Abstract
Diabetic retinopathy (DR) is a major concern for blindness all over the world. Diabetic retinopathy is associated with thickening of basement membrane, retinal thinning, retinal detachment, and pericyte death. Advanced glycation end products (AGEs) mediate the progression of DR by stimulating the expression of RAGE and VEGF which subsequently damages the blood-retinal barrier. Employing a set of in vitro protein glycation systems, earlier we demonstrated antiglycating potential of ellagic acid (EA). In this study, we evaluated the efficacy of EA to prevent in vivo accumulation of AGE and to ameliorate retinal changes in diabetic rats. Streptozotocin-induced diabetic rats were fed either with 0.2 or 2% EA in the diet for 12 weeks. Effect of EA on retinal function was assessed with electroretinogram (ERG). At the end of the experiment, rats were scarified and retina was collected. Histology was carried out with H&E staining and immunohistochemistry. Formation of AGE product (CML) and activation of RAGE was analyzed by immunoblotting and immunohistochemistry. Expression of GFAP, VEGF, Bax and HIF-1α was assessed by qRT-PCR and immunoblotting. Dietary supplementation of EA to diabetic rats resulted in: (1) inhibition of accumulation of CML and activation of RAGE in retina, (2) attenuation of expression of GFAP, VEGF, and HIF-1α in retina, (3) attenuation of cell death by reducing proapoptic mediator Bax and (4) amelioration of retinal thickness and function. In conclusion, EA attenuated the retinal abnormalities including angiogenesis, hypoxia and cell death by inhibiting AGE-RAGE mediated cellular events.
Keywords: AGE, Diabetic retinopathy, Electroretinogram, Ellagic acid, Oscillatory potentials, Type 1 diabetes
Introduction
The global prevalence of diabetes is rapidly rising at an alarming rate. Uncontrolled or poorly controlled diabetes can lead to various long-term complications such as micro (blindness, nephropathy and neuropathy) and macrovascular (cardiovascular and stroke) problems in diabetic patients (Brownlee 2001). Long-term secondary complications are the main causes of morbidity and mortality in diabetic patients all over the world. One of the most common microvascular complications of diabetes is diabetic retinopathy (DR), and it remains a major cause of blindness worldwide (Marshall and Flyvbjerg 2006). The characteristic feature of DR is the appearance of vascular lesions of increasing severity, ending up in the growth of new vessels. While, the prevalence of DR varied (20–60%) in different studies, DR occurs in 70% of the population having diabetes for more than 15 years.
The long-term complications of diabetes are thought to be a result of the accumulation of tissue macromolecules that have been progressively modified by non-enzymatic glycation, which subsequently leads to the formation of advanced glycation end products (AGE) (Ahmed 2005; Singh et al. 2001). Formation of AGE plays a key role in the development of several pathophysiologies associated with aging and diabetes such as arthritis, atherosclerosis, chronic renal insufficiency, Alzheimer’s disease, nephropathy, neuropathy, cataract and retinopathy. Microvascular lesions, such as microaneurysms, blood barrier dysfunction, and capillary dropout, are key features of DR (Curtis et al. 2009; Frank 2004). However, it should be appreciated that the sole purpose of the retinal circulation is to support the metabolic demands of the inner retinal neurons and glia and that these cells are also damaged appreciably during diabetes. Such neuronal and glial dysfunction occurs in unison with blood flow abnormalities and often before the appearance of overt microvascular damage (Antonetti et al. 2006). The early stages of retinopathy result from retinal ischemia as a result of nonperfusion of the retina or a decrease in oxygen tension. In the late stages, the ischemia-induced pathologic growth of new blood vessels can cause catastrophic loss of vision.
Hyperglycemia induced accumulation of AGE leads to intramural pericyte death and thickening of the basement membrane lead to incompetence of the vascular walls. These damages change the formation of the blood-retinal barrier and make the retinal blood vessels become more permeable. AGE also stimulates the receptor for AGE (RAGE) expression which activates vascular endothelial growth factor (VEGF) production that leads to new blood vessel formation. In addition, AGE induces apoptotic cell death of pericytes through interaction with RAGE and induction of VEGF (Yamagishi et al. 2002). Studies reported, using retinal vascular cells, that AGEs can trigger pathogenic events including decreased endothelial nitric oxide synthase (eNOS) activities, loss of pericytes (Chibber et al. 1997), nerve growth factor (NGF) and increased expression of glial acidic fibrallary protein (GFAP) and VEGF (Lu et al. 1998). These changes act through specific RAGE. Further, injection of AGE into non-diabetic animals induced thickening of the basement membrane of retinal vessels (Clements et al. 1998), increased leukocyte adhesion (Moore et al. 2003), and increased breakdown of the blood retinal barrier (Stitt et al. 2000).
This raises the possibility that inhibition of AGE formation may prevent the progression of DR. However, designing a drug having anti-AGE activity is a challenge due to the complexity of reactions involved in the formation of AGE. A number of compounds such as aminoguanidine, pyridoxamine, carnosine, ALT-711 and phenyl thiazolium bromide have been investigated in several in vitro and in vivo studies (Stitt et al. 2002; Rahbar and Figarola 2003). Aminoguanidine has been evaluated in a multicenter clinical trial where it failed to achieve statistically significant lowering of serum creatinine, and urinary albumin but showed a positive trend toward slowing the progression of overt nephropathy and retinopathy (Bolton et al. 2004). Another well-known AGE inhibitor pyridoxamine ameliorated DR in experimental diabetic rats and it also passed phase III clinical trials (Stitt et al. 2002). However, excepting pyridoxamine, none of the other compounds passed through the clinical trials.
Hence, identification and testing of new antiglycating agents with higher levels of efficacy and safety in humans is very much needed. In the course of screening and identifying new antiglycating agents, we have evaluated a number of traditional and very common dietary sources and found that some spice principles, fruits and vegetables have the potential to inhibit AGE formation under in vitro conditions and in animal models (Saraswat et al. 2009, 2010). Flavonoids are abundantly found in many of these dietary sources, and ellagic acid (EA) is one of the commonly found dietary polyphenols. Apart from the greatest sources, such as berries and pomegranate, EA is also present in those dietary sources that were reported to have antiglycating potential such as apples, grapes, orange, guava and cumin (Landete 2011). Employing a battery of in vitro protein glycation systems and lens organ culture model, we recently reported on the antiglycating activity of EA (Muthenna et al. 2012). Further, we showed that feeding of EA to diabetic rats ameliorated proteinurea through inhibition of AGE (Raghu et al. 2016). Therefore, present study was designed to see potential of EA in inhibiting the AGE-RAGE mediated cellular events thereby ameliorating retinal abnormalities including angiogenesis, hypoxia, and cell death in streptozotocin (STZ) induced diabetic rat model.
Materials and methods
Materials
Streptozotocin, EDTA, BSA, Tri-reagent, Triton X-100, acrylamide, bis-acrylamide, PMSF, aprotinin, HRP-conjugated anti-rabbit (A6154), and anti-mouse (A9044) secondary antibodies were purchased from Sigma Chemicals (St. Louis, MO). Immobilon-NC membrane was from Millipore (Bedford, MA). All other chemicals and solvents were of analytical grade and were obtained from local companies.
Experimental design and dietary regimen
Three-month-old male Wistar-NIN rats with average body weight of 230 ± 23 g (obtained from National Center for Laboratory Animal Sciences National Institute of Nutrition) were maintained at a temperature of 22 ± 2 °C, 50% humidity, and 12-hour light/dark cycle as described previously (Akileshwari et al. 2014) and the eyeball/retina of the same animals were used. All the animals were fed AIN-93 diet ad libitum. The control rats received 0.1 M citrate buffer, pH 4.5 as a vehicle whereas the experimental rats received a single intraperitoneal injection of STZ (32 mg/kg) in citrate buffer. After 72 h, fasting blood glucose levels were monitored. Animals having blood glucose levels <150 mg/dl were excluded from the experiment and the rest were distributed into three groups (diabetic). A group of diabetic animals received only AIN-93 diet (D) whereas rest of the diabetic animals received the AIN-93 diet containing either 0.2 or 2% ellagic acid (EA1 or EA2). A pilot study was conducted with 0.1, 0.2, 1 and 2% EA to fix optimal dose of EA and accordingly 0.2 and 2% EA was used in the diet. Each group consisted of eight animals.
Animal care
Institutional and national guidelines for the care and use of animals were followed and all experimental procedures involving animals were approved by the IAEC (Institutional Animal Ethical Committee) of the National Institute of Nutrition. Animals were housed in individual cages and had free access to water. Food intake (daily) and bodyweights (weekly) were monitored. We adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Blood, retina collection and processing
Blood was collected once a week from the retro-orbital plexus for glucose estimation, HbA1c formation and RBC-IgG binding analysis. At the end of 12 weeks, animals were sacrificed by CO2 asphyxiation. The retinas were dissected from eye, snap frozen and stored at −80 °C for subsequent analysis. Four eye balls were fixed in 4% paraformaldehyde for subsequent histological studies.
Biochemical estimations
Serum glucose was measured by the glucose oxidase–peroxidase (GOD-POD) method using a commercially available kit (BioSystems, S.A.Costa Brava 30, Barcelona, Spain). The HbA1cwas estimated in whole blood by using commercially available kit (BioSystems, S.A.Costa Brava 30, Barcelona, Spain).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from retina (three rats from each group) using Tri-reagent according to the manufacturer instructions. Isolated RNA was further purified by RNeasy Mini Kit and quantified by measuring the absorbance at 260 and 280 nmusing Nano drop. Two to four µg of total RNA was reverse transcribed using High Capacity cDNA Reverse Transcription kit as described previously (Reddy et al. 2013). Real-time PCR (ABI-7500) was performed in triplicates with 25 ng cDNA templates using SYBR green master mix with the following gene specific primers: β-actin forward 5′-gagaagagctatgagctgcc-3′, β-actin reverse 5′-ctcaggaggagcaatgatct-3′, GFAP forward 5′-tttctccaacctccagatcc-3′, GFAP reverse 5′-agctttaggccctcacactg-3′, VEGF forward 5′- tcaccaaagccagcacatag- 3′, VEGF reverse 5′-gggagtctgtgtttttgc ag-3′, HIF-1α forward 5′-gaa accgcctatgacgtgct- 3′, HIF-1α reverse 5′-atcgaggcttgtcgactga-3′. Normalization and validation of data were carried using β-actin as an internal control and data were compared between control and diabetic samples according to comparative threshold cycle (2−∆∆ct) method (Reddy et al. 2013). The reaction conditions were as follow: 40 cycles of initial denaturation temperature at 95 °C for 30 s followed by annealing at 52 °C for 40 s and extension at 72 °C for 1 min and product specificity was analyzed by melt curve analysis.
Whole tissue lysate preparation
Retina (three animals from each group) was homogenized in a buffer containing 20 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM DTT (TNE buffer; pH 7.5) and protease inhibitors. Homogenization of retina was performed on ice using a glass homogenizer and the homogenate was centrifuged at 12,000 g at 4o C for 20 min (Reddy et al. 2013). The protein concentrations were measured by BCA method.
SDS–PAGE and immunoblotting
Electrophoresis was performed with retinal lysate (80 µg of protein) under reducing conditions on 10% polyacrylamide gels using Tris–glycine as running buffer. After electrophoresis, the proteins were transferred onto nitrocellulose membrane. Immunoblotting was performed with, anti-GFAP (1:25,000), anti-VEGF (1:1000), anti-HIF-1α (1:1000), anti-NGF (1:500) and anti-CML (N-ε-carboxymethyl lysine; 1:100) monoclonal antibodies and after blocking with non-fat milk powder and then incubated with peroxidase tagged anti-rabbit IgG antibody or anti-mouse secondary antibody (1:3500) conjugated with HRP. Beta-actin was used as a loading control. The immunoblots were developed using enhanced chemiluminiscence (ECL) detection kit and digital images were recorded by Image analyzer (G:BoxiChemi XR, Syngene, UK).Quantification of band intensity was performed with Image J software.
Immunohistochemistry
The dissected eyeball was immediately placed in 4% paraformaldehyde in phosphate buffer (pH-7.2), fixed overnight, embedded in paraffin blocks, and cut into 4 µm sections. Sections were deparafinized on a hot plate for 45 min at 60 °C, followed by incubating in xylene for 5 min further dehydration in decreasing grades of ethanol (100, 95, and 70%). Antigen retrieval was performed using microwave, sections were boiled in 0.01 M Na-citrate pH 6.0 for 10 min and blocked with blocking solution (3% goat serum or 3% horse serum in 0.3% TritonX in PBS). Slides were washed 3 times with PBS and incubated in PBS with goat serum with anti-GFAP (rabbit1:500), anti-HIF1α (1:100), anti-VEGF, anti-Bax, (mouse, 1:50) antibodies overnight at 4 °C. Slides were washed 3times with PBS and the binding of primary antibodies was visualized by Alexafluor-488 conjugated anti-rabbit (1:2000) and Alexafluor-555 conjugated anti-mouse IgG antibody (1:1000) for 1 h. Sections were mounted in medium containing DAPI and visualized using a Leica laser microscope (LMD6000, Leica microsystems, Germany).
Electroretinogram
Electroretinograms (ERG) were recorded on anesthetized animals to determine retinal function. Twelve-hours prior to recording ERG, animals were weighed and allowed to dark adapt overnight (food and water are available ad libitum). All subsequent procedures were performed in the dark with a dark-room safelight and night vision goggles as reported previously (Machida et al. 2000). An ERG-JET electrode was placed on the cornea sclera 1 mm from the temporal limbus of each eye. The ground electrode was on the tail. Responses were amplified at 10,000 gain at 0.3–500 Hz, filtered to remove 60 Hz noise, and digitized at a 10-kHz rate. Scotopic ERG responses were computer-averaged with stimulus intervals of 1–180 s depending on intensity, and photopic responses were averaged with stimulus intervals of 1 s. For photopic ERGs, animals were light-adapted for 10 min before photopic recordings.
Statistical analysis
Data were expressed as mean ± SEM unless otherwise stated. One-way analysis of variance (ANOVA) with post hoc Tukey’s method was used in this study. Differences were considered significant if the p value was less than 0.05.
Results
Food intake and body weights
As reported by us earlier (Saraswat et al. 2010; Reddy et al. 2013; Suryanarayana et al. 2007), there was an increase in the food intake in all the diabetic animals compared with the control animals. Despite the increased food intake, the body weight of diabetic animals decreased significantly when compared to control animals. However feeding of EA to diabetic rats did not influence food intake and the body weights to a significant extent (Table 1).
Table 1.
Average food intake, body weight, blood glucose and glycosylated hemoglobin data experimental rats
| Parameters | Control (C) | Diabetic (D) | D + EA1 | D + EA2 |
|---|---|---|---|---|
| Food intake (g/day) | 16.5 ± 1.92 | 31.4 ± 4.51** | 30.6 ± 2.57 | 29.3 ± 4.24 |
| Body weight (g) | 309 ± 14.0 | 196 ± 23.8** | 199 ± 22.6** | 206 ± 28.6** |
| Blood glucose (mg/dL) | 90.5 ± 12.51 | 372 ± 54.3** | 312 ± 53.7** | 234 ± 63.2** |
| HbA1c (%) | 6.03 ± 0.98 | 11.6 ± 2.03** | 9.2 ± 1.70** | 7.58 ± 0.52 |
Data are mean ± SE (n = 8). ** p < 0.001 represents level of significance of difference compared with non-diabetic rats
Blood glucose and HbA1c levels
The mean blood glucose levels of diabetic rats were significantly higher when compared with control rats (Table 1). Further, diabetic rats showed significantly higher levels of HbA1c compared with the controls (Table 1). Though, blood glucose levels were decreased to a marginal level due to 2% EA feeding to diabetic rats, glucose levels were unaffected by 0.2% EA.
Decreased CML and RAGE levels upon ellagic acid feeding
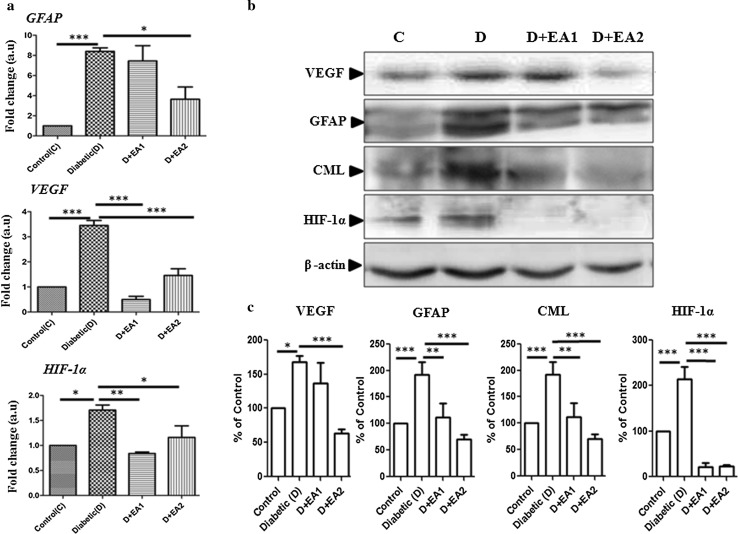
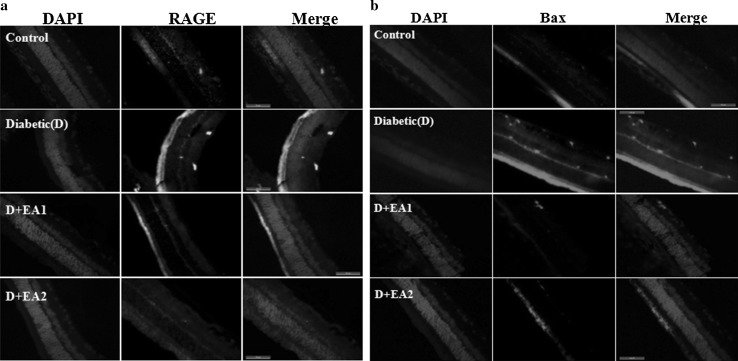
To assess the accumulation of AGE in retinal tissues of diabetic rats, immunoblotting was performed using an antibody for the predominant AGE, CML. As expected, there was an increased level of CML in retinal lysates of diabetic rats compared to control rats (Fig. 1b). Further, increased immunostaining of RAGE in diabetic retinal section compared to control rats (Fig. 2a) substantiates the increased accumulation of AGE in retina as shown by immunoblotting. Interestingly, in support of our previous in vitro data (Muthenna et al. 2012), feeding of EA to diabetic rats inhibited CML formation in a dose dependent formation (Fig. 1b).The effect was more pronounced with 2% EA dose. In accord with CML data, immunostaining of RAGE also decreased in retina of EA-fed diabetic rats (Fig. 2a).
Fig. 1.
Expression of GFAP, VEGF, and HIF-1α Panel a qRT-PCR analysis of GFAP, VEGF and HIF-1α in retina of control, diabetic rats, and diabetic rats fed with EA (D + EA1 and D + EA2). Relative expression pattern was analyzed by comparative threshold cycle (2−∆∆ct) method. Expression values were represented as fold change over control on an arbitrary scale after normalization with actin. Data represent mean ± SEM of three independent experiments. (Panel b) Representative immunoblots of VEGF, GFAP, CML, HIF-1α in retina of control (C), diabetic rats (D), and diabetic rats treated with EA (D + EA1 & D + EA2); β-actin used as loading control. Quantification of immunoblots (panel c); expression was normalized for β-actin and are represented as percent of control. Data represent mean ± SE of three independent experiments. Level of significance: *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 2.
Immunostaining of RAGE and Bax. Panel a Immunostaining of RAGE in retina of control, diabetic rats, and diabetic rats fed with EA (D + EA1 and D + EA2). Immunostaining of RAGE is shown in green and nuclear staining with DAPI in blue. Panel b Immunostaining of Bax in retina of control, diabetic rats, and diabetic rats fed with EA (D + EA1 and D + EA2). Immunostaining of Bax is shown in red and nuclear staining with DAPI in blue. Scale bar equals 50 μm. Original magnification at 630×
Pericyte death and retinal functional abnormalities
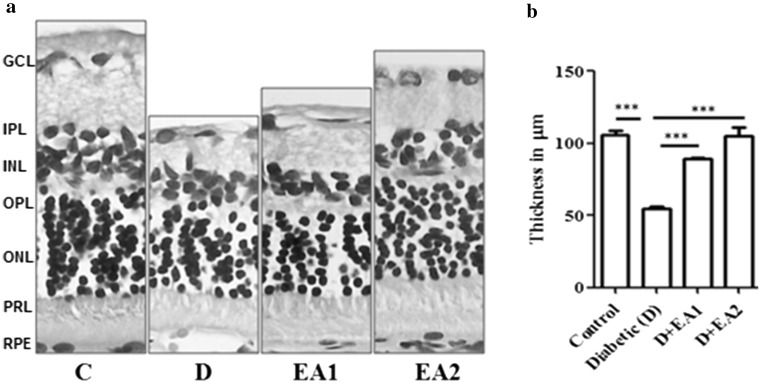
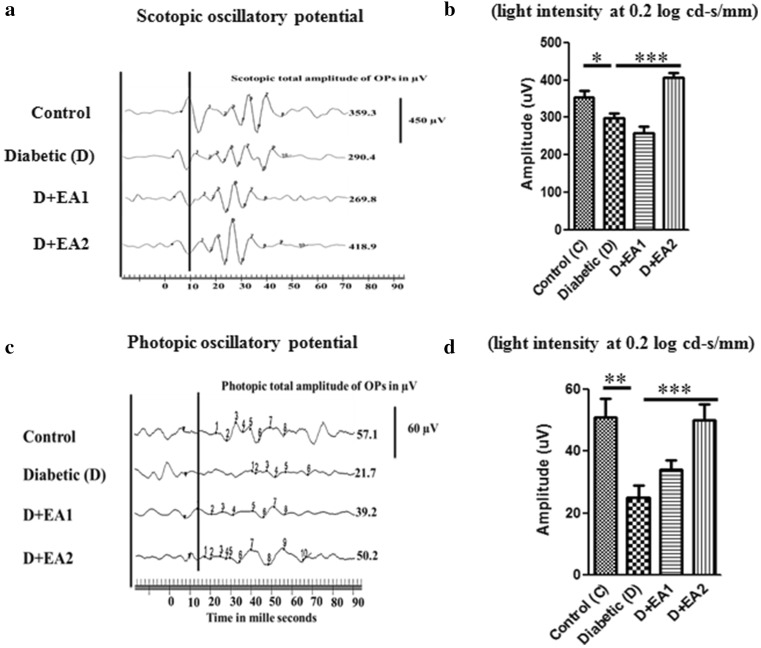
Alteration in the protein profile due to CML aggregates lead to the insolubilization of protein that have been considered to be the ultimate change that results in retinal cell death and retinal basement membrane thickening. Thus, we determined the expression of proapoptotic protein Bax by immunohistochemistry. While, the immunostaining of Bax was increased in retina of diabetic rats compared to control rats, feeding of EA to diabetic rats showed lesser immunostaining in inner layers of retina (Fig. 2b). These results suggest that AGE-mediated cell death in diabetic retina could be attenuated by EA. Further, retinal thickness was remarkably decreased in diabetic rats, compared with control rats and feeding of EA to diabetic rats improved the thickness of retina (Fig. 3a, b). Improvement of functional abnormalities in diabetic retina upon EA feeding as determined of ERG recording support these findings. There was significant decrease in both scotopic and photopic oscillatory potentials in diabetic rats compared to control rats and feeding of EA improved the OP (Fig. 4a–d).
Fig. 3.
Histology of retina. Panel a Representative retinal sections of control (C), diabetic (D) and diabetic rats fed with EA (D + EA1& D + EA2) were stained with H&E. GCL,-ganglion cell layer; IPL-inner plexiform layer; INL-inner nuclear layer; OPL-outer plexiform layer; ONL-outer nuclear layer; RPE-retinal pigment epithelium layer. Original magnifications 630× and Scale bars, 50 μm. Panel b Quantification of retinal thickness and values are mean ± SEM (n = 6). Level of significance: ***p < 0.001
Fig. 4.
Electroretinogram data from control (C), diabetic rats (D) and diabetic rats fed with EA (D + EA1 & D + EA2). Panel a Representative wave forms of scotopic oscillatory potentials (SOPs). Panel b Normalized variance of sum of peaks of SOPs. Panel c Representative wave forms of photopic oscillatory potentials (POPs). Panel d Normalized variance of sum of peaks of POPs. Data in panels B and D are mean ± SEM (n = 8); Level of significance: *p < 0.05, **p < 0.01, ***p < 0.001
Expression of HIF-1α, VEGF and GFAP
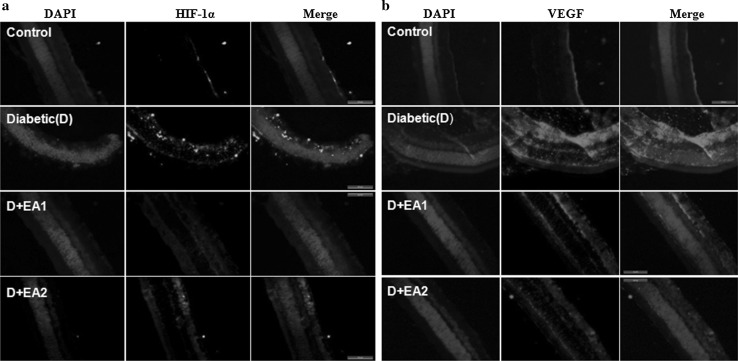
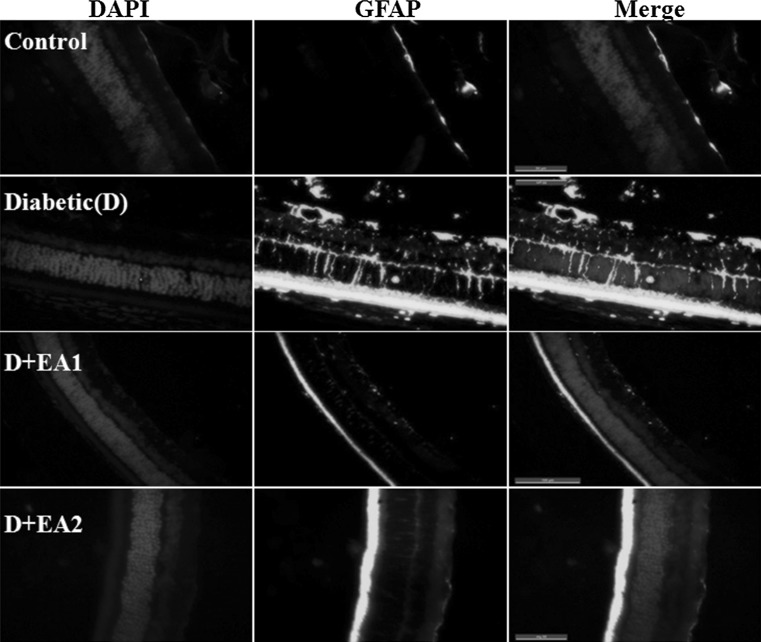
Neovascularization stimulated by hyperglycemia-mediated induction of vascular endothelial growth factor (VEGF) has been implicated inthe pathogenesis of diabetic retinopathy. Activation of AGE-RAGE signaling is known to induce VEGF expression in diabetic retina (Lu et al. 1998). The expression of VEGF under hyperglycemia is regulated by hypoxia inducible factor 1α (HIF-1α) due to local hypoxia (Forsythe et al. 1996). Hence, we determined the effect of EA feeding on the expression of VEGF, HIF-1α, and GFAP in diabetic retina. Results of qRT-PCR and immunoblots analysis indicate that there was a significant increase in VEGF and HIF-1α in diabetic rats compared to control animals and interestingly this was attenuated in EA treated diabetic rats (Fig. 1a–c). Further, immunohistochemistry analysis supports the qRT-PCR and immunoblot data. In the present study, we observed that in diabetic retina nuclear HIF-1α staining was present in ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL) of retina and whereas it was almost negligible in the EA treated diabetic rats (Fig. 5a). Similarly, immunostaining indicate that while in diabetic rat retina VEGF expression increased over control rats and EA fed groups showed a lowered VEGF expression (Fig. 5b). Results also suggest that gliosis marker, GFAP was up-regulated in diabetic rat and treatment with EA inhibited the GFAP expression as shown by qRT-PCR, immunoblotting and Immunostaining (Figs. 1, 6).
Fig. 5.
Immunostaining of HIF-1α and VEGF. Panel a Immunostaining of HIF-1α in retina of control, diabetic rats, and diabetic rats fed with EA (D + EA1 and D + EA2). Immunostaining of HIF1α is shown in green and nuclear staining with DAPI in blue. Panel b Immunostaining of VEGF in retina of control, diabetic rats, and diabetic rats fed with EA (D + EA1 and D + EA2). Immunostaining of VEGF is shown in red and nuclear staining with DAPI in blue. Scale bar equals 50 μm. Original magnification at 630×
Fig. 6.
Immunostaining of GFAP in retina of control, diabetic rats, and diabetic rats fed with EA (D + EA1 and D + EA2). Immunostaining of GFAP is shown in green and nuclear staining with DAPI in blue. Scale bar equals 50 μm. Original magnification at 630×
Discussion
Chronic complications of diabetes are a major health problem, and it has become a priority to characterize further their pathophysiological mechanisms to develop novel, rational therapeutic strategies. Even though prolonged exposure of the tissues to hyperglycemia seems to be the primary causative factor, some other factors may play a role as far as intensive blood glucose control reduces chronic complications but does not prevent them altogether (The Diabetes Control and Complications Trial Research Group 1993; UK Prospective Diabetes Study (UKPDS) Group 1998). Although there have been major advances in the control of hyperglycemia (diabetes) through dietary changes, hypoglycemic agents, insulin and islet transplantation, the management of long-term complications of diabetes, such as blindness remain serious problems to be dealt with. Since a considerable amount of evidence has shown the contribution of AGEs to the development of diabetic complications, inhibition of AGEs is considered to be one promising approach for the prevention and treatment of diabetic complications. The currently available knowledge of the mechanisms involved in AGE formation has led to attempts to inhibit the formation of AGEs.
A variety of compounds have been investigated and some have shown promising results (Stitt et al. 2002; Rahbar and Figarola 2003; Bolton et al. 2004; Vasan et al. 1996). Nonetheless, except for one or two, there are not many antiglycating agents available for clinical use. Previously, we have identified some common dietary agents that have the potential to inhibit AGE formation and showed their potential to prevent or treat diabetic complications (Saraswat et al. 2009, 2010; Kumar et al. 2009). Ellagic acid (EA) is one of the commonly found dietary polyphenols in many of these sources and we recently reported the antiglycating activity of EA (Muthenna et al. 2012). Further, we have also shown that EA delays cataract and attenuates proteinurea in diabetic rats (Raghu et al. 2016; Akileshwari et al. 2014).
Therefore, in the present study, we investigated the potential of EA against DR using diabetic rat model and provide the evidence for its action. The results indicate that there was an increase in CML levels in STZ-induced diabetic rat retina as compared to control rat retina. Interestingly, feeding of EA to diabetic rats results in considerable inhibition of CML formation. The expression of RAGE was also observed to have reduced upon EA feeding further supports anti-AGE activity of EA. While EA is known to have various health benefits (Landete 2011), the results described in the present study provide information regarding potential of EA with respect to DR. A number of AGEs have been identified in tissue proteins and among all AGEs, CML is most abundant in diabetic patients, and hence EA could be a potential molecule that needs to be explored further for its prospects for diabetic complications. It is noteworthy to mention that a recent study reported that EA ameliorates renal function in experimental diabetic nephropathy animal model (Ahad et al. 2014).
Accumulation of AGE in chronic diabetes is known to cause pericyte death and thickening of the basement membrane lead to incompetence of the vascular walls. AGE also stimulates expression of RAGE which leads to activation of VEGF production which in turn triggers angiogenesis in retina. In addition, AGE induces apoptotic cell death of pericytes through interaction with RAGE and induction of VEGF.It has become increasingly evident that hypoxia plays an important role in all diabetes complications as it has been postulated that hyperglycemia induces a pseudohypoxia state. Adaptive responses of cells to hypoxia are mediated by the hypoxia-inducible factor-1α (HIF-1α).A small further increase of hypoxia caused by the diabetic milieu and/or a compromised vasculature is likely to have pathological consequences through upregulation of the expression of VEGF and other factors (Hughes et al. 2010).VEGF is a potent mitogen for endothelial cells that triggers proliferation, migration and tube formation leading to growth of new blood vessels that, however, in the diabetic retina are fragile and may break and it act mainly paracrine signaling. Moreover, AGE induces VEGF expression in cell cultures and animal models, thus being considered to be involved in the pathogenesis of diabetic retinopathy (Lu et al. 1998). Glial fibrillary acidic protein (GFAP) is an intermediate filament protein present in retinal glial cells (ganglion cells) and retinal astrocytes (Barber et al. 2000). Retinal glia (astrocytes, Muller cells, and microglia) are sensitive indicators of retinal tissue injury and have been shown to be greatly affected STZ induced diabetes because of formation of AGE (Barber et al. 2000). Therefore, we have assessed the possibility of EA modulating the expression of HIF-1α, VEGF and GFAP in diabetic retina due to its AGE inhibition. Interestingly, the findings of the present study show that treatment with EA indeed modulated the expression of HIF-1α, VEGF and GFAP in diabetic retina and support the protection offered by EA.
Ganglion cells in diabetic retinas express several proapoptotic molecules including Bax, suggesting that these cells are the most vulnerable population (Abu-El-Asrar et al. 2004). Bax antisense oligonucleotides reduced axotomy-induced retinal ganglion cell death by reduction of Bax protein expression, indicating that Bax induction is a prerequisite for the execution of retinal ganglion cell apoptosis (Isenmann et al. 1999). This further proves that the retinal ganglion cell apoptosis due to enhanced Bax expression was inhibited by EA feeding in these STZ rats. We observed that both scotopic as well as phototopic OP were reduced in the diabetic rats. Though the EA1 group did not show much recovery in the OP, significant improvement was seen in the EA2 group suggesting the protective nature of EA in the retinal function and thereby its physiology. Further increased expression of proapoptotic protein Bax was significantly decreased in retina of diabetic rats by feeding of EA. These results suggest the correlation between the cellular interactions (Bax positive cells) and physiological response in the form of oscillatory potentials in ERG. We have shown that EA has antiglycating action and aldose reductase inhibition in our in vitro studies (Muthenna et al. 2012; Akileshwari et al. 2014). These two pathways contribute majorly to the development of diabetic pathologies including DR. We observed that EA delayed progression and maturation of cataract, despite not altering the hyperglycemia in the rats. Further, we have also shown that EA inhibits vascular smooth muscle cell proliferation and prevents atheroma formation in STZ-induced diabetic rats (Rani et al. 2013). These results thus suggest that EA may act downstream to glucose-mediated changes in preventing diabetic complications.
In conclusion, in this study we have demonstrated that EA improved retinal thickness and retinal function under hyperglycemic conditions. Further, dietary EA attenuated the angiogenesis and hypoxia by decreasing the expression of GFAP, VEGF, and HIF-1α in diabetic retina. In addition, EA decreased the cell death by reducing the Bax levels. All these effects are attributable to inhibiting the AGE-RAGE mediated cellular events as EA is potent antiglycating agent.
Acknowledgements
GBR received grants from the Department of Biotechnology, India under 7th FP of Indo-EU collaborative grant on functional foods (Grant agreement #245030). GR was supported by a postdoctoral fellowship from the grant.
Abbreviations
- AGE
Advanced glycation end product
- CML
Carboxy methyl lysine
- DR
Diabetic retinopathy
- EA
Ellagic acid
- ERG
Electroretinogram
- STZ
Streptozotocin
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interests.
References
- Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45(8):2760–2766. doi: 10.1167/iovs.03-1392. [DOI] [PubMed] [Google Scholar]
- Ahad A, Ganai AA, Mujeeb M, Siddiqui WA. Ellagic acid, an NF-kappaB inhibitor, ameliorates renal function in experimental diabetic nephropathy. Chem Biol Interact. 2014;219C:64–75. doi: 10.1016/j.cbi.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Ahmed N. Advanced glycation endproducts–role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Akileshwari C, Raghu G, Muthenna P, Mueller NH, Suryanarayana P, Petrash JM, et al. Bioflavonoid ellagic acid inhibits aldose reductase: implications for prevention of diabetic complications. J Funct Foods. 2014;6:374–383. doi: 10.1016/j.jff.2013.11.004. [DOI] [Google Scholar]
- Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn state retina research group. Invest Ophthalmol Vis Sci. 2000;41(11):3561–3568. [PubMed] [Google Scholar]
- Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24(1):32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Chibber R, Molinatti PA, Rosatto N, Lambourne B, Kohner EM. Toxic action of advanced glycation end products on cultured retinal capillary pericytes and endothelial cells: relevance to diabetic retinopathy. Diabetologia. 1997;40(2):156–164. doi: 10.1007/s001250050657. [DOI] [PubMed] [Google Scholar]
- Clements RS, Jr, Robison WG, Jr, Cohen MP. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J Diabetes Complicat. 1998;12(1):28–33. doi: 10.1016/S1056-8727(97)00051-2. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond) 2009;23(7):1496–1508. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/MCB.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- Hughes JM, Groot AJ, van der Groep P, Sersansie R, Vooijs M, van Diest PJ, et al. Active HIF-1 in the normal human retina. J Histochem Cytochem. 2010;58(3):247–254. doi: 10.1369/jhc.2009.953786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenmann S, Engel S, Gillardon F, Bahr M. Bax antisense oligonucleotides reduce axotomy-induced retinal ganglion cell death in vivo by reduction of Bax protein expression. Cell Death Differ. 1999;6(7):673–682. doi: 10.1038/sj.cdd.4400538. [DOI] [PubMed] [Google Scholar]
- Kumar PA, Reddy PY, Srinivas PN, Reddy GB. Delay of diabetic cataract in rats by the antiglycating potential of cumin through modulation of alpha-crystallin chaperone activity. J Nutr Biochem. 2009;20(7):553–562. doi: 10.1016/j.jnutbio.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Landete JM. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int. 2011;44(5):1150–1160. doi: 10.1016/j.foodres.2011.04.027. [DOI] [Google Scholar]
- Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, et al. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest. 1998;101(6):1219–1224. doi: 10.1172/JCI1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida S, Kondo M, Jamison JA, Khan NW, Kononen LT, Sugawara T, et al. P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci. 2000;41(10):3200–3209. [PubMed] [Google Scholar]
- Marshall SM, Flyvbjerg A. Prevention and early detection of vascular complications of diabetes. BMJ. 2006;333(7566):475–480. doi: 10.1136/bmj.38922.650521.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TC, Moore JE, Kaji Y, Frizzell N, Usui T, Poulaki V, et al. The role of advanced glycation end products in retinal microvascular leukostasis. Invest Ophthalmol Vis Sci. 2003;44(10):4457–4464. doi: 10.1167/iovs.02-1063. [DOI] [PubMed] [Google Scholar]
- Muthenna P, Akileshwari C, Reddy GB. Ellagic acid, a new antiglycating agent: its inhibition of N-(carboxymethyl)lysine. Biochem J. 2012;442(1):221–230. doi: 10.1042/BJ20110846. [DOI] [PubMed] [Google Scholar]
- Raghu G, Jakhotia S, Yadagiri Reddy P, Kumar PA, Bhanuprakash Reddy G. Ellagic acid inhibits non-enzymatic glycation and prevents proteinuria in diabetic rats. Food Funct. 2016;7(3):1574–1583. doi: 10.1039/C5FO01372K. [DOI] [PubMed] [Google Scholar]
- Rahbar S, Figarola JL. Novel inhibitors of advanced glycation endproducts. Arch Biochem Biophys. 2003;419(1):63–79. doi: 10.1016/j.abb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Rani U, Kesavan R, Ganugula R, Avaneesh T, Kumar U, Reddy G, et al. Ellagic acid inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and prevents atheroma formation in streptozotocin-induced diabetic rats. J Nutr Biochem. 2013;24(11):1830–1839. doi: 10.1016/j.jnutbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Raghu G, Reddy SS, Pasupulati AK, Suryanarayana P, Reddy GB. Response of small heat shock proteins in diabetic rat retina. Invest Ophthalmol Vis Sci. 2013;54(12):7674–7682. doi: 10.1167/iovs.13-12715. [DOI] [PubMed] [Google Scholar]
- Saraswat M, Reddy PY, Muthenna P, Reddy GB. Prevention of non-enzymic glycation of proteins by dietary agents: prospects for alleviating diabetic complications. Br J Nutr. 2009;101(11):1714–1721. doi: 10.1017/S0007114508116270. [DOI] [PubMed] [Google Scholar]
- Saraswat M, Suryanarayana P, Reddy PY, Patil MA, Balakrishna N, Reddy GB. Antiglycating potential of Zingiber officinalis and delay of diabetic cataract in rats. Mol Vis. 2010;16:1525–1537. [PMC free article] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Bhaduri T, McMullen CB, Gardiner TA, Archer DB. Advanced glycation end products induce blood-retinal barrier dysfunction in normoglycemic rats. Mol Cell Biol Res Commun. 2000;3(6):380–388. doi: 10.1006/mcbr.2000.0243. [DOI] [PubMed] [Google Scholar]
- Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, et al. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51(9):2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- Suryanarayana P, Saraswat M, Petrash JM, Reddy GB. Emblica officinalis and its enriched tannoids delay streptozotocin-induced diabetic cataract in rats. Mol Vis. 2007;13:1291–1297. [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- Vasan S, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, et al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382(6588):275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Inagaki Y, Amano S, Okamoto T, Takeuchi M, Makita Z. Pigment epithelium-derived factor protects cultured retinal pericytes from advanced glycation end product-induced injury through its antioxidative properties. Biochem Biophys Res Commun. 2002;296(4):877–882. doi: 10.1016/S0006-291X(02)00940-3. [DOI] [PubMed] [Google Scholar]