Abstract
Bacillus spp. are widely used in animal production for their probiotic properties. In many animal species, feed supplementation with specific Bacillus strains can provide numerous benefits including improvement in digestibility, the gut microbiota and immune modulation, and growth performance. Bacilli are fed to animals as spores that can sustain the harsh feed processing and long storage. However, the spores are metabolically quiescent and it is widely accepted that probiotics should be in a metabolically active state to perform certain probiotic functions like secretion of antimicrobial compounds and enzymes, synthesis of short chain fatty acids, and competition for essential nutrients. These functions should become active in the host gastrointestinal tract (GIT) soon after digestion of spores in order to contribute to microbiota and host metabolism. Considering that bacterial spores are metabolically dormant and many health benefits are provided by vegetative cells, it is of particular interest to discuss the life cycle of Bacillus in animal GIT. This review aims to capture the main characteristics of spores and vegetative cells and to discuss the latest knowledge in the life cycle of beneficial Bacillus in various intestinal environments. Furthermore, we review how the life cycle may influence probiotic functions of Bacillus and their benefits for human and animal health.
Keywords: Bacillus, Spore, Vegetative cells, Germination, Probiotic, Gastrointestinal tract
Introduction
Recently, the International Scientific Association for Probiotics and Prebiotics (ISAPP) emphasised the need for the clarification of the definition of the term probiotic. In a consensus opinion published in 2014, the expert panel re-inforced the importance of viability of probiotic microorganisms by defining them as: “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al. 2014). In their opinion, viability is being implicitly linked to some mechanisms of probiotic action suggesting that the probiotic should be metabolically active at the site of action i.e. in the gut. Traditionally a large variety of microbial species used as probiotics in food and feed are already metabolically active even before entering the host, including yeast (Saccharomyces) and non sporulating bacteria (Bifidobacterium, Enterococcus, Lactobacillus, Pediococcus, and Streptococcus). For decades, scientists have investigated solutions and technologies to improve the survival rate and the delivery of probiotics to the gastrointestinal tract (GIT) given the harsh environmental conditions in the feed process and the upper GIT. Strategies that have been used include sub-lethal stresses (Nguyen et al. 2016), use of protectants (Riaz and Masud 2013), drying technology (Broeckx et al. 2016), encapsulating materials (Opara 2017; Huq et al. 2013) and immobilisation techniques (Mitropoulou et al. 2013) that improve stability, survival, long-term preservation and biotherapy of probiotics. However, by nature bacterial spores don’t require these technologies to resist the food and feed processing effects. The sporulating probiotic bacteria (Bacillus, Clostridium) are highly resilient and retain their viability during distribution and storage (Cutting 2011), but they need to regain their metabolic activity after entering the host to provide benefits. Thus, it is of interest to understand the dynamics of Bacillus spp. germination in the gut including the fate of ingested Bacillus spores and the ratio of vegetative cells and spores in the different gut sections across different hosts, the length of time that the Bacillus persist in the gut after their withdrawal from the diet, and the influence of GIT physiology and Bacillus strain.
While the life cycle of pathogenic Bacillus in human GIT has been extensively researched (Hutchison et al. 2014; Narula et al. 2016), little has been published on the life cycle of beneficial spore formers during the last decade. The aim of this review is to capture the main characteristics of spores and vegetative cells and to discuss the latest knowledge in the life cycle of beneficial Bacillus in various intestinal environments. Furthermore, we review how the life cycle may influence probiotic functions of Bacillus and their benefits for human and animal health.
Characteristics of spores and vegetative cells
Bacillus cells have two distinct morphologies, endospore and vegetative cell, depending on the environmental stimuli (Piggot and Hilbert 2004). Endospores are the most durable type of cells found in nature, and represent a survival strategy of the bacteria. Bacillus endospores, or more traditionally referred to as Bacillus spores, have been isolated from amber that is more than 25 million years old, demonstrating the metabolic dormancy and protective capability of spores (Cano and Borucki 2012). The spores protect the bacterium by attaining a highly defensive state that is resistant to environmental conditions such as extreme temperature, radiation, pH, pressure and toxic chemical agents that would harm the vegetative form (Pedraza-Reyes et al. 2012). Some parts of the GIT represent a toxic environment for Bacillus with anoxia, low pH, and bile salts, along with an extremely high concentration of commensal bacteria (reaching 1012. g−1 of faecal content in the colon) that compete for nutrients and ecological space. These conditions challenge the growth of the vegetative bacterium, yet the spore would be inert to these conditions and transit unimpeded through the GIT.
The outermost structure of endspores, the exosporium, consists of proteins, lipids and carbohydrates that provide hydrophobic characteristics to the spore and make it insoluble. The extremely low permeability of the inner membrane prevents both hydrophobic and hydrophilic molecules from entering the core. Bacterial spores contain a high concentration of dipicolinic acid (DPA), the exact content of which varies between different species yet ranges from 5 to 15% in dry weight. The unique and intrinsic composition of Bacillus spores provides high tolerance to the acidic conditions in the stomach upon ingestion as described in Table 1.
Table 1.
Differences between the resistance profiles of Bacillus spores and vegetative cells against adverse environmental and gastrointestinal tract conditions
| Condition | Vegetative cells | Spores | References |
|---|---|---|---|
| Extreme pH | Inactivated with variable kinetics depending on the specific nutrients composition and strains used | Not inactivated between 1.0 and 5.0 in gastric medium | Clavel et al. (2004) |
| Stomach acid | Highly sensitive | Trigger germination | Ciffo et al. (1987) |
| No effect on spore | Duc Le et al. (2003a) | ||
| Bile salts | Highly sensitive <1 mmol−1 Bacteriocidal on VC | No effect on spores | Hyronimus et al. (2000) |
| Modest inhibition of germination | Duc Le et al. (2003a) | ||
| Intestinal conditions | Suitable growth environment in the absence of intestinal microbiota exerting competitive pressure | Suitable growth environment in the absence of intestinal microbiota exerting competitive pressure | Ceuppens et al. (2012d) |
The spore will germinate in the presence of germinants, nutrients or non-nutrients (Swick et al. 2016) and other favorable environmental conditions. Nutrients activate the germinant receptors causing the release of ions including Ca2+ and DPA from the spore core. In addition to nutrients, spores are germinated by other agents, including lysozyme, salts, high pressures and cationic surfactants such as dodecylamine (Setlow 2003). Binding of germinants to the receptors present on the inner membrane triggers the release of the DPA, allowing water to enter the spore core. This re-hydration activates spore enzymes that hydrolyse the spore cortex, allowing the spores to start germination. Cortex hydrolysis and subsequent cortex expansion allows full hydration of the spore core. DPA is rapidly released during germination, but its function remains uncertain. Studies have shown that spores lacking DPA have significantly increased water, and decreased heat and H2O2 resistance (Balassa et al. 1979). In addition, it is possible that Ca2+-DPA released from one spore may stimulate the germination of other neighboring spores (Setlow 2003). This enables the restart of metabolism and macromolecular synthesis in a process termed outgrowth, eventually leading to the resumption of vegetative growth (Setlow 2003). However, when the dormant spore breaks hibernation and begins to be metabolically active, it also loses resistance to environmental stress. Thus, the structure of the spores and vegetative cells have adapted to specific environments at different stages of the life cycle that are controlled by sophisticated molecular mechanisms. The sporulation capability of probiotic Bacillus would be a perfect advantage but only if germination occurs fast enough to generate enough live cells that could reach the appropriate location within the gut and display beneficial effects.
Life cycle of Bacillus in various GITs
Globally, available data indicates that Bacillus spores may have similar life cycles in mammals (mice, pigs) and avian species (chicken). They can germinate in variable proportions and at different locations depending on the physiological characteristics encountered in the gut as described in further detail below for each model.
Animal models
Laboratory animal models, mainly mice (Table 2), have been used to study the Bacillus life cycle in more depth. It has been shown that the spores administered by the intragastric route to mice could not germinate and on the other hand, ingested spores that did not germinate prior to reaching the large intestine remained metabolically dormant during the rest of the transit (Table 2; Spinosa et al. 2000). These results suggest that the stressful conditions encountered during the upper digestive tract may be the key for triggering the germination process and that the time window for germination is limited. One of the key triggering factors for sporulation is the low pH in the stomach (Ciffo et al. 1987) that, conveniently, activates the spores before they reach the small intestine which is rich in nutrients. Further evidence on the importance of the small intestine as the main sporulation site was shown in a mice study that used recombinant Bacillus subtilis. The authors showed that the spores, upon exiting the stomach, can germinate, proliferate, and then resporulate in the small intestine of mice (Tam et al. 2006).
Table 2.
Overview of studies of germination and overgrowth of sporulated Bacillus added to mice diet or administered intragastrically)
| Animal Target | Strain | Dose, Physiological status | Germination (G) or not | Results | References |
|---|---|---|---|---|---|
| Mice | B. subtilis (natto) | G | Enhancement of the growth of Lactobacillus murinus seemed to be linked to metabolites produced during germination and (or) outgrowth of spores of the strain Natto | Hosoi et al. (1999) | |
| Mice |
B. subtilis MO 1099 B. clausii (Enterogermina®-Sanofi) |
1 × 109 spores/animal intragastric | No G | Elimination of SP from the intestine reflecting solely its intake from food (logarithmic phenomenon) No VC: explained by the sensitivity to taurodeoxycholic acid Probiotic effect due to SP only or extra-intestinal life cycle (VC found in mesenteric lymph noodles) |
Spinosa et al. (2000) |
| Mice | B. subtilis | 5.87 × 108 SP/mice 2 × 109 SP/mice |
Only evidence of G | Only evidence that SP may germinate. SP are not disseminated in significant numbers beyond GIT High persistence rate in the gut that could be ascribed to a small proportion of the SP germination in upper tract of intestinal tract, multiplying once or twice and then resporulating in the distal part of the gut |
Hoa et al. (2001) |
| Mice BALB/c | B. subtilis SC2288 resistant to chloramphenicol | 6 × 108 SP/mice | 1–12% G | G found in jejunum and ileum using a competitive RT-PCR targeting a genetically engineered chimeric gene ftsH-lacZ only expressed in VC. No VC was found in duodenum | Casula and Cutting (2002) |
| Mice | B. subtilis SC2362 carries rrnO-lacZ gene as well as cat gene | 2.4 × 1010 VC/mice 2.1 × 108 SP/mice |
G | % of recovery of VC in feces: 0.00016% of inoculating dose % of recovery of SP in feces: 12% of inoculating dose Germination was found associated to as systemic anti-beta galactosidase IgG response in small intestine |
Duc Le et al. (2003a) |
| Mice |
B. subtilis PY79 B. pumilus BiosubtylNT B. clausii Enterogermina® B. cereus var. vietnami Subtyl B. cereus BiosubtylDL B. cereus Bactilsubtil |
109 SP/mice per gavage | P | Except the wild-type strain PY79 which rapidly cleared from mouse gut, all other probiotic strains persist until 18 days after inoculation. All B. cereus strains persist longer in the GIT of mice: after 15 days significant numbers are still present |
Duc Le et al. (2004b) |
| Mice C57BL/6 | 3 strains of B. subtilis
B. subtilis PY79; RH103 carries a chimeric gene tetanus toxin fragment C); UL12 with Cmr All strains are GMO |
2 × 1010 SP/dose on days 1, 2, 3, 24, 25, 26, 49, 50 and 51 | G | Declining fecal shed. Strains do not permanently colonize the GIT Based on gene expression in the GIT of mouse Persistence from 15 to 27 days |
Tam et al. (2006) |
| Gnotobiotic rats | B. thuringiensis subsp. israelensis | G | Wilcks et al. 2008 | ||
| Mice Balb/c AnNHsd | B. polyfermenticus CJ6 | 109 SP/mouse | G P >11 days |
VC and SP resist to in vitro simulated gastric juice, bile salt and acid SP germinate and persist in GIT of mice as fecal excretion was 4-fold higher compared to inocula |
Jung et al. (2012) |
| Balb/c inbred mice |
B. subtilis HU58 human origin B. subtilis PXN21 (Protexin Ltd) B. subtilis PY79 laboratory strain |
1 × 109 SP/mice | P of PY79; PXN21 and HU58 is 7, 18 and 21 days respectively | Transit period for B. subtilis varies considerably between different strains Persistence of HU58 and PXN21 is correlated with their abilities to form biofilm |
Permpoonpattana et al. (2012) |
VC vegetative cell, G germination, SP spore, GIT gastrointestinal tract, P persistence, RT-PCR reverse transcription polymerase chain reaction
Despite the important use of probiotic Bacillus in animal nutrition, only a few studies have been published on the germination potential of Bacillus spores in farm animals (Table 2). In chickens, it was observed that 10–90% of spores germinated in the small intestine (Latorre et al. 2014) (Table 3). In vitro, crop and intestinal models have shown that up to 90% of the spores germinate within 60 min (Latorre et al. 2014). In addition to highlighting that Bacillus have adapted to different hosts, Hong et al. (2009) reports that there is a mixture of spores and vegetative cells in the small intestine and that upon exiting the stomach or crop Bacillus are mostly still in their spore form. However, it seems that the germination is not systematic and that the host, the intestinal location of germination as well as the bacterial species may be crucial contributing factors for the survival and metabolic activity of vegetative cells along the GIT.
Table 3.
Overview of studies of germination and overgrowth of sporulated Bacillus fed to pigs and broilers
| Animal Target | Strain | Dose/Physiological status | Germination (G) or not | Results | References |
|---|---|---|---|---|---|
| Pigs | B. subtilis BN | G | Germinate and multiply in GI tract | Ozawa et al. (1981) | |
| Piglet stomach |
B. cereus var toyoi Toyocerin |
G | Rapid germination in stomach of piglets, cells undergo repeated germination and sporulation during intestinal passages | Jadamus et al. (2001) | |
| Piglets |
B. subtilis CH201 B. licheniformis CH200 |
1.28 × 108 SP/g feed for 14 days (1:1) | G | Stability of SP all along the GIT (gut samples) Both strains germinate in the GIT, particularly in the stomach, but only limited outgrowth of the VC occurs |
Leser et al. (2008) |
| Grower finisher pigs |
B. subtilis CH201 B. licheniformis CH200 |
1.6 × 108 SP/g feed from 60 to 120 days and then no supplementation for 7 days | G | Excretion of SP with linear regression, persistence of SP for 10 to 11 days after withdrawal of the supplementation Evidence of germination (x 2.14) but no outgrowth |
Leser et al. (2008) |
| Pigs 65–70 kg |
B. subtilis CH201 B. licheniformis CH200 |
SP added intragastrically in a dialyzed membrane and followed for 4, 6, 8 and 24 h | G | % of SP decreases and the % of VC increases The duodenum and the colon are more convenient for germination Germination occurs in stomach but VC are quickly killed Resporulation occurs in intestine No colonization |
Leser et al. (2008) |
| Day-of-hatch, off-sex broiler chickens | B. subtilis PHL-NP122 | Adminsitrated either as a single gavage dose or constantly in the feed SP group:106 spores per gram of feed or gavaged with a single dose of 106 spores suspended in 0.25 mL of PBS per chick using a sterile ball-ended gavage needle |
G | Continuous administration: the number of recovered spores was constant through 120 h in each of the enteric regions from chickens receiving spores supplemented in the feed. However, the number of recovered B. subtilis spores was consistently about 105 spores per gram of digesta, which is about a 1-log10 reduction of the feed inclusion rate, suggesting approximately a 90% germination rate in the GIT when fed Persistence: recovered B. subtilis spores decreased with time, with only approximately 102 spores per gram of sample by 120 h |
Latorre et al. (2014) |
| Chicken crops |
B. cereus var toyoi Toyocerin |
G | Rapid germination in chicken crops | Jadamus et al. (2001) | |
| 1 day-old chick | B. subtilis SC2362 rrnO-lac + cat genes | 1 × 109 SP/chick | G | Number of VC was found to surpass the number of SP after 20 h postdosing in all regions of the GI tract of chick → no answer whether it is germination +/− replication in the gut? VCs were found in all regions of the GIT SP are transient members of the GIT of chick and do not colonize Fast germination of SP: 20 h after SP doses were administrated |
Cartman et al. (2008) |
| Chicken | B. thuringiensis subsp. kurstaki transformed Bt HD-73GFP | 10 ml VC 10 ml SP |
G? | Bt harbors the marker gene gfp and erythromycin resistant Bt was found for the first 4 days after being fed VC of Bt Bt was found from day 1 to 13 in chicken feces of group fed with SP Bt |
Zhang et al. (2012) |
VC vegetative cell, G germination, SP spore, GIT gastrointestinal tract, P persistence, RT-PCR reverse transcription polymerase chain reaction
There are species and strain specific differences in the persistence of Bacillus strains in the GIT. In mice, persistence of different species ranges from 7 to 27 days indicating differences in the life cycle of the Bacillus strains (Tam et al. 2006). In another study using mice, all Bacillus cereus tested strains appeared to be more persistent than B. subtilis, B. pumilus and B. clausii (Duc Le et al. 2004b; Table 2). The rationale for these findings may be found in metabolic differences between species. Some Bacillus species are facultative anaerobes with anaerobic respiration and fermentative growth capabilities (Clements et al. 2001) and could survive in various GIT locations independent of the O2 conditions. On top of nutritional and aerobic specificities occurring across species, differences in germination are the result of the genetic makeup, resulting in significant differences between various strains belonging to the same species and the heterogeneous behaviour of individual cells within an isogenic population (Wells-Bennik et al. 2016).
In addition to strains, the host appears to impact the Bacillus life cycle. When comparing work done in mammals like mice (Table 2) and pigs (Table 3) and in chickens (Table 3), it seems that in avian species, the vegetative cells are found in every part of the GIT whereas in mammals, they are mainly recovered in the distal GIT as for mice (Cartman et al. 2008) and for pigs (Leser et al. 2008). Interestingly, the work done by Cartman et al. (2008) in mice suggests that the maturity of the GIT may be important in the germination process of spores. In addition, it seems that the resident intestinal bacteria (mainly from the large intestine) may limit or suppress the outgrowth of the vegetative cells.
Studies also highlight that survival and persistence of Bacillus in the GIT seems to be related to the inoculated form and dose in a species-specific manner. Comparative analysis of survival rates of ingested spores versus vegetative cells of B. subtilis in mice revealed that ingestion of only the sporulated form enables sufficient faecal recovery (Duc Le et al. 2004b). The recovery rate of the spores was 75,000-fold higher than the recovery rate of the vegetative cells (Duc Le et al. 2004b) suggesting both germination and proliferation occured since it is thought that cells must proliferate, at least one cell division before sporulation can commence.
Essential for the life cycle of Bacillus is also timely sporulation before exiting the GIT. In the 1970s, conditions encountered in the large intestine were shown to re-activate the sporulation process, requiring both time and energy (Piggot and Coote 1976). This finding is supported by the long persistence time (27 days) of Bacillus in the gut of mice (Tam et al. 2006) and the excretion of a higher dose of Bacillus spores in the faeces of pigs (Leser et al. 2008) or mice (Hoa et al. 2001) compared to the inoculum originally ingested by the animals. These findings indicate that the re-sporulation process in the gut and especially the large intestine plays a key role in completing the life cycle. It has been shown that the formation of endospores in Bacillus are promoted by conditions such as, a high cell density (Grossman and Losick 1988), nutrient limitation (Schaeffer et al. 1965), high mineral composition, or neutral pH that are in line with conditions found in the large intestine. There is however strain dependent differences in the response to these environmental cues (Bernlohr and Leitzmann 1969). Although sporulation of B. subtilis is induced by starvation, sporulation is not immediately initiated when growth slows due to nutrient limitation. Other alternative responses can occur, such as activation of flagellar motility to seek new food sources by chemotaxis, or secretion of hydrolytic enzymes to scavenge extracellular proteins and polysaccharides (González-Pastor 2012). Sporulation is the ultimate response to starvation and is thus suppressed until alternative responses are shown to be inadequate. The sporulation in the intestine and exit from the host as an environment resistant spore completes the life cycle of the Bacillus.
Human and human GIT models
Data obtained from human studies has been treated separately from that of mammals, since the majority of data has been generated from artificial GIT models. Only one recent study has involved human volunteers. Although, the evidence from animals has shed light on the life cycle of Bacillus, the data from humans is more scarce. Assessment of Bacillus spore germination has been studied in vitro in a variety of human GIT models, from batch compartments (Ceuppens et al. 2012a) to complex dynamic interconnected systems like the Intestinal Models (TIM) developed by The Netherlands Organisation (TNO) for applied scientific research, (Hatanaka et al. 2012) and the Simulator of Human Intestinal Microbial Ecosystem (SHIME) from Prodigest (Ceuppens et al. 2012b) (Table 4). These models have been used to evaluate Bacillus from two different perspectives; on one hand, B. cereus pathogenic strains were assessed for the health risks (Ceuppens et al. 2010; Ceuppens et al. 2012a, b, c); and on the other hand, beneficial probiotic Bacillus candidates were applied in the models to assess the beneficial effects in human nutrition (Duc Le et al. 2004a; Hatanaka et al. 2012; Maathuis et al. 2010). These models are interesting since they allow you to determine phase when and where in the gut, spores germinate. Based on the studies above, it may be concluded that in most human GIT models, germination may be detected. However, the number of vegetative cells detected varies depending on specific gut sections. Bile appears to be major limiting factor, since an early germination of the spores in the upper part may reduce the final proportion of vegetative cells reaching the ileum and colon (Ceuppens et al. 2012b). Hatanaka et al. (2012) demonstrated that only 8% of the spores germinated after passage through the upper part of the GIT model, but it was enough to influence a shift in the colon microbiota and exercise a probiotic effect. Dose, strain, experimental design, inter-experimental variation and how the Bacillus cultures were prepared may also explain varying results. Thus, the use of Human GIT models suggests that the life cycle of Bacillus spores is comparable to that of mice and pigs. These in vitro data have recently been supported by a clinical study (Ghelardi et al. 2015); four strains of B. clausii were orally administrated to healthy adult volunteers and the fate of the spores was investigated. The authors used specific antibiotic resistance profiles of each of the probiotic Bacillus strains to detect them in faeces. The study demonstrated that the strains not only survived in vivo GIT conditions, but also underwent germination, outgrowth and multiplication as vegetative forms.
Table 4.
Overview of studies of germination and overgrowth of sporulated Bacillus in human or human gastrointestinal tract models
| Human or GIT Model | Strain | Dose Physiological status | Germination (G) or not | Results | Reference |
|---|---|---|---|---|---|
| Healthy adult volunteers | Mixture of 4 B. clausii strains Enterogermina® | 2 × 109 SP/capsule or vial | G, outgrowth and multiplication | The unique human in vivo experiment demonstrating germination of spores | Ghelardi et al. (2015) |
| Simulated gastric fluid (SGF) and Simulated intestinal fluid (SIF) |
B. subtilis PY79 B. pumilus BiosubtylNT B. clausii Enterogermina® B. cereus var. vietnami Subtyl B. cereus BiosubtylDL B. cereus Bactilsubtil |
From 107 to 5 × 108 SP/ml | Poor Resistance | Sensitivity to SGF: BiosubtylNT, Subtyl (0.02% survival after 1 h) and Bactilsubtil Sensitivity to SIF: Subtyl (0.2% survival after 3 h) and Bactilsubtil mainly to bile |
Duc Le et al. (2004a) |
| TIM-1 Human (model for upper part: stomach + small intestine) | B. coagulans GBI-30 (GanedenBC30) | 2×109 SP per compartment | No G | 70.4% of SP survived after 6 h in TIM-1. Not able to determine the amount of VC due to overgrowth of endogenous microbiota. No effect of SP supplementation on protein digestion of the diet but improvement of lactose and fructose digestion. Supplementation of diet with lactose and fructose on top of the SP may facilitate quick germination of SP. However, quick germination may induce higher mortality of VC in upper part of the GIT | Maathuis et al. (2010) |
| SHIME model | B. cereus NHV 1230-88 | 5.7 log CFU/ml of VC 4.6 log/ml of SP |
G | All VC were inactivated during gastrointestinal passage. Bacillus SP germinated and grew out during the gastrointestinal simulation experiment | Ceuppens et al. (2010) |
| TIM-1 and TIM-2 | B. subtilis C-3102 Calsporin® | 9 × 107 SP/g of meal in TIM-1 4 × 109 SP in TIM-2 |
G (8%) | In TIM-1 (stomach → ileum), survival of SP is 99 and 8% have germinated In TIM-2 (colon) → effluent from TIM-1 containing SP is more efficient in increasing Bifidobacterium and decreasing Clostridium compare to SP added directly to TIM-2 suggesting that oral passage is essential and germination even small may be required |
Hatanaka et al. (2012) |
| GIT model of human SHIME |
B. cereus NVH 1230-88 B. cereus 14579 + Ery® plasmid |
107 Exp. VC 107 Stat. VC 107 SP |
G in duodenum only |
Gastric part Exponential VC dyed; Stationary VC less sensitive but dyed after 4 h; SP are not inactivated but do not germinate after 8 h Duodenum Major part of the exp. VC is inactivated but residual VC are rapidly able to grow; stat. VC can grow; SP germinate within 1 h Ileum (only DNA copy, no distinction between SP or VC): Inactivation of VC and stability of SP: no growth and no germination |
Ceuppens et al. (2012b) |
| In vitro model for bile resistance test |
B. cereus ATCC 14579 B. cereus NVH1230-88 B. cereus FF73 |
VC and SP | No G | Bacteriocidal effect of bile is pH dependant VC that resist to gastric passage are inactivated in the intestinal environment by bacteriocidal effect of bile SP are not inactivated by bile but do not germinate until [bile] <5.0 g l−1 SP germination is bile concentration dependant |
Ceuppens et al. (2012a) |
| In vitro model with 5 compartments |
B. cereus RIVM 9903295-4 B. cereus NVH1230-88 B. cereus LFMFP 381 B. cereus LFMFP 710 |
1 × 107 SP/ml | G No outgrowth of VC | Survival and germination of SP Outgrowth of VC is suppressed by human intestinal bacteria |
Ceuppens et al. (2012c) |
VC vegetative cell, G germination, SP spore, GIT gastrointestinal tract, P persistence, Exp exponential Stat stationary
In conclusion, whatever the model and physiological characteristics, there is evidence of possible germination of Bacillus spores in the GIT especially in the stomach and small intestine. The rapid germination just after ingestion in the stomach is not the ideal pathway due to the presence of gastric juices and bile, however, germination in the first part of the small intestine which is nutrient rich and possesses a low microbial pressure would provide a good environment for reproduction. Furthermore, the sensitivity of vegetative cells to this stress is highly dependent on the growth status. Vegetative cells in the exponential phase are more sensitive to gastric and duodenal conditions compared to vegetative cells in the stationary phase. Conversely, a late germination in the large intestine reduces the opportunities of outgrowth due to: (1) the increased sensitivity of vegetative cells in the exponential phase and (2) the shorter transit time. Also, Bacillus strains and the dose administrated appear to be crucial determinants that impact the entire life cycle in the GIT.
It seems clear that Bacillus spores can germinate in conditions present in the upper GIT, but it is also obvious that not all spores will germinate before excretion, particularily in species with a short transit time of 2 to 4 h such as shrimp (Beseres et al. 2006). Also, germinated Bacillus may sporulate in the small intestine and in essence a mixture of spores and vegetative cells may be found in the intestine. Ingested spores of Bacillus transit through the GIT according to a ratio of vegetative cells to spores which may vary in different gut sections according to transit time, gut motility, microbiota, environmental conditions (e.g. pH, oxygen level, nutrient availability) and the nature of the feed. These parameters have not been fully assessed in the probiotic efficacy studies with Bacillus and would be important to include in future studies in order to better understand the correlation between the efficacy and life cycle of the Bacillus and to align observed probiotic benefits in the host to a physiological Bacillus status. Therefore, we decided to review the probiotic mechanisms associated with Bacillus and the potential differences between spores and vegetative cells in regards to probiotic benefits.
Probiotic efficacy of spores and vegetative cells
Bioactive secondary metabolites
Bioactive microbial secondary metabolites are components which regulate growth processes, replications, and/or exhibit responding (regulating, inhibiting, stimulating) action to the metabolism of cells at the biochemical level, in minimal concentration and Bacillus strains are well-known to produce secondary metabolites (Sansinenea and Ortiz 2011). These metabolites are exclusively produced by vegetative cells and generally at the highest levels during the transition from active growth to the stationary phase and thus not by the dormant spores.
An important category of secondary metabolites for the probiotic function of the Bacillus is the production of antimicrobial compounds, which is also commercially utilized. About half of the bacterial biocontrol agents are prepared from Bacillus strains. The antimicrobials include well documented molecules like bacteriocins and bacteriocin-like inhibitory substances (e.g., subtilin and coagulin) or antibiotics (e.g., surfactin, iturins A, C, D, E, and bacilysin) (Mondol et al. 2013). These bioactive secondary metabolites are often a key factor in the screening process of probiotic candidates for food production animals, since the prevention of microbial diarrhea is a key aspect of animal performance.
Vegetative Bacillus cells also have a capacity to secrete extracellular carbohydrate-, lipid-, and protein-degrading enzymes that digest food and feed (Schallmey et al. 2004). Although the efficacy of animal growth performance resulting from feed supplementation with Bacillus spores is inferior to that achieved with exogenous enzyme supplementation, Bacillus species are considered attractive sources of various extracellular hydrolytic enzymes that could aid in nutrient digestion and feed utilization. For example, feed supplementation with protease, amylase, and lipase secreting Bacillus, improved the growth performance of broiler chicks (Santoso et al. 2001). In shrimp, Bacillus added to feed resulted in increased specific activity of lipase, protease and amylase in the digestive tract (Ziaei-Nejad et al. 2006). These studies indicate that spores in feed germinate and become metabolically active in the GIT to secrete digestive enzymes. However, it can be difficult to distinguish between the activity of enzymes endogenous to shrimp and enzymes synthesized by Bacillus and it has been suggested that the presence of the probiotic strain could stimulate endogenous enzyme production by the shrimp.
Detoxification
Toxic compounds found in feed that may threaten animal health include heavy metals and mycotoxins. Extensive use of pesticides during crop production and exposure of the environment to industrial toxic waste results in the deposition of heavy metals in soil and water, and this practice can pose serious threats to crop production and animal health through contaminated feed. Due to their high surface area per unit weight, Bacillus spores and vegetative cells can act as biological adsorbents for dissolved toxins and metals. For example, it has been demonstrated that mature spores of a marine Bacillus sp. strain SG1 can oxidize Mn (II) to MnO2, and that vegetative cells of the same strain reduce MnO2 in low-oxygen conditions (Mondol et al. 2013). The binding of ions is closely linked to the characteristic structure of the cell surface and therefore spores and vegetative cells act differently. It appears that the teichoic acids linked to the head groups of membrane lipids (lipoteichoic acids, LTAs) contribute to the adsorption of ions onto the cell walls of vegetative B. subtilis (Moriwaki et al. 2013); however, the LTAs are mainly absent in the spore and thus they have a different binding characteristic.
Mycotoxins are toxic secondary metabolites produced by fungi (moulds) that are formed during the growth phase of crops, but they can also be produced during storage or processing, when humid conditions occur. A wide range of microorganisms, including bacteria, moulds and yeasts, have demonstrated the ability to biotransform mycotoxins. Such microbes act in the animal GIT before absorption of the mycotoxins by the host can occur. Enzyme production by Bacillus strains has also been associated with the reduction of mycotoxins (Karlovsky 2011), and in vitro studies have demonstrated the removal of deoxynivalenol (DON) after incubation with culture supernatants of Bacillus licheniformis and B. subtilis (Cheng et al. 2010). The degradation of zearalenone by supernatants of B. subtilis 168 and Bacillus natto CICC 24640 has also been shown (Tinyiro et al. 2011). Based on in vitro screening, Bagherzadeh Kasmani et al. (2012) isolated hundreds of strains of vegetative forms of Bacillus with aflatoxin-binding capacity. Their best candidate (in fact a Brevibacillus strain) was fed in vegetative form to quails recieving an aflatoxin contaminated diet, and was shown to restore performance, serum biochemistry, and immune responses in vivo. Overall, these data indicate that vegetative cells are the key for detoxification. Nevertheless, most of the in vivo studies on detoxifcation have not assessed the Bacillus forms in the GIT. These studies showed that B. subtilis was able to detoxify aflatoxin B1-contaminated feed and facilitate animal growth (Petchkongkaew et al. 2008) and that a preparation of B. subtilis ANSB060 had a protective effect on growth performance and meat quality of broilers, and reduced aflatoxin residues in the liver of broilers that were fed naturally moldy peanut meal (Fan et al. 2013). A Bacillus strain isolated from fish intestines was successfully used to detoxify deoxynivalenol in moldy corn that was fed to pigs (Li et al. 2011). On the other hand, a commercial product containing spores of B. licheniformis CH200 and B. subtilis CH201 at a density of 2.3 × 106 colony-forming units per g diet, failed to prevent absorption and toxic effects of the Fusarium toxin DON in piglets, suggesting that the probiotic bacteria neither bound nor degraded DON prior to absorption (Dänicke and Döll 2010) and that detoxification may depend on the strain of Bacillus. Although the majority of the in vivo studies did not assess the physiological status of the Bacillus, based on the studies reviewed above, it is likely that both vegetative cells and spores were present in the GIT and that vegetative cells are responsible for the efficacy. Without further data, it is difficult to conclude if spores in the GIT have a role in detoxification or what could be their contribution as absorbents.
Competition for ecological niches
Beneficial, non-pathogenic, and mature microbial cultures can be introduced into the intestinal tract of an animal, to create an environment where the beneficial microbiota outcompete opportunistic pathogens and reduce the chance of pathogen colonization. Mechanisms widely recognized for probiotic strains are competition for nutrients and intestinal space, and adherence to intestinal surfaces that can reduce pathogen colonization. Adhesion properties are general traits of probiotic Bacillus strains, and it has been reported that B. indicus HU36, B. subtilis var. Natto, B. subtilis PY79, B. subtilis BS3 and B. licheniformis BL31 all adhere to a human epithelial colorectal adenocarcinoma cells model—Caco-2 cells (Jung et al. 2012). The capacity of adhesion, on the other hand, seems to be a strain specific property and dependent on the form of Bacillus. Spores and vegetative cells have been shown to attach in vitro to Caco-2 cells (Sánchez et al. 2009). In vivo, both vegetative cells and spores of B. polyfermenticus CJ6 could adhere and colonize the small and large intestines of mice (Jung et al. 2012). The different cell surface compositions of vegetative cells and spores may interfere with the capability of the strain to reside temporarily in the intestinal tract. Harimawan et al. (2013) studied the adhesion of B. subtilis spores and vegetative cells onto metal surfaces, as it indicates biofilm formation. Their data showed that compared to vegetative cells, spores had greater adhesion on stainless steel. Although the surfaces of spores and vegetative cells were hydrophilic, the former displayed higher hydrophobicity, which may arise from a significant amount of proteins on the spore’s surface. The higher hydrophobicity and the lower energy barrier of spores results in significantly higher adhesion forces, compared to vegetative cells (Harimawan et al. 2013). Bacteria at different growth stages are known to have different local surface heterogeneity properties, such as surface charge density, electrophoretic mobility, and hydrophobicity. Spores contain significantly more proteins and appendages on their surface (e.g. proteinaceous coat, exosporium) compared to vegetative cells (Sirec et al. 2014). It seems that both vegetative cells and spores may have adhesion abilities, but at different levels, thus the probiotic mechanism may be dependant on the life cycle of the Bacillus strain in the intestine.
Immune effects of spores and vegetative cells
Bacillus genus is also used as probiotics in animal nutrition to promote immunity against various commercially relevant pathogens. In vivo studies revealed that Bacillus can beneficially promote markers of immunity in both chickens and pigs (Scharek-Tedin et al. 2013; Schierack et al. 2007), but association with improved animal performance has not always been established (Lee et al. 2010, 2014). Only few studies have assessed the differences between vegetative cells and spores in generating immune responses and the experiments have been focused mostly on the functions of dendritic cells (DCs) and macrophages that are capable of phagocytosis.
Intestinal macrophages are important for maintaining homeostasis in the intestine by phagocytosis and killing of bacteria. In a murine study, Bacillus spores were shown to survive inside the macrophages for a longer time than the vegetative cells (Duc le et al. 2004b) and, interestingly, spores could germinate inside the cells before they were released (Huang et al. 2008). This leads to a longer retention time in the macrophages (Huang et al. 2008) and later activation of some cellular effector pathways. The authors showed that the vegetative cells upregulated the expression of Toll-like receptor (TLR) 2 and TLR4 in macrophages after 4 h of incubation, whereas it took 6 h with spores (Huang et al. 2008). Non-viable spores could not upregulate TLR’s, which suggests that it is the vegetative cell that has the greatest influence on macrophage function (Huang et al. 2008). Taken together, it seems that the immune response is delayed with spores, and is different to vegetative cells.
The prototypical function of DCs is to guide the type of immune response by phagocytosing bacteria, travelling to lymphoid tissues, and presenting antigens to naïve T-cells. In vitro, B. subtilis has been shown to drive the maturation of avian bone marrow derived DCs and induce antigen presentation (Liang et al. 2013). In addition, B. subtilis has been found in Peyers’s Patches, mesenteric lymph nodes and the spleen of mice (Duc le et al. 2003a), showing that Bacilli also induce the adaptive immune response. Although both spores and vegetative cells are carried by DCs, the form of Bacillus seems to influence the type of immune response, T helper cell 1 (Th1) or 2 (Th2) dominated, that is generated by the DCs. It was elegantly shown that Clostridium tetani antigens on a spore coat, induced Th1 bias, yet expression on a germinating spore (vegetative cells) induced Th2 bias of the immune response (Uyen et al. 2007). The authors suggested that longer persistence time of spores inside the phagocytes favors intracellular (Th1) responses (Uyen et al. 2007) In addition, it has been shown that non-viable (unable to germinate) B. subtilis spores can enhance expression of Th1 type cytokines in mice DCs and confer protection against H5N1 avian influenza (Song et al. 2012).
Although in experimental settings spores may favor Th1 and vegetative cells Th2 responses, in vivo, a mix of vegetative cells and spores is present as described above, and it may be more difficult to observe a clear-cut bias in the immune response. One study showed that orally administered B. subtilis spores to mice induced systemic IgG and secretory IgA with Th1 bias (Duc le et al. 2003b). Similarly, it has been found that spore or spore coat induce Th1 bias and production of interferon-γ (IFN-γ) (Duc le et al. 2003b). On the other hand, live and heatkilled spores have been shown to induce both Th1 and Th2 cytokines in Th1 prone C57BL6 mice and in Th2 prone BALB/c mice (de Souza et al. 2014). Also, Barnes et al. (2007) showed that feeding of mice with killed spores resulted in a balanced Th1/Th2 response. These studies above suggest that in vivo, the resulting immune response is dependent on multiple factors, including the host and the strain. Overall, it seems that Bacillus probiotics can have a wide effect on immune response that have features of both Th1 and Th2 responses.
Conclusion
In general, there is clear evidence that Bacillus spores can germinate in GIT conditions. Both the spores and the vegetative cells exhibit functional properties in conjunction with each other in the GIT, and this functionality seems to depend on the ratio between the spores and the vegetative forms. From a probiotic perspective, vegetative cells and spores have separate, but complementary functions. Some properties like immunomodulation and adsorption can be promoted by both VCs and SPs, while others, like antimicrobial activity, are a unique and specific trait of vegetative cells, as summarized in Fig. 1.
Fig. 1.
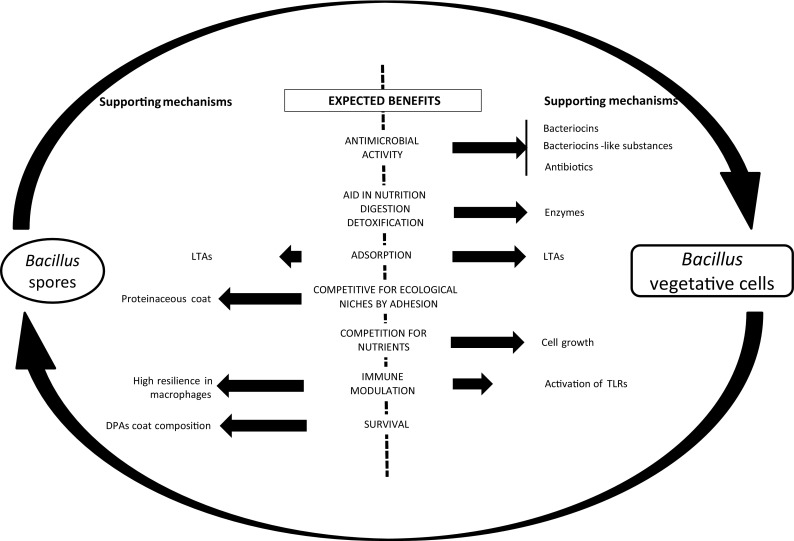
Overview of specific and non-specific beneficial mechanisms of probiotic Bacillus attributable to viable vegetative cells and spores in the gastrointestinal of animals and associated metabolites or cell wall components (black arrow) when expected to occur (LTA: lipoteichoic acids)
The germination process that guarantees metabolic activity (e.g. secretion of antimicrobial compounds, enzymes, synthesis of SCFAs and competition for essential nutrients) is highly dependent on the strain itself, GIT location, environmental conditions, age of the animal and intestinal maturity, as well as the conditions applied during the sporulation process prior to ingestion. Only a few studies have demonstrated spore germination of Bacillus spp. from in vitro and in vivo approaches and more studies are warranted. However, the available studies involve, studying the germination process of spores using non-specific and non-accurate culture methods, that may fail to detect specific strains in complex environments of the GIT or on the other hand, highly performant molecular tools that use GMO or toxigenic strains. Thus, even though germination seems to be obvious, it is difficult to establish the ratio between spores and vegetative cells that influence their probiotic properties.
Since spores must germinate to exert most of their beneficial effects, it would be interesting to identify compounds that effectively stimulate spore germination in the gut. The need for effective decontamination regimens for spores of organisms such as Clostridium difficile and B. anthracis, has renewed interest in this area, as stimulating spore germination would be the first step towards increasing vulnerability to elimination (Setlow 2014). It has been demonstrated previously that the sporulation of Bacillus strains may influence the germination potential of Bacillus spores. B. subtilis spores prepared under different conditions have been shown to exhibit different germination kinetics with nutrient germinants (Wells-Bennik et al. 2016). Temperature during sporulation and composition of the sporulation media has been shown to effect the relative composition of the spore, especially DPA and calcium (Baweja et al. 2008). In vitro, the same authors have demonstrated that early sporulation at an alkaline pH can generate spores that were found to be more resistant to both acidic and basic pH conditions, which may help the survival of a higher percentage of viable spores in such a microenvironment. Furthermore, technology and specific conditions applied during sporulation appear to modify the nature of the coat protein cross-linking (Abhyankar et al. 2016) that may have implications on the adhesion capability of the spores to specific gut ecological niches, as they have been shown to be proteinaceaous in nature (Fig. 1). It is likely that the manufacturing process, as well as stresses that probiotic Bacillus spores undergo during feed processing and storage, can directly influence spore survival, germination, and outgrowth in the gut as highlighted for food (Warda et al. 2015). Until now, little has been done in this research area, but the increasingly growing importance of the spore-surface display, which is a powerful technology that can effectively express bioactive molecules on the surface of Bacillus spores, should help to better understand the factors influencing sporulation (Wang et al. 2017). It is suggested that more investigation is done on how the technical sporulation step influences the spore behavior in vivo in the gut and how it affects the overall modes of action and benefits of the probiotic strains.
Finally, little research has been done in vivo in pigs and broilers or in animal in vitro GIT models, which would be necessary in order to develop accurate methods to trace Bacillus spores and vegetative cells along the GIT, to better understand the influence of vegetative cell and spore ratio on beneficial effects along the GIT. The inter-strain variation in sporulation, and germination properties, make the prediction and control of spore behavior challenging as recently demonstrated for 17 strains of B. subtilis including nine isolates from the same origin (Krawczyk et al. 2017). Further research is needed to clearly understand and monitor the life cycle of probiotic Bacillus at strain level that would help in screening and developing new probiotic products with improved benefits for animal.
Acknowledgements
The authors would like to express their thanks to Kirsty Gibbs and Laura Payling from DuPont Industrial Biosciences for review and comments on the manuscript.
Contributor Information
M. Bernardeau, Phone: + 33 (0) 231.565.631 Email: marion.bernardeau@dupont.com
P. Nurminen, Phone: + 358 40 5789 594, Email: paivi.nurminen@dupont.com
References
- Abhyankar WR, Kamphorst K, Swarge BN, van Veen H, van der Wel NN, Brul S, de Koster CG, de Koning LJ. The influence of sporulation conditions on the spore coat protein composition of Bacillus subtilis Spores. Front Microbiol. 2016;7:1636. doi: 10.3389/fmicb.2016.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagherzadeh Kasmani F, Karimi Torshizi MA, Allameh A, Shariatmadari F. A novel aflatoxin-binding Bacillus probiotic: performance, serum biochemistry, and immunological parameters in Japanese quail. Poult Sci. 2012;91:1846–1853. doi: 10.3382/ps.2011-01830. [DOI] [PubMed] [Google Scholar]
- Balassa G, Milhaud P, Raulet E, Silva MT, Sousa JCF. A Bacillus subtilis mutant requiring dipicolinic acid for the development of heat-resistant spores. J Gen Microbiol. 1979;110:365–379. doi: 10.1099/00221287-110-2-365. [DOI] [PubMed] [Google Scholar]
- Barnes AG, Cerovic V, Hobson PS, Klavinskis LS. Bacillus subtilis spores: a novel microparticle adjuvant which can instruct a balanced Th1 and Th2 immune response to specific antigen. Eur J Immunol. 2007;37:1538–1547. doi: 10.1002/eji.200636875. [DOI] [PubMed] [Google Scholar]
- Baweja RB, Zaman MS, Mattoo AR, Sharma K, Tripathi V, Aggarwal A, Dubey GP, Kurupati RK, Ganguli M, Chaudhury NK, Sen S, Das TK, Gade WN, Singh Y. Properties of Bacillus anthracis spores prepared under different environmental conditions. Arch Microbiol. 2008;189:71–79. doi: 10.1007/s00203-007-0295-9. [DOI] [PubMed] [Google Scholar]
- Bernlohr RW, Leitzmann C. Control of sporulation. In: Gould GW, Hurst A, editors. The bacterial spore. London: Academic Press; 1969. pp. 183–213. [Google Scholar]
- Beseres JJ, Lawrence AL, Feller RJ. Practical equivalence of laboratory and field measurements of gut passage time in two penaeid shrimp species. Mar Ecol-Prog Ser. 2006;309:221–231. doi: 10.3354/meps309221. [DOI] [Google Scholar]
- Broeckx G, Vandenheuvel D, Claes IJ, Lebeer S, Kiekens F. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int J Pharm. 2016;505:303–318. doi: 10.1016/j.ijpharm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Cano RJ, Borucki MK. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican Amber. Science. 2012;268:1060–1064. doi: 10.1126/science.7538699. [DOI] [PubMed] [Google Scholar]
- Cartman ST, La Ragione RM, Woodward MJ. Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl Environ Microb. 2008;74:5254–5258. doi: 10.1128/AEM.00580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula G, Cutting SM. Bacillus probiotics: spore germination in the gastrointestinal tract. Appl Environ Microb. 2002;68:2344–2352. doi: 10.1128/AEM.68.5.2344-2352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens S, Boon N, Rajkovic A, Heyndrickx M, Van de Wiele T, Uyttendaele M. Quantification methods for Bacillus cereus vegetative cells and spores in the gastrointestinal environment. J Microbiol Meth. 2010;83:202–210. doi: 10.1016/j.mimet.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Ceuppens S, Uyttendaele M, Drieskens K, Heyndrickx M, Rajkovic A, Boon N, Van de Wiele T. Survival and germination of Bacillus cereus spores without outgrowth or enterotoxin production during in vitro simulation of gastrointestinal transit. Appl Environ Microbiol. 2012;78:7698–7705. doi: 10.1128/AEM.02142-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens S, Uyttendaele M, Drieskens K, Rajkovic A, Boon N, Wiele TV. Survival of Bacillus cereus vegetative cells and spores during in vitro simulation of gastric passage. J Food Prot. 2012;75:690–694. doi: 10.4315/0362-028X.JFP-11-481. [DOI] [PubMed] [Google Scholar]
- Ceuppens S, Uyttendaele M, Hamelink S, Boon N, Van de Wiele T. Inactivation of Bacillus cereus vegetative cells by gastric acid and bile during in vitro gastrointestinal transit. Gut Pathogens. 2012;3:11. doi: 10.1186/1757-4749-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens S, Van de Wiele T, Rajkovic A, Ferrer-Cabaceran T, Heyndrickx M, Boon N, Uyttendaele M. Impact of intestinal microbiota and gastrointestinal conditions on the in vitro survival and growth of Bacillus cereus. Int J Food Microbiol. 2012;155:241–246. doi: 10.1016/j.ijfoodmicro.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Cheng BC, Wan CX, Yang SL, Yu HY, Wei H, Liu JS, Tian WH, Zeng M. Detoxification of deoxynivalenol by Bacillus strains. J Food Saf. 2010;30:599–614. [Google Scholar]
- Ciffo F, Dacarro C, Giovanetti M, Mazza PG. Gastric resistance of Bacillus subtilis spores used in oral bacteriotherapy: in vitro studies. Farmacia Terapia. 1987;4:163–169. [Google Scholar]
- Clavel T, Carlin F, Lairon D, Nguyen-The C, Schmitt P. Survival of Bacillus cereus spores and vegetative cells in acid media simulating human stomach. J Appl Microbiol. 2004;97:214–219. doi: 10.1111/j.1365-2672.2004.02292.x. [DOI] [PubMed] [Google Scholar]
- Clements LD, Miller BS, Streips UN. Comparative growth analysis of the facultative anaerobes Bacillus subtilis, Bacillus licheniformis, and Escherichia coli. Syst Appl Microbiol. 2001;25:284–286. doi: 10.1078/0723-2020-00108. [DOI] [PubMed] [Google Scholar]
- Cutting SM. Bacillus probiotics. Food Microbiol. 2011;28(2):214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Döll S. A probiotic feed additive containing spores of Bacillus subtilis and B. licheniformis does not prevent absorption and toxic effects of the Fusarium toxin deoxynivalenol in piglets. Food Chem Toxicol. 2010;48:152–158. doi: 10.1016/j.fct.2009.09.032. [DOI] [PubMed] [Google Scholar]
- de Souza RD, Batista MT, Luiz WB, Cavalcante RCM, Amorim JH, Pereira Bizerra RS, Gimens Martins E, de Souza Ferreira LC. Bacillus subtilis spores as vaccine adjuvants: further insights into the mechanisms of action. PLoS ONE. 2014;9:e87454. doi: 10.1371/journal.pone.0087454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc le H, Hong HA, Cutting SM. Germination of the spore in the gastrointestinal tract provides a novel route for heterologous antigen delivery. Vaccine. 2003;21:4215–4224. doi: 10.1016/S0264-410X(03)00492-4. [DOI] [PubMed] [Google Scholar]
- Duc le LH, Hong HA, Fairweather N, Ricca E, Cutting SM. Bacterial spores as vaccine vehicles. Infect Immun. 2003;71:2810–2818. doi: 10.1128/IAI.71.5.2810-2818.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc le H, Hong HA, Barbosa TM, Henriques AO, Cutting SM. Characterization of Bacillus probiotics available for human use. Appl Environ Microb. 2004;70:2161–2171. doi: 10.1128/AEM.70.4.2161-2171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc le H, Hong HA, Uyen NQ, Cutting SM. Intracellular fate and immunogenicity of B. subtilis spores. Vaccine. 2004;22:1873–1885. doi: 10.1016/j.vaccine.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Fan Y, Zhao L, Ma Q, Li X, Shi H, Zhou T, Zhang J, Ji C. Effects of Bacillus subtilis ANSB060 on growth performance, meat quality and aflatoxin residues in broilers fed moldy peanut meal naturally contaminated with aflatoxins. Food Chem Toxicol. 2013;59:748–753. doi: 10.1016/j.fct.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Ghelardi E, Celandroni F, Salvetti S, Gueye SA, Lupetti A, Senesi S. Survival and persistence of Bacillus clausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J Appl Microbiol. 2015;119:552–559. doi: 10.1111/jam.12848. [DOI] [PubMed] [Google Scholar]
- González-Pastor JE. Multicellularity and social behaviour in Bacillus subtilis. In: Graumann P, editor. Bacillus: cellular and molecular biology. 2. Norfolk: University of Freiburg, Caister Academic Press; 2012. pp. 351–376. [Google Scholar]
- Grossman AD, Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci USA. 1988;85:4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harimawan A, Zhong S, Lim CT, Ting YP. Adhesion of B. subtilis spores and vegetative cells onto stainless steel-DLVO theories and AFM spectroscopy. J Colloid Interf Sci. 2013;405:233–241. doi: 10.1016/j.jcis.2013.05.031. [DOI] [PubMed] [Google Scholar]
- Hatanaka M, Nakamura Y, Maathuis AJ, Venema K, Murota I, Yamamoto N. Influence of Bacillus subtilis C-3102 on microbiota in a dynamic in vitro model of the gastrointestinal tract simulating human conditions. Benef Microbes. 2012;3:229–236. doi: 10.3920/BM2012.0016. [DOI] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Berni Canani R, Flint HJ, Salminen S, Calder PC, Sanders ME. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Hoa TT, Duc LH, Isticato R, Baccigalupi L, Ricca E, Van PH, Cutting SM. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl Environ Microb. 2001;67:3819–3823. doi: 10.1128/AEM.67.9.3819-3823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HA, To E, Fakhry S, Baccigalupi L, Ricca E, Cutting SM. Defining the natural habitat of Bacillus spore-formers. Res Microbiol. 2009;160:375–379. doi: 10.1016/j.resmic.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Ametani A, Kiuchi K, Kaminogawa S. Changes in fecal microflora induced by intubation of mice with Bacillus subtilis (natto) spores are dependent upon dietary components. Can J Microbiol. 1999;45:59–66. doi: 10.1139/w98-206. [DOI] [PubMed] [Google Scholar]
- Huang JM, La Ragione RM, Cooley WA, Todryk S, Cutting SM. Cytoplasmic delivery of antigens, by Bacillus subtilis enhances Th1 responses. Vaccine. 2008;26:6043–6052. doi: 10.1016/j.vaccine.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Huq T, Khan A, Khan RA, Riedl B, Lacroix M. Encapsulation of probiotic bacteria in biopolymeric system. Crit Rev Food Sci Nutr. 2013;53:909–916. doi: 10.1080/10408398.2011.573152. [DOI] [PubMed] [Google Scholar]
- Hutchison EA, Miller DA, Angert ER. Sporulation in bacteria: beyond the standard model. Microbiol Spectr. 2014 doi: 10.1128/microbiolspec.TBS-0013-2012. [DOI] [PubMed] [Google Scholar]
- Hyronimus B, Le Marrec C, Sassi AH, Deschamps A. Acid and bile tolerance of spore-forming lactic acid bacteria. Int J Food Microbiol. 2000;61:193–197. doi: 10.1016/S0168-1605(00)00366-4. [DOI] [PubMed] [Google Scholar]
- Jadamus A, Vahjen W, Simon O. Growth behaviour of a spore forming probiotic strain in the gastrointestinal tract of broiler chicken and piglets. Arch Tierernahr. 2001;54:1–17. doi: 10.1080/17450390109381962. [DOI] [PubMed] [Google Scholar]
- Jung JH, Lee MY, Chang HC. Evaluation of the probiotic potential of Bacillus polyfermenticus CJ6 isolated from Meju, a Korean soybean fermentation starter. J Microbiol Biotechn. 2012;22:1510–1517. doi: 10.4014/jmb.1205.05049. [DOI] [PubMed] [Google Scholar]
- Karlovsky P. Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl Microbiol Biotechnol. 2011;91:491–504. doi: 10.1007/s00253-011-3401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk AO, de Jong A, Omony J, Holsappel S, Wells-Bennik MH, Kuipers OP, Eijlander RT (2017). Spore heat-activation requirements and germination responses correlate with sequences of germinant receptors and with the presence of a specific spoVA2mob operon in food-borne strains of Bacillus subtilis. Appl Environ Microbiol 27. pii: AEM.03122-16 [DOI] [PMC free article] [PubMed]
- Latorre JD, Hernandez-Velasco X, Kallapura G, Menconi A, Pumford NR, Morgan MJ, Layton SL, Bielke LR, Hargis BM, Téllez G. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult Sci. 2014;93:1793–1800. doi: 10.3382/ps.2013-03809. [DOI] [PubMed] [Google Scholar]
- Lee KW, Lillehoj HS, Jang SI, Li G, Lee SH, Lillehoj EP, Siragusa GR. Effect of Bacillus-based direct-fed microbials on Eimeria maxima infection in broiler chickens. Comp Immunol Microb. 2010;33:e105–e110. doi: 10.1016/j.cimid.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Lee KW, Lillehoj HS, Jang SI, Lee SH. Effects of salinomycin and Bacillus subtilis on growth performance and immune responses in broiler chickens. Res Vet Sci. 2014;97:304–308. doi: 10.1016/j.rvsc.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Leser TD, Knarreborg A, Worm J. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J Appl Microbiol. 2008;104:1025–1033. doi: 10.1111/j.1365-2672.2007.03633.x. [DOI] [PubMed] [Google Scholar]
- Li XZ, Zhu C, Lange CFM, Zhou T, He J, Yu H, Gong J, Young JC. Efficacy of detoxification of deoxynivalenol-contaminated corn by Bacillus sp. LS100 in reducing the adverse effects of themycotoxin on swine growth performance. Food Addit Contam. 2011;28:894–901. doi: 10.1080/19440049.2011.576402. [DOI] [PubMed] [Google Scholar]
- Liang J, Fu J, Kang H, Lin J, Yu Q, Yang Q. The stimulatory effect of TLRs ligands on maturation of chicken bone marrow-derived dendritic cells. Vet Immunol Immunopathol. 2013;155:205–210. doi: 10.1016/j.vetimm.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Maathuis AJ, Keller D, Farmer S. Survival and metabolic activity of the GanedenBC30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Benef Microbes. 2010;1:31–36. doi: 10.3920/BM2009.0009. [DOI] [PubMed] [Google Scholar]
- Mitropoulou G, Nedovic V, Goyal A, Kourkoutas Y. Immobilization technologies in probiotic food production. J Nutr Metab. 2013;2013:716861. doi: 10.1155/2013/716861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondol MA, Shin HJ, Islam MT. Diversity of secondary metabolites from marine Bacillus species: chemistry and biological activity. Mar Drugs. 2013;11:2846–28472. doi: 10.3390/md11082846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki H, Koide R, Yoshikawa R, Warabino Y, Yamamoto H. Adsorption of rare earth ions onto the cell walls of wild-type and lipoteichoic acid-defective strains of Bacillus subtilis. Appl Microbiol Biotechnol. 2013;97:3721–3728. doi: 10.1007/s00253-012-4200-3. [DOI] [PubMed] [Google Scholar]
- Narula J, Fujita M, Igoshin OA. Functional requirements of cellular differentiation: lessons from Bacillus subtilis. Curr Opin Microbiol. 2016;34:38–46. doi: 10.1016/j.mib.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Truong DH, Kouhoundé S, Ly S, Razafindralambo H, Delvigne F (2016) Biochemical engineering approaches for increasing viability and functionality of probiotic bacteria. Int J Mol Sci 17: pii: E867 [DOI] [PMC free article] [PubMed]
- Opara EC. Applications of cell microencapsulation. Methods Mol Biol. 2017;1479:23–39. doi: 10.1007/978-1-4939-6364-5_2. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Yokota H, Kimura M, Mitsuoka T. Effects of administration of Bacillus subtilis strain BN on intestinal flora of weanling piglets. Nihon Juigaku Zasshi. 1981;43:771–775. doi: 10.1292/jvms1939.43.771. [DOI] [PubMed] [Google Scholar]
- Pedraza-Reyes M, Ramírez-Ramírez N, Vidales-Rodríguez LE, Robleto EA. Chapter 6: mechanisms of bacterial spore survival. In: Abel-Santos E, editor. Bacterial spores: current research and applications. Norfolk: Caister Academic Press; 2012. p. 282. [Google Scholar]
- Permpoonpattana P, Hong HA, Khaneja R, Cutting SM. Evaluation of Bacillus subtilis strains as probiotics and their potential as a food ingredient. Benef Microbes. 2012;3:127–135. doi: 10.3920/BM2012.0002. [DOI] [PubMed] [Google Scholar]
- Petchkongkaew A, Taillandier P, Gasaluck P, Lebrihi A. Isolation of Bacillus spp. from Thai fermented soybean (Thua-nao): screening for aflatoxin B-1 and ochratoxin A detoxification. J Appl Microbiol. 2008;104:1495–1502. doi: 10.1111/j.1365-2672.2007.03700.x. [DOI] [PubMed] [Google Scholar]
- Piggot PJ, Coote JG. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot PJ, Hilbert DW. Sporulation of Bacillus subtilis. Curr Opin Microbiol. 2004;7:579–586. doi: 10.1016/j.mib.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Riaz QU, Masud T. Recent trends and applications of encapsulating materials for probiotic stability. Crit Rev Food Sci Nutr 53:231-44 Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL (2004) Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2013;172:1118–1124. doi: 10.4049/jimmunol.172.2.1118. [DOI] [PubMed] [Google Scholar]
- Sánchez B, Arias S, Chaignepain S, Denayrolles M, Schmitter JM, Bressollier P, Urdaci MC. Identification of surface proteins involved in the adhesion of a probiotic Bacillus cereus strain to mucin and fibronectin. Microbiology. 2009;155:1708–1716. doi: 10.1099/mic.0.025288-0. [DOI] [PubMed] [Google Scholar]
- Sansinenea E, Ortiz A. Secondary metabolites of soil Bacillus spp. Biotechnol Lett. 2011;33:1523–1538. doi: 10.1007/s10529-011-0617-5. [DOI] [PubMed] [Google Scholar]
- Santoso U, Tanaka K, Ohtania S. Effect of fermented product from Bacillus subtilis on feed efficiency, lipid accumulation and ammonia production in broiler chicks. Asian Aust J Anim Sci. 2001;14:333–337. doi: 10.5713/ajas.2001.333. [DOI] [Google Scholar]
- Schaeffer P, Millet J, Aubert JP. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallmey M, Singh A, Ward OP. Developments in the use of Bacillus species for industrial production. Can J Microbiol. 2004;50:1–17. doi: 10.1139/w03-076. [DOI] [PubMed] [Google Scholar]
- Scharek-Tedin L, Pieper R, Vahjen W, Tedin K, Neumann K, Zentek J. Bacillus cereus var. Toyoi modulates the immune reaction and reduces the occurrence of diarrhea in piglets challenged with Salmonella Typhimurium DT104. J Anim Sci. 2013;91:5696–5704. doi: 10.2527/jas.2013-6382. [DOI] [PubMed] [Google Scholar]
- Schierack P, Wieler LH, Taras D, Herwig V, Tachu B, Hlinak A, Schmidt MF, Scharek L. Bacillus cereus var. toyoi enhanced systemic immune response in piglets. Vet Immunol Immunopathol. 2007;118:1–11. doi: 10.1016/j.vetimm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Setlow P. Spore germination. Curr Opin Microbiol. 2003;6:550–556. doi: 10.1016/j.mib.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Setlow P. Germination of spores of Bacillus species: what we know and do not know. J Bacteriol. 2014;196:1297–1305. doi: 10.1128/JB.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirec T, Cangiano G, Baccigalupi L, Ricca E, Isticato R. The spore surface of intestinal isolates of Bacillus subtilis. FEMS Microbiol Lett. 2014;358:194–201. doi: 10.1111/1574-6968.12538. [DOI] [PubMed] [Google Scholar]
- Song M, Hong HA, Huang JM, Colenutt C, Khang DD, Nguyen TV, Park SM, Shim BS, Song HH, Cheon IS, Jang JE, Choi JA, Choi YK, Stadler K, Cutting SM. Killed Bacillus subtilis spores as a mucosal adjuvant for an H5N1 vaccine. Vaccine. 2012;30:3266–3277. doi: 10.1016/j.vaccine.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Spinosa MR, Braccini T, Ricca E, De Felice M, Morelli L, Pozzi G, Oggioni MR. On the fate of ingested Bacillus spores. Res Microbiol. 2000;151:361–368. doi: 10.1016/S0923-2508(00)00159-5. [DOI] [PubMed] [Google Scholar]
- Swick MC, Koehler TM, Driks A. Surviving between hosts: sporulation and transmission. Microbiol Spectr. 2016 doi: 10.1128/microbiolspec.VMBF-0029-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam NK, Uyen NQ, Hong HA, Duc le H, Hoa TT, Serra CR, Henriques AO, Cutting SM. The intestinal life cycle of Bacillus subtilis and close relatives. J Bacteriol. 2006;188:2692–2700. doi: 10.1128/JB.188.7.2692-2700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinyiro SE, Wokadala C, Xu D, Yao W. Adsorption and degradation of zearalenone by Bacillus strains. Folia Microbiol (Praha) 2011;56:321–327. doi: 10.1007/s12223-011-0047-8. [DOI] [PubMed] [Google Scholar]
- Uyen NQ, Hong HA, Cutting SM. Enhanced immunisation and expression strategies using bacterial spores as heat-stable vaccine delivery vehicles. Vaccine. 2007;25:356–365. doi: 10.1016/j.vaccine.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang Y, Yang R. Recent progress in Bacillus subtilis spore-surface display: concept, progress, and future. Appl Microbiol Biotechnol. 2017;101:933–949. doi: 10.1007/s00253-016-8080-9. [DOI] [PubMed] [Google Scholar]
- Warda AK, den Besten HMW, Sha N, Abee T, Nierop Groot MN. Influence of food matrix on outgrowth heterogeneity of heat damaged Bacillus cereus spores. Int J Food Microbiol. 2015;201:27–34. doi: 10.1016/j.ijfoodmicro.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Wells-Bennik MH, Eijlander RT, den Besten HM, Berendsen EM, Warda AK, Krawczyk AO, Nierop Groot MN, Xiao Y, Zwietering MH, Kuipers OP, Abee T. Bacterial spores in food: survival, emergence, and outgrowth. Annu Rev Food Sci Technol. 2016;7:457–482. doi: 10.1146/annurev-food-041715-033144. [DOI] [PubMed] [Google Scholar]
- Wilcks A, Smidt L, Bahl MI, Hansen BM, Andrup L, Hendriksen NB, Licht TR. Germination and conjugation of Bacillus thuringiensis subsp. israelensis in the intestine of gnotobiotic rats. J Appl Microbiol. 2008;104:1252–1259. doi: 10.1111/j.1365-2672.2007.03657.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Peng Y, Wu S, Sun L, Huang E, Huang T, Xu L, Wu C, Gelbič I, Guan X. Microbial ecology and association of Bacillus thuringiensis in chicken feces originating from feed. Curr Microbiol. 2012;65:784–791. doi: 10.1007/s00284-012-0231-3. [DOI] [PubMed] [Google Scholar]
- Ziaei-Nejad S, Habibi Rezaei M, Azari Takami G, Lovett DL, Mirvaghefi AR, Shakouri M. The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture. 2006;252:516–524. doi: 10.1016/j.aquaculture.2005.07.021. [DOI] [Google Scholar]


