chemical byproducts formed during cooking at very high temperatures are a group of complex heterogeneous compounds known as Advanced glycation end products (AGEs). The AGEs are glycotoxins formed by non-enzymatic reaction of reducing sugars with free amino acids of proteins, lipids or DNA. The AGEs in diet are believed to contribute significantly to total body pool of AGEs. The dietary AGEs (dAGEs) have been implicated in the etio-pathogenesis of several common inflammatory and lifestyle disorders like diabetes mellitus, atherosclerosis, cardiovascular diseases, rheumatoid arthritis and Alzheimer’s disease, aging, cataracts and cancer etc. (Uribarri et al. 2010; Rasheed et al. 2009, Chandra et al. 2009; Rachel et al. 2016). Various methods have been used for quantification of AGEs like ELISA, HPLC, LCMS etc. however none of these is universally accepted. The fluorescence property of AGEs was first described by Monnier. Since then, the later method has also been used to characterize and quantify the AGEs (Uribarri et al. 2010; Yu et al. 2015; Goldberg et al. 2004). In this study we intend to compare the AGE specific fluorescence (ASF) of repeatedly heated used cooking oil (RHUO) with that of unused cooking oils (UO) obtained from different street food vendors in the Chandigarh city. Also we have tried to assess the effect of adding substances like vinegar, vitamin C and Lime juice on ASF of RHUOs.
Materials and methods
One hundred and two samples of RHUO along with the corresponding samples of fresh (not subjected to heating) oil were obtained from street food vendors after taking their informed consents for participation in the above study. Code numbers were assigned to different oil samples and identity of shops was kept confidential. The samples were transported immediately to laboratory in the Department of Experimental Medicine and Biotechnology. All the work was carried out after approval from Institute Ethics Committee. After collection, the sample was processed on the same day for the extraction of AGEs. For this, first of all, the oil samples (3 ml) were mixed and incubated with distilled water. The AGEs being water soluble came in the aqueous layer. Subsequently, the proteins were precipitated out with Trichloroacetic acid (TCA, 13.5 M, 1 ml at 4 °C for 10 min). The sample was then centrifuged (Thermo electron corporation; Sorvall®RC6PLUS) at 14,000 rpm for 10 min at room temperature. The supernatant was carefully separated from the protein pellet and equal volume of chloroform was added (to remove the lipids) followed by centrifugation at 14,000 rpm for 10 min at room temperature (Maza et al. 2012). The aqueous layer containing the AGEs was analyzed using fluorescence spectrophotometer (Elegant; Varian, Cary Eclipse, EL 05073911) at excitation wavelength 355 nm and emission wavelength 440 nm. Three readings were taken and mean was calculated to give the final value. BSA-Glucose (50 mg/ml BSA + Glucose 0.5 M in PBS 0.2 M, Ph7.4) ripened at 37 °C in dark conditions for 60 days was used as standard in our experiments (Bhatwadekar and Golen 2005). The RHUO samples were then incubated with substances like Lime concentrate, Non-fruit Vinegar (purchased from the market and used as such) and Vitamin C (0.1 mg/ml in distilled water) (1 part: 1000 parts oil each) for 10 min. For studying the effects of repeated heating the fresh oil samples were purchased from the market.
Results
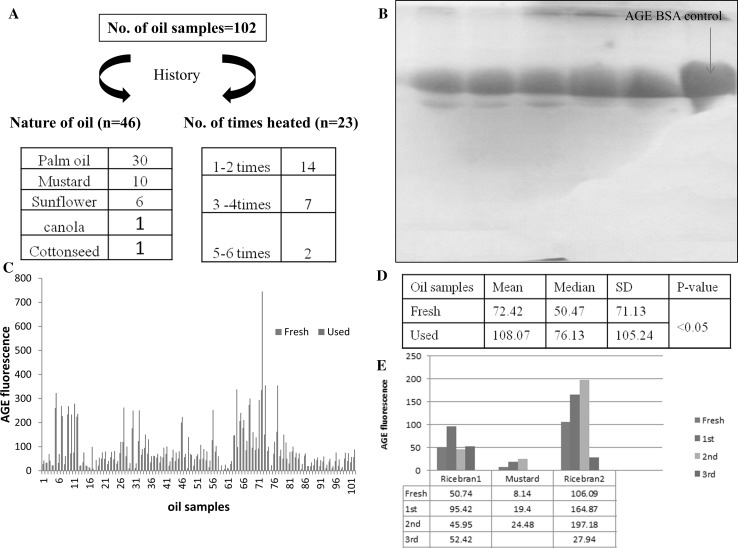
The oil samples collected had been used for deep frying of various snacks which are commonly consumed in North India like Samosa, Pakoda, fried chappatis, Spring rolls, momos, etc. Although many different types of vegetable oils were used, palm oil was most frequently used (62.5%) amongst the above. Most of the oil samples were subjected to repeated heating on an average 2–4 times/day (range 1–6 times) however the history regarding episodes of heating or the nature of the oil was available only in 23 and 48 cases respectively (Fig. 1a).
Fig. 1.
a Although 102 samples each of fresh and used oils were collected, history regarding origin of the oils and number of times they were heated was available in few samples only (48 and 23 cases respectively). b SDS-PAGE analysis of the used oil samples after PAS staining. Note the staining of glycoproteins only with PAS. c AGE specific fluorescence of used and corresponding fresh cooking oil. Note the higher fluorescence of used as compared to fresh cooking oils in majority of the samples. d Table below shows comparison of statistical parameters in above two categories. e Comparison of ASF of different oils after repeated heating for 2–3 times for 15 min each. An increase was noted in all samples after 1st heating, however, the effect was found to vary in different oils on subsequent heating
To confirm the presence of AGEs in our extracts we performed the SDS PAGE analysis followed by Per Iodic Acid Schiff (PAS staining) which specifically stains glycoproteins in 10 of our RHUO samples. The SDS PAGE showed the presence of PAS positive bands in 45–67 KDa region thus confirming the presence of AGEs (Fig. 1b).
Eighty-eight (86.27%) out of 102 samples analyzed showed a significantly higher ASF (paired T-test, p value <0.05) in RHUO as compared to the corresponding UO. The mean fluorescence level of UO and RHUO samples were found to be 72.39 ± 71.13 (range = 2.62–338) and 108.07 ± 105.24 (range = 8.5–745) respectively (BSA-Glucose standard = 525.46) (Fig. 1c, d). A history regarding number of heating times was available in 23 samples only. A statistical correlation of difference in ASF of RHUO & UO with number of times the oil has been heated was attempted in them; however, no correlation was found (Pearson’s correlation analysis, p value >0.05).
In order to check the effect of multiple heating times on the level of AGEs in oil under controlled conditions, we subjected the different fresh oil samples purchased from market to repeated heating (2–3 times for 15 min each). We observed an increase in ASF up to single heating period in one oil sample (Rice bran1, Fresh = 50.74, 1st heating = 95.42) whereas in other two oil samples (Rice bran2 and Mustard; Fresh = 106.09, 8.14; 1st heating = 164.87, 19.4; 2nd heating = 197.18, 24.48 respectively) increased fluorescence was observed up to two heating periods (Fig. 1e).
Nine RHUO samples were incubated with vinegar, 11 with vitamin C and 14 with lime concentrate. The ASF was found to be reduced in 10/11 (90.90%) and 10/14 (71.4%, p value ≤0.05) samples incubated with vitamin C and lime concentrate respectively. However, on incubation with vinegar the reduction was noticed only in 4/10 (40%) samples analyzed (Fig. 2a–c).
Fig. 2.
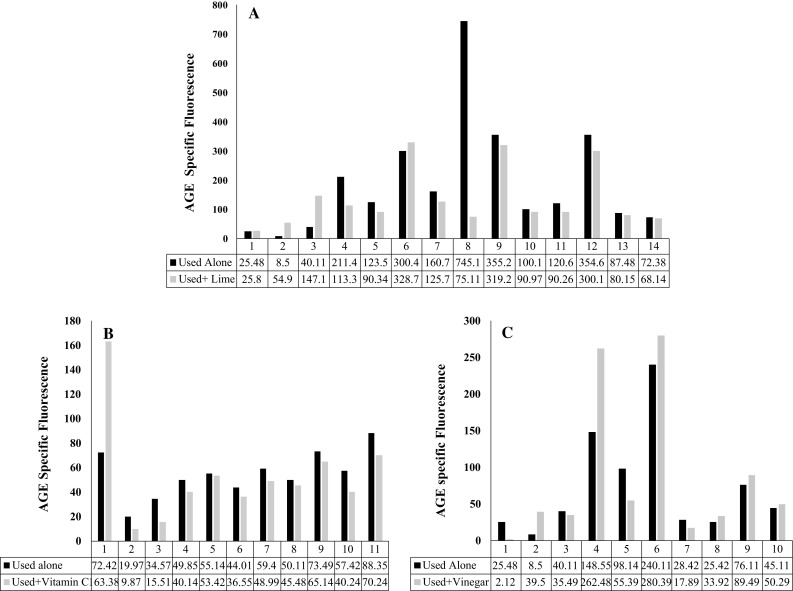
a–c AGE specific fluorescence before and after treatment with inhibitors Lime concentrate, vitamin C and vinegar respectively. Note the significant reduction in ASF upon treatment with first 2 in 10/14 and 10/11 samples respectively whereas with vinegar it is seen in only few cases
Discussion
Refined vegetable cooking oils are a commonly used medium for preparation of fried snacks. Deep frying of different foodstuffs involves use of high heating temperatures which not only lead to dehydration of foodstuffs but also result in generation of volatile and non-volatile degradation products from the cooking oils. The later may remain in the oil as toxins which may be absorbed in the body along with the food fried in them (Muhizi 2014). With increased reliance on consumption of outdoor foodstuffs and fried snacks there has been a spurt in the oil consumption throughout the world (Statistica 2015). In many instances the oil in which these are cooked is subjected to repeated heating throughout the day.
AGEs have received increasing attention in recent years due to their proposed association with several chronic diseases as well as the aging process (Luevano-Contreras and Chapman-Novakofski 2010). Although AGEs may be formed as a part of normal metabolism in the body, their excess may result in accumulation in the tissues. This in turn, may lead to tissue damage due to structural alterations or via receptor mediated activation of various intracellular signaling pathways. Whereas, the former effects are due to increased cross linking of proteins like collagen thus reducing their susceptibility to proteolysis, the later effects involve activation of pro-inflammatory pathways like NFκB with increased production of molecules like VCAM-1, IL-6, and TNF-α etc. The end result is increased vulnerability of the tissues to injury (Basta et al. 2005; Ott et al. 2014). dAGEs generated in cooking oils can therefore contribute significantly to total body AGE content and thus enhance the risk of lifestyle related diseases associated with them (Uribarri et al. 2005; Yamagashi et al. 2007). Many type of AGEs are found to fluoresce between 355 and 440 nm. Thus, in this work, for the first time we have used fluorescence method to assess the quality of RHUO with special emphasis on AGE content.
We found extreme variability in ASF of both RHUO and UO in our study. This may be due to heterogeneous nature of oils that we collected from the vendors. The ASF of UO was also high in many cases. This may be due to exposure of some fresh oils to high temperatures during their purification process (Uribarri et al. 2010). We found a significant increase in ASF of RHUO as compared to the UO which may indicate increased AGE formation in oil samples subjected to repeated heating. The impact of high cooking temperature and cooking practices on AGE content of foods has been highlighted in previous studies as well (O’Brien and Morrissey 1998). The processes like frying, grilling, roasting, barbecuing (Temperatures 180 to >200 °C) have been associated with increased AGE formation in the foodstuffs (Yamagishi et al. 2007; Dyer 1993). Although, previous studies have provided AGE content of many cooking oils, they have used specific AGE-ELISA to determine the concentration of AGEs like Carboxymethyl lysine (CML) or Methylglyoxal (Uribarri et al. 2010). The studies using fluorescent assay to measure the content of fluorescent AGEs in cooking oil have not been carried out. Though AGEs have been classified into the fluorescent and non-fluorescent types in previous studies it is not known which of these are more prevalent in foodstuffs. Also, the proportion of the three most studied AGEs CML, pyrraline and pentosidine in different diets is not known. The increased accumulation of pentosidine, a fluorescent AGE, however has been linked to diseases like diabetes (Dyer 1993).
A direct association between ASF and repeated heating however could not be deduced from our study neither in samples obtained from food vendors nor in samples heated for different number of times in laboratory conditions. In our opinion this may be due to sample heterogeneity, variable nature of items being fried and lack of information regarding episodes of heating/mixing with fresh oil in majority of the cases. The laboratory based experiments on oils purchased from grocery shops could not recapitulate the conditions of heating in the food shops as nothing was cooked in them at the time of repeated heating. Perhaps, a more detailed study with a proper history regarding heating time, temperature and duration after sub categorization of samples according to their origin may help to show the true link between them.
Many compounds are known to inhibit AGE formation. These include synthetic compounds like aminoguanidine, drugs like metformin, vitamins like thiamine, pyridoxamine, chelating agents like desferoxamine and d-penicillamine. AGE-breakers like alagebrium have been shown to break the cross- links formed by AGEs. Besides these, many naturally occurring substances like lime/lemon juice, vinegar, vitamin C etc. have also been found to inhibit the AGE formation. Marinating the foodstuffs with above before cooking has been shown to reduce their AGE content post-cooking (Bhatwadekar and Gholen 2005; Rahbar and Figarola 2003). AGE formation by Maillard reaction is favored by a pH ≥ 7. The above substances are believed to provide an acidic pH which inhibits the condensation reaction leading to reduced early AGE/Schiffs base formation. However, their effect on different stages of AGE formation or AGE cross links has not been dissected out. In the present study, we found that vitamin C and lime juice were especially effective in reducing the ASF in RHUO samples. This may suggest their effectiveness in reducing already formed AGEs also, however, the exact mechanism of the same remains to be deciphered. Further, we have used commercially available non-fruit vinegar prepared from acetic acid in the above study, which was found to be less effective when compared to vitamin C and lime juice. Although previous studies have highlighted the effectiveness of vinegar in reducing the AGE content of foods, the difference between the effects of different types of vinegars (synthetic verses natural) on AGE formation have not been well documented (Uribarri et al. 2010).
Conclusion
In this study, fluorescence method to assess the quality of RHUO with special emphasis on their AGE content was used. The ASF was significantly high in majority of RHUO samples as compared to UO. The later may indicate increased fluorescent AGE formation in RHUO samples. Further, in our work we found common household substances like lime concentrate to be effective in reducing the ASF of the RHUO. If the fluorescence values may be taken as paralleling the AGE concentrations then creating awareness amongst the people regarding the use of acidic substances like lime juice while cooking/frying may prove to be useful in reducing the dAGE content.
Acknowledgements
The authors acknowledge the financial support provided by Department of Science & Technology, Chandigarh for the above work.
References
- Basta G, Lazerrani G, Del TS, Ratto GM, Schimdt AM, De CR. At least two distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25:1401–1407. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- Bhatwadekar AD, Gholen VS. Rapid method for the preparation of an AGE-BSA standard calibrator using thermal glycation. J Clin Lab Anal. 2005;19:11–15. doi: 10.1002/jcla.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra KP, Shiwalkar A, Kotecha J, Thakkar P, Srivastava A, Chauthaiwale V, et al. Phase I clinical studies of the advanced glycation end-product (Age)-breaker Trc4186: safety, tolerability And pharmacokinetics in healthy subjects. Clin Drug Investig. 2009;29:559–575. doi: 10.2165/11315260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Dyer DG. Accumulation of maillard reaction products in skin collagen in diabetes and aging. J Clin Investig. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg T, Cai W, Peppa M, Dardane V, Baliga BS, Uribarri J, et al. Advanced gly-coxidation end products in commonly consumed foods. J Am Dietetic Assoc. 2004;104:1287–1291. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2:247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maza MP, Garrido F, Escalante N, Leiva L, Barrera G, Schnitzler S, et al. Fluorescent advanced glycation end-products (ages) detected by spectro-photofluorimetry, as a screening tool to detect diabetic microvascular complications. J Diabetes Mellitus. 2012;2:221–226. doi: 10.4236/jdm.2012.22035. [DOI] [Google Scholar]
- Muhizi T. NMR and FTIR analysis of overheated cooking oil. Orient J Chem. 2014;30:643–649. doi: 10.13005/ojc/300233. [DOI] [Google Scholar]
- O’Brien J, Morrissey PA. Nutritional and toxicological aspects of Maillard browning reaction in foods. Crit Rev Food Sci Nutr. 1998;28:211–248. doi: 10.1080/10408398909527499. [DOI] [PubMed] [Google Scholar]
- Ott C, Jacobs K, Haucke E, Santos AN, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachel EC, Aimee LD, Tan SM, Ryan L, Coughlan MT. Dietary advanced glycation end products and risk factors for chronic disease: a systematic review of randomised controlled trials. Nutrients. 2016;8:1–26. doi: 10.3390/nu8030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar S, Figarola JL. Novel inhibitors of advanced glycation end products. Arch Biochem Biophys. 2003;419:63–79. doi: 10.1016/j.abb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Rasheed Z, Kumar L, Sajid Prasad I, Ansari NA, Ahmad R. Advanced glycation end products (AGEs) damaged IgG, a target for circulating autoantibodies in patients with type 1 diabetes mellitus. Open Glycosci. 2009;2:1–8. doi: 10.2174/1875398100902010001. [DOI] [Google Scholar]
- Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann NY Acad Sci. 2005;1043:461–466. doi: 10.1196/annals.1333.052. [DOI] [PubMed] [Google Scholar]
- Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–916. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Ueda S, Okuda S. Food-derived advanced glycation end products (AGEs): a novel therapeutic target for various disorders. Curr Pharm Des. 2007;13:2832–2836. doi: 10.2174/138161207781757051. [DOI] [PubMed] [Google Scholar]
- Yu LL, Wang S, Sun B. Food safety chemistry: toxicant occurrence, analysis and mitigation. Boca Raton: CRC Press; 2015. [Google Scholar]