Abstract
Antioxidant activity of Chlorella (Chlorella vulgaris) was evaluated in virgin olive oil (VOO) at different concentrations of 0.5, 1.0, and 1.5% (w/w) under accelerated storage conditions. Antioxidant activity of Chlorella was compared with those of BHT and β-carotene. Chlorella samples significantly retarded the formation of primary, secondary, and total oxidation products in comparison with those of the control. The stability increased as concentrations of Chlorella increased. Samples containing 0.5, 1.0, and 1.5% Chlorella significantly improved VOO stability by 19.99, 28.83, and 33.14%, respectively. Observed effects can be related to the release in the assortment of bioactive compounds from Chlorella algae to the VOO. Among the different antioxidants evaluatedy, BHT exhibited the highest antioxidant activity. On the contrary, β-carotene had no preventive effect against the oxidation of VOO. It also proved incapable of limiting the progress of VOO oxidation and played role as pro-oxidant. In conclusion, Chlorella enhanced VOO oxidative stability. Thus it can be considered as a promising source of natural antioxidants.
Keywords: Chlorella vulgaris, Natural antioxidant, Oxidative stability, Virgin olive oil
Introduction
Virgin olive oil (VOO) is a highly appreciated vegetable oil from a nutritional standpoint. Its annual global production was 2.83 million tons in 2013 (FAOSTAT 2013). VOO is obtained from the olive fruit only by the application of physical processes and treatments. Accordingly, the pleasant taste and aroma are intrinsic to VOO. VOO is mainly characterized by the abundant presence of minor natural antioxidants and high amounts of monounsaturated fatty acids (MUFAs). Consequently, these components generate more stability in the VOO and help reduce oxidation (Frankel 2010). Despite its distinctive features, VOO may be oxidized due to its polyunsaturated fatty acids (PUFAs) composition (Morales and Przybylski 2013). When exhibiting a progressive manner, oxidation decreases the shelf life and nutritional value through PUFA oxidation. It changes the color of food, deteriorates its quality (Wanasundara and Shahidi 2005; Shahidi and Ambigaipalan 2015), and develops relatively high levels of rancidity, unpleasantness, and undesirable flavors in the VOO (Frankel 2010).
The most widely used synthetic antioxidants are butylated hydroxyanisol (BHA) and butylated hydroxytoluene (BHT). Currently, the use of these synthetic antioxidants has been brought to limits due to their potential carcinogenic and toxic effects (Namiki 1990). To overcome this limitation and to fulfill consumer demands, natural antioxidants are commonly safer alternatives that can prolong the shelf life of foods containing lipids. Their applications can become more extensive than synthetic antioxidants. The use of natural antioxidant compounds from different sources is currently growing to become an attractive subject for original research (Yanishlieva and Marinova 2001).
Chlorella (Chlorella vulgaris) is an edible green microalga. Its annual production is 2000 tons (of dry weight) in the global industry. The main producers of Chlorella are Japan, Germany, and Taiwan (Safi et al. 2014). A wide range of investigations have already demonstrated that Chlorella has various in vitro and in vivo biological functions including anti-proliferative qualities (Wu et al. 2005), anti-cancer qualities (Mohd Azamai et al. 2009) and antioxidant potentials (Vijayavel et al. 2007). Chlorella is composed of a vast spectrum of chemicals. It produces a rich cornucopia of favorable proteins which are comparable to the high quality of plant proteins. It synthesizes lipids containing essential fatty acids, polysaccharides, notable levels of pigments, sterols, minerals, and vitamins C and E (Safi et al. 2014; Plaza et al. 2012). Chlorella contains bioactive substances comprising phenolic compounds, carotenoids and chlorophyll (Plaza et al. 2012; Cha et al. 2010), which are known for their considerable antioxidant activities, and it acts as in vitro agents that serves as scavenger of free radicals (Vijayavel et al. 2007), metal chelator, and reducing agent (Hajimahmoodi et al. 2010; Wang et al. 2010). Accordingly, Chlorella can be regarded as a promising source by which natural antioxidants can be produced safely. To establish various functions for the microalga, some studies have referred to the involvement of Chlorella and its components in a variety of food products. Parallel to that end, Gouveia et al. (2006) prepared and experimented mayonnaises by using Chlorella in the medium with the purpose of studying antioxidant activity. They found that the addition of microalga can be a way to improve the stability of food emulsions, besides being a coloring agent. Gouveia et al. (2007) evaluated the stability of colored soybean oil with carotenoids extracted from Chlorella and reported that those carotenoids in the oil can be maintained well over time.
The main objective of this study was to evaluate the effects of different Chlorella concentrations on the oxidative stability of VOO. Likewise, antioxidant activities of Chlorella were compared to those of β-carotene and BHT.
Materials and methods
Chemicals
Gallic acid, ascorbic acid, citric acid, quercetin, β-carotene, BHT, Folin–Ciocalteu’s phenol reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and p-anisidine (4-Methoxyaniline) were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). All solvents were of analytical or chromatography grade and were purchased from Merck Company (Darmstadt, Germany).
Chlorella
Spray-dried Chlorella packed in the absence of oxygen was purchased from Lifestream International Ltd. (Aukland, New Zealand) and were stored under dry and dark conditions until further use. The chemical composition of Chlorella was also determined according to the Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC 1997).
Antioxidant properties of Chlorella
To determine the bioactive compounds in Chlorella and its antioxidant properties, the methanolic extract of Chlorella was prepared which consisted of several consecutive steps as follows: five grams of Chlorella ground powder was added to a 25 ml methanol, shaken for 2 min, and was centrifuged for 5 min at 5000 rpm. Then, the supernatant was filtered through Whatman No.1 filter paper. The extraction procedure was repeated on the residue twice, and each supernatant was filtered through filter paper. Finally, the collected supernatants were brought to a 100 ml volume by methanol. The extract was immediately stored at 4 °C prior to further analysis.
Total phenolic content (TPC) of Chlorella extract (10 mg/ml) was measured according to the method described by Habibi et al. (2016). Gallic acid solutions at concentrations of 0.02–0.10 mg/ml were used to obtain a calibration curve. TPC was expressed as mg gallic acid equivalent (GAE) per g Chlorella (mg GAE/g). Total flavonoid content (TFC) of Chlorella extract (10 mg/ml) was determined using the method of Di Marco et al. (2014). The TFC was expressed as mg quercetin equivalent (QE) per g Chlorella by using a calibration curve in 0.02–0.10 mg/ml quercetin concentration range. Carotenoid and chlorophyll contents of Chlorella were measured according to the method described by Dere et al. (1998) and results were expressed as mg per g Chlorella.
Antiradical and cupric ion reducing antioxidant capacities (CUPRAC) of Chlorella extract were analyzed. The ability of Chlorella extract was evaluated at a series of concentrations (0.01–1.00 mg/ml) to scavenge the stable DPPH radical according to the method described by Shalaby and Shanab (2013). In this assay, BHT solutions (0.01–0.10 mg/ml) were used as the positive control. The results were given in the form of IC50 value, which was defined as the extract concentration needed to scavenge 50% of DPPH radicals. The IC50 value was expressed in terms of mg/ml. Furthermore, the relative IC50 equivalent to the ratio percentage of IC50 value of BHT to IC50 value of Chlorella was estimated. CUPRAC of Chlorella extract (1 mg/ml) was determined based on the procedure described by Apak et al. (2004). The calibration curve was constructed with ascorbic acid at various concentrations (0.01–0.10 mg/ml). CUPRAC was expressed as mg ascorbic acid equivalent per gram of Chlorella.
Fatty acid composition of Chlorella
Chlorella fatty acids were converted to fatty acid methyl esters (FAMEs) according to the method described by Golmakani et al. (2012a). Gas chromatography (GC) of FAMEs was carried out using a gas chromatograph apparatus (SP-3420 A, Beijing, China), equipped with a flame ionization detector (FID), and a BPX-70 fused silica capillary column (30 m × 0.25 mm, 0.25 µm film thickness). Nitrogen was used as a carrier gas. The injected volume was 1 µl and split ratio was 1:10. Injector and detector temperatures were set at 250 and 300 °C, respectively. The oven temperature was programmed from 140 to 200 °C as follows: the initial oven temperature was kept at 140 °C for 5 min. It was then increased to 180 °C at a rate of 20 °C/min and held for 9 min, followed by a ramp to 200 °C at a rate of 20 °C/min and was then maintained at 200 °C for 3 min. Fatty acids were identified by comparing their retention times with those of respective standards. The results are expressed as percentage of relative peak area.
VOO
VOO was provided from the Edible Oil Industries Group of Etka Organization. The VOO was stored in a dark bottle without headspace at 4 °C until further analysis was due
Initial characteristics of VOO
Free fatty acids (Ca 5a–40), peroxide value (PV; Cd 8–53), para-anisidine value (AV; Cd 18–90), and specific extinction coefficients at 232 nm (K232) and 268 nm (K268) (Ch 5–91) were measured following the Official Methods of the American Oil Chemists’ Society (AOCS 1998). Totox value (TV) was calculated as 2PV + AV. Carotenoid and chlorophyll contents in VOO (Minguez-Mosquera et al. 1991) were measured and the results were expressed in terms of mg lutein and pheophytin-a per kg of VOO, respectively. TPC of VOO was determined by the method described by Polavarapu et al. (2011). The calibration curve of TPC was constructed with gallic acid solutions at concentrations of 0.02–0.10 mg/ml. TPC was expressed as mg GAE/g. Refractive index (Tp 1a–64) and specific gravity (To 1a–64) were determined according to the Official Methods of the American Oil Chemists’ Society (AOCS 1998).
Fatty acid composition of VOO
VOO fatty acids were esterified according to the method of Golmakani et al. (2012b). GC of FAMEs was carried out based on the same technical features and settings of gas chromatograph apparatus which was formerly mentioned for fatty acid composition of Chlorella. Results are expressed as the percentage for relative peak area of total FAMEs.
Oxidative stability of VOO
Chlorella was finely crushed by using a mill grinder (MJW176P, Matsushita Electric Industrial Company, Ltd. Osaka, Japan). Thereafter, Chlorella was added to the VOO at concentrations of 0.5, 1.0, and 1.5% (w/w), then being dissolved and dispersed by an ultrasonic processor (Ultrasonic homogenizer equipped with a cylindrical microprobe, GmbH, Berlin, Germany) of 50 W ultrasonic power, 10 s of interval time, 20 s of ultrasonic time, and 10 min of total working time at 25 °C. β-Carotene and BHT samples were prepared at concentrations of 0.01% (w/w). All samples (even the control) contained 0.005% (w/w) citric acid.
Samples (50 ml per each sample) were kept in open amber glass bottles (75 ml capacity and 3 cm internal diameter). The heating process was carried out in an incubator (Memmert GmbH + Co.KG, Schwabach, Germany) set at 70 ± 1 °C for 42 days and samples were placed in darkness. To evaluate oxidative stability of samples, known methods for measuring oxidation levels such as PV, AV, TV, K232, and K268 were monitored every week. In addition to that, carotenoid and chlorophyll contents were measured. Indices of induction period (IP), protection factor (PF), antioxidant power (AOP), antioxidant activity (AA), and the improved oxidative stability (IOS) were characterized by valued measurements. IPs of samples were calculated as the number of days necessary to reach an established maximum PV level for VOO, namely 20 meq/kg (IOC 2015). PF is equivalent to the ratio of IPsample to IPcontrol in a normative sense. AOP (Silva et al. 2001), AA, and IOS (%) were calculated according to the following equations:
| 1 |
| 2 |
| 3 |
Statistical analysis
The experiments and analyses were performed in triplicate. Data were presented as mean values with ±standard deviation. A general linear model (GLM) procedure from Statistical Analysis Software (SAS) version 9.1 (SAS Institute Inc., Cary, NC) was applied to compare the mean values at p < 0.05. Multiple comparisons of mean values were carried out by using the Duncan’s multiple range tests.
Results and discussion
Chlorella
Chlorella showed 64.20% protein, 13.21% fat, 10.70% carbohydrate, 7.22% total ash, and 4.67% moisture. It was predominantly rich in protein but had low moisture content.
Antioxidant properties of Chlorella
TPC of Chlorella extract was found to be 23.74 ± 1.50 mg GAE/g. Similar to our results, Hajimahmoodi et al. (2010) evaluated antioxidant properties of Chlorella and reported TPC of 19.46 mg GAE/g in extracellular substance extracts of Chlorella. They elucidated antioxidant capacities of microalgae characterized by their phenolic compounds. Cha et al. (2010) studied bioactive compounds extracted from Chlorella and detected TPC of 15 mg GAE/g.
Extract of Chlorella exhibited the presence of flavonoid compounds, providing TFC of 9.16 ± 0.10 mg QE/g. Similarly, Wang et al. (2010) showed that the TFC of Chlorella as 3.18 mg QE/g.
In this experiment, the initial carotenoid and chlorophyll contents of Chlorella were 2.7 and 32.6 mg/g dry weight, respectively. Chlorophyll was known to be the major pigment in Chlorella (Safi et al. 2014).
The IC50 value of Chlorella extract was 0.745 ± 0.023 mg/ml, indicating the ability of Chlorella in scavenging free radicals.
The protective behavior of Chlorella against DPPH radicals depended on the assortment of bioactive components such as phenolic compounds and pigments. When compared with Chlorella, the positive control BHT exhibited higher scavenging activity, with an IC50 value of 0.0363 ± 0.002 mg/ml. More specifically, the IC50 value of Chlorella extract accounted comparatively to be only 4.87% of the IC50 value for BHT.
In the CUPRAC method, hydrophilic antioxidants are also measured (Apak et al. 2004). Chlorella extract possessed a reductive capacity of 29.42 ± 0.77 mg ascorbic acid equivalent/g Chlorella. In the presence of a reducing agent, here Chlorella, the cupric ion was reduced. This result can confirm the reducing power of natural components in Chlorella.
Fatty acid composition of Chlorella
Palmitic acid (28.02%), α-linolenic acid (24.95%), and linoleic acid (15.11%) were quantified as the major fatty acids in Chlorella (Table 1). Plaza et al. (2012) evaluated fatty acids in extracts of Chlorella obtained from different conditions. They identified palmitic acid (11.74–28.87%), α-linolenic acid (13.37–21.36%), and linoleic acid (11.62–20.65%) as the main fatty acids. In this study, Chlorella was observed to be rich in SFA (52.94%) and PUFA (40.06%). Negligible amounts of PUFAs (0.026–0.079%) were liberated from different concentrations of Chlorella (0.5–1.5%) into the VOO. Therefore, PUFA content VOO did not change significantly with the addition of Chlorella.
Table 1.
Fatty acid composition of Chlorella and virgin olive oil
| Fatty acids | Relative peak area (%) | |
|---|---|---|
| Chlorella | Virgin olive oil | |
| Myristic acid (C14:0) | 4.16 ± 0.49* | ND** |
| Palmitic acid (C16:0) | 28.02 ± 1.63 | 17.14 ± 0.05 |
| Margaric acid (C17:0) | 5.92 ± 1.00 | ND** |
| Stearic acid (C18:0) | 14.85 ± 0.02 | 0.42 ± 0.16 |
| Oleic acid (C18:1 ω-9) | 7.01 ± 0.22 | 75.44 ± 3.77 |
| Linoleic acid (C18:2 ω-6) | 15.11 ± 1.34 | 5.71 ± 3.03 |
| α-Linolenic acid (C18:3 ω-3) | 24.95 ± 0.45 | 1.29 ± 0.53 |
| ∑ Saturated fatty acid (SFA) | 52.94 ± 1.11 | 17.59 ± 0.16 |
| ∑ Monounsaturated acid (MUFA) | 7.01 ± 0.22 | 74.90 ± 2.75 |
| ∑ Polyunsaturated fatty acid (PUFA) | 40.06 ± 0.90 | 7.51 ± 2.60 |
* Mean ± SD (n = 3)
** Not detected
VOO
Initial characteristics of VOO
According to Table 2, physical properties of VOO were within the limits set by Codex Alimentarius Commission (Codex 2001). Also, quality criteria such as free fatty acids, PV, K232, and K268 were within the range established by the International Olive Council (IOC 2015). Therefore, taking into account the limits set by the International Olive Council for olive oil classification, the oil that was used in this study could fall in the virgin category. Carotenoid and chlorophyll contents of VOO were 6.16 and 15.49 mg/kg, respectively. Furthermore, the TPC of VOO was 0.294 mg GAE/g.
Table 2.
Initial characteristics of virgin olive oil
| Parameter | Amount | Standard range |
|---|---|---|
| Refractive index | 1.4706 ± 0.0021* | 1.4677–1.4705** |
| Specific gravity | 0.910 ± 0.000 | 0.910–0.916** |
| Free fatty acid (% oleic acid) | 1.76 ± 0.15 | 2≥*** |
| Peroxide value (meq O2/kg) | 4.18 ± 0.21 | 20≥*** |
| p-Anisidine value (mg/kg) | 3.77 ± 0.05 | |
| Totox value | 12.13 ± 0.05 | |
| K232 | 1.25 ± 0.13 | 2.60≥*** |
| K268 | 0.10 ± 0.00 | 0.25≥*** |
| Total phenolic content (mg gallic acid equivalent/g) | 0.294 ± 0.027 | |
| Carotenoid content (mg/kg) | 6.16 ± 0.04 | |
| Chlorophyll content (mg/kg) | 15.49 ± 0.22 |
Fatty acid composition of VOO
Fatty acid composition met the range established by the International Olive Council (IOC 2015) for VOO category (Table 1). Oleic acid (75.44%), palmitic acid (17.14%) and linoleic acid (5.71%) were the major fatty acids in the VOO. MUFA was the most abundant group of fatty acids in the VOO, contributing to 75.44% of the total fatty acids. PUFA comprised only 7.51% of the total fatty acids.
Oxidative stability of VOO
Carotenoid and chlorophyll contents
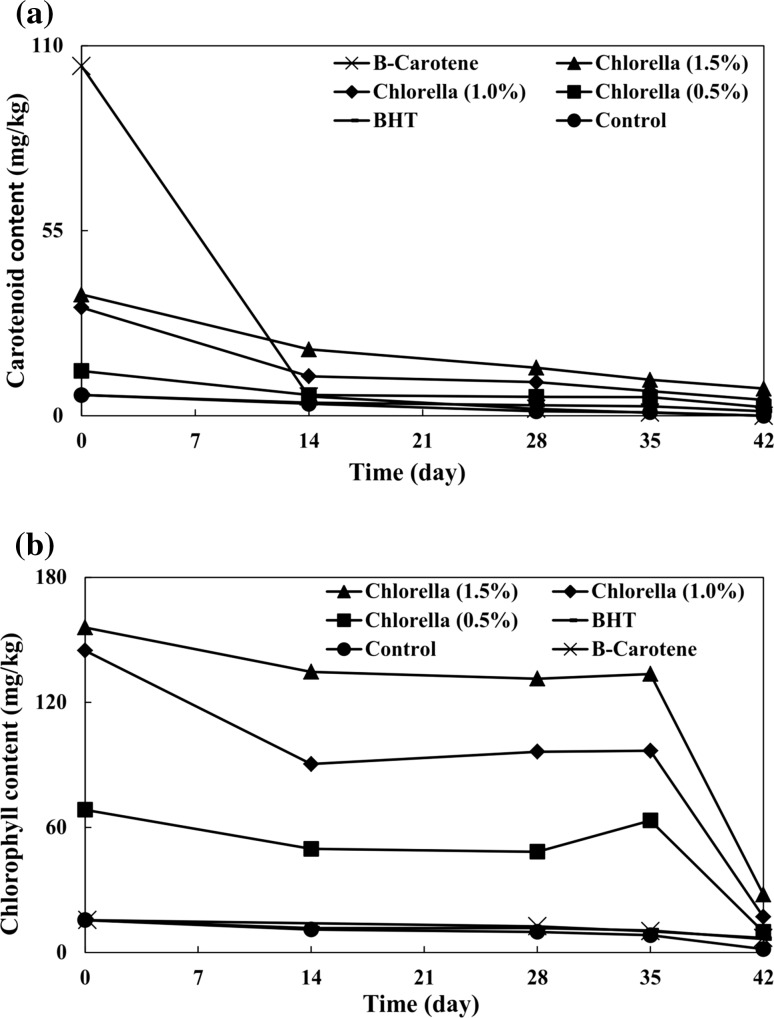
There were significant correlations between chlorophylls, carotenoid contents and antioxidant capacities in the Chlorella (Cha et al. 2010). Initial carotenoid content of the control group was 6.16 mg/kg. The oxidation phenomenon occurred exhaustively for the carotenoid content in the control group, and carotenoids were completely degraded (Fig. 1a). The β-carotene sample had the highest amount of carotenoid (103.38 mg/kg), being 16.78 times higher than the carotenoid content of the control. A rapid decrease in carotenoid content of the β-carotene sample was observed until the content disappeared completely toward the end of the storage period. β-Carotene was proposed to be a pro-oxidant and was decomposed relying upon cumulative actions of temperature, darkness and tense atmospheric oxygen conditions (Lee and Kim 1992). Carotenoid contents of samples containing 0.5, 1.0 and 1.5% Chlorella were 2.16, 5.22 and 5.85 times higher than that of the control, respectively. Carotenoid contents of samples containing 0.5, 1.0 and 1.5% Chlorella were reduced by 80.95, 85.27 and 77.57%, respectively during the storage period, indicating that carotenoid consumption can help protect VOO against oxidation. Gouveia et al. (2007) evaluated the stability of soybean oil with carotenoids extracted from Chlorella that were stored at room temperature (and were protected against light) during 6 weeks in a manner by monitoring total carotenoid content. Their results showed that carotenoids possessed good stability which could be maintained over time. They argued that carotenoids can contribute to soybean oil stability because of their antioxidant properties.
Fig. 1.
Effects of different concentrations of Chlorella on a carotenoid and b chlorophyll contents of virgin olive oil
In this experiment, the carotenoid content of BHT sample was 6.16 mg/kg, which was reduced by 78.08% during the storage period, and ended up being 1.35 mg/kg.
Chlorophyll can behave as an antioxidant agent in darkness (Gutiérrez-Rosales et al. 1992). Initial chlorophyll content of the control sample was 15.49 mg/kg. Through the storage period, the chlorophyll content of the control decreased by 89.67% (Fig. 1b). Chlorella samples were characterized by high proportions of chlorophyll. Initial chlorophyll contents of samples containing 0.5, 1.0 and 1.5% Chlorella were respectively 4.42, 9.35 and 10.07 times higher than that of the control. Generally, all Chlorella samples showed the same trend in their decreasing chlorophyll contents up to 28 days of storage period, then a subsequent increase in the content by day 35 of storage and, finally, another decrease in the chlorophyll content toward the end of storage. Storage at high temperature (70 °C) caused chlorophyll liberation from Chlorella, resulting in higher chlorophyll content during days 28–35 of the storage period. Chlorophyll contents of Chlorella samples were significantly higher than those of other samples throughout the storage period. In β-carotene and BHT samples, chlorophyll contents were reduced by 97.93 and 58.36%, respectively. This difference can be due to the different antioxidant capacities of β-carotene and BHT. In comparison with the control, the highest degradation of chlorophyll was observed in the β-carotene sample, indicating poorer performance of β-carotene during storage.
PV, AV and TV
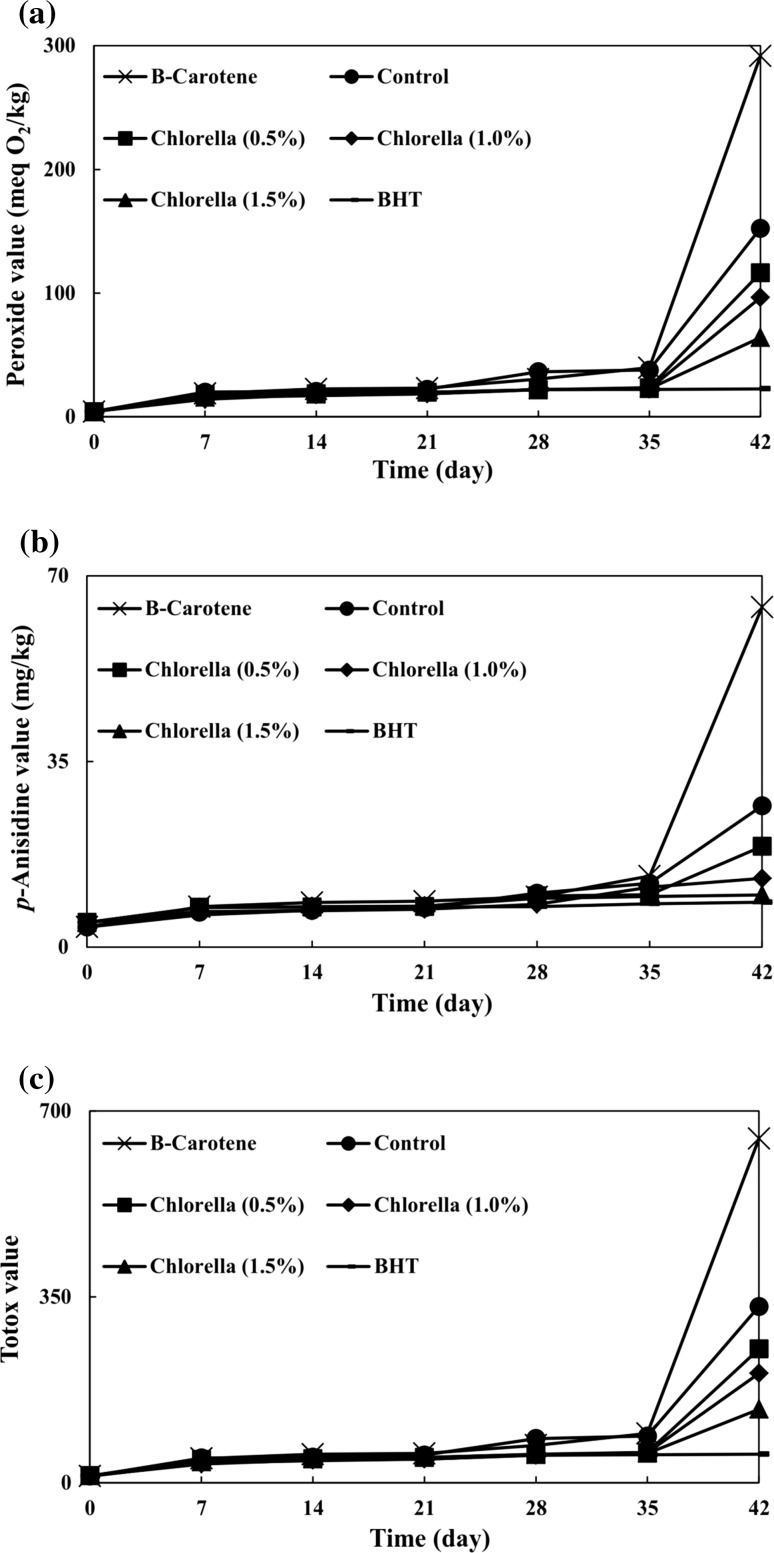
In this study, oxidation was triggered by heating, and oxidative stability was tested by measuring the primary, secondary and total oxidation products. PV increased by increasing the oxidation period in all samples (Fig. 2a). The PV of control sample gradually increased until day 35 of storage. Subsequently, a definite faster increase was observed, and the PV of control sample reached 152.45 meq O2/kg. As far as the heating process is concerned, the PVs of Chlorella samples were significantly lower than that of the control. At the end of the storage period, PVs of different concentrations of Chlorella (0.5, 1.0, and 1.5%) were significantly lower (by 23.67, 36.74, and 57.91%, respectively) than that of the control. This finding can be attributed to favorable roles of bioactive compounds of the Chlorella such as phenolic compounds and pigments that inhibit the formation of hydro-peroxides which are gradually released from Chlorella when being in contact with VOO during storage. Rao et al. (2007) evaluated the antioxidant capacity of astaxanthin extracted from the microalga Haematococcus pluvialis in several edible oils (palm, gingelly, groundnut, rice bran, sunflower, and mustard oils) which were stored at different temperatures (25, 70, and 90 °C). Evaluations were made by determining their PVs. They found that PVs of samples with astaxanthin were 40–50% lower in comparison with the control.
Fig. 2.
Effects of different concentrations of Chlorella on a peroxide value, b para-anisidine value, and c Totox value of virgin olive oil during storage at 70 °C
Higher concentrations of Chlorella resulted in significantly lower hydro-peroxide contents at the end of storage period, indicating the release of higher amounts of bioactive compounds into the VOO. Wang et al. (2012) investigated antioxidant capacity of astaxanthin extracted from H. pluvialis at different concentrations (0.2, 0.6, and 1.0 mg/g) in sunflower oil stored at 60 °C. Here too the antioxidant capacity was examined by determining the PV. Their results showed that higher concentrations of astaxanthin led to higher inhibitory effects on hydro-peroxide formation.
Sample containing β-carotene showed higher PV (291.80 meq O2/kg) than samples containing Chlorella (64.16–116.37 meq O2/kg) and also the control sample (152.45 meq O2/kg) at the end of the storage period.
According to Fig. 2a, the PV of BHT sample reached 22.52 meq O2/kg, and a slow rate of hydro-peroxide formation was detected for BHT sample in comparison with those of other samples. Lima Araújo et al. (2006) compared antioxidant capacities of extracts from cyanobacterium Anabaena PCC 7119 (1000 ppm) with those of BHT (100 and 200 ppm) in soybean oil stored at 63° C by measuring the PV. They observed that PV of Anabaena extract was significantly lower than that of the 200 ppm for BHT, but nonetheless it was comparable to that of the 100 ppm of BHT.
The AVs of all samples increased by a prolonged storage periods (Fig. 2b). The AV of the control increased slowly and linearly until day 35 of the storage. Thereafter, a much faster increase was observed. At the end of the storage period, there were significant differences between the AVs of the control (26.64 mg/kg) and the Chlorella samples (9.84–19.00 mg/kg). It is noteworthy that at this time, the AVs of different concentrations of Chlorella (0.5, 1.0, and 1.5%) were significantly lower (by 28.68, 51.31, and 63.06%, respectively) in comparison with that of the control. Progressive release of Chlorella bioactive compounds was effective in reducing the development of secondary oxidation products. Limón et al. (2015) applied the Rancimat method to study the oxidative stability of extra virgin olive oil (EVOO) containing extracts of the microalga Scenedesmus almeriensis. They reported that S. almeriensis can increase oxidative stability of EVOO from 11.85 to 42.06%. They attributed the improvement of EVOO stability to the chemical composition of S. almeriensis extracts, being rich in β-carotene.
With increase in Chlorella concentration, the AVs of VOO significantly decreased at the end of storage period. Sample containing 1.5% Chlorella had the lowest AV at the end of the assay and showed limited progress in the production of secondary oxidation products. However, even in comparison with lower concentrations of Chlorella, β-carotene showed lower antioxidant capacity. The AV of BHT sample reached 8.46 mg/kg, being statistically similar to that of 1.5% Chlorella.
Similar to PV, TVs of all samples increased during longer oxidation period (Fig. 2c). TV of the control was gradually increased until 35 days of storage. The onwards storage led to a drastic increase in TV. During storage, TVs of different concentrations of Chlorella samples were significantly lower than that of the control. At the end of the storage period, TVs of different concentrations of Chlorella (0.5, 1.0, and 1.5%) were significantly lower (by 24.07, 37.92, and 58.33%, respectively) in comparison with that of the control. Gouveia et al. (2006) evaluated antioxidant effects of green and orange Chlorella in food emulsions through 6 weeks of storage by determining the PV and AV. Their results demonstrated that emulsions show an enhanced resistance to oxidation because of the pigments in Chlorella.
With increase in Chlorella concentration, TVs decreased significantly at the end of storage period. Even though the TV of β-carotene sample was significantly higher than those of different concentrations of Chlorella, yet the TV of BHT sample was significantly lower.
Antioxidant indices
To ascertain whether different antioxidants contributed to the stability of VOO, several indices were estimated (Table 3). Chlorella samples had significantly higher IPs in comparison with the control, indicating a higher efficiency of Chlorella samples. The presence of bioactive compounds therefore helps elucidate the antioxidant potential of Chlorella in VOO. Ayadi et al. (2009) determined IPs of EVOO flavored with rosemary, thyme, lemon, and basil stored at 60 °C and thereby detected higher IPs in EVOOs containing rosemary, thyme, and lemon compared to the control. They attributed this finding to the fact that natural antioxidants such as phenolic compounds, chlorophyll, and carotenoids exude from the medicinal plants and are thus released into the EVOO.
Table 3.
Effects of different concentrations of Chlorella on antioxidant indices of virgin olive oil
| Samples | Induction period (day) | Protection factor | Antioxidant power (%) | Antioxidant activity | Improved oxidative stability (%) |
|---|---|---|---|---|---|
| Control | 16.94 ± 0.12e* | 1.00 ± 0.00e | – | – | – |
| Chlorella (0.5%) | 20.33 ± 0.04d | 1.20 ± 0.00d | 16.66 ± 0.17d | 0.40 ± 0.00b | 19.99 ± 0.24d |
| Chlorella (1.0%) | 21.82 ± 0.04c | 1.29 ± 0.00c | 22.37 ± 0.14c | 0.29 ± 0.00b | 28.83 ± 0.24c |
| Chlorella (1.5%) | 22.55 ± 0.09b | 1.33 ± 0.01b | 24.89 ± 0.30b | 0.22 ± 0.00b | 33.14 ± 0.54b |
| β-Carotene | 16.34 ± 0.02f | 0.96 ± 0.01e | −3.70 ± 0.13e | −3.56 ± 0.12c | −3.56 ± 0.12e |
| BHT | 29.07 ± 0.49a | 1.72 ± 0.03a | 41.71 ± 0.99a | 71.59 ± 2.89a | 71.59 ± 2.89a |
* Mean ± SD (n = 3). Means with different letters in each column are significantly different (p < 0.05)
The IP value of VOO increased significantly by higher Chlorella concentrations, which can be due to the higher release of bioactive compounds from Chlorella into the VOO. Contrariwise, the IP of β-carotene sample was significantly lower than that of the control. The negative impact of β-carotene can be explained by its pro-oxidant behavior under oxygen tensions. The IP of BHT sample was 29.07 days, being significantly higher than those of Chlorella samples.
According to Table 3, Chlorella samples exhibited significant PFs, ranging from 1.20 to 1.33, compared with the control (1.00). PFs increased significantly by higher Chlorella concentrations. The PF of β-carotene sample measured 0.98 which is statistically similar to that of the control (1.00), indicating that β-carotene had no protective effects against oxidation. Furthermore, BHT sample showed a significantly higher PF value of 1.72 in comparison with lower values attributed to Chlorella samples.
AOP values of Chlorella samples ranged from 16.66 to 24.89% (Table 3). The AOP patterns of Chlorella samples were similar to patterns staged by IP and PF values. Higher concentrations of Chlorella showed significantly higher amounts of AOP. β-Carotene sample had significantly lower AOP than Chlorella samples. Furthermore, the AOP of BHT sample was significantly higher than those of Chlorella samples.
Contrary to the above mentioned indices (IP, PF, and AOP), the AA index is a function of antioxidant concentration (Antolovich et al. 2002). Accordingly, the Chlorella samples were identical in terms of their AAs (Table 3). On the contrary, β-carotene showed a pro-oxidant activity with regard to its negative AA value (Antolovich et al. 2002). The AA of BHT sample was significantly higher than those of all other samples (even Chlorella samples).
The oxidative stabilities of samples containing 0.5, 1.0, and 1.5% Chlorella were significantly improved by 19.99, 28.83, and 33.14%, respectively (Table 3). IOS of Chlorella increased by increasing its concentration. Conversely, IOS of β-carotene samples was significantly lower than that of the control. Therefore, the β-carotene cannot be considered as an efficient antioxidant, since it plays its role as a pro-oxidant. Amongst the antioxidants, BHT was characterized with the highest IOS (71.59%).
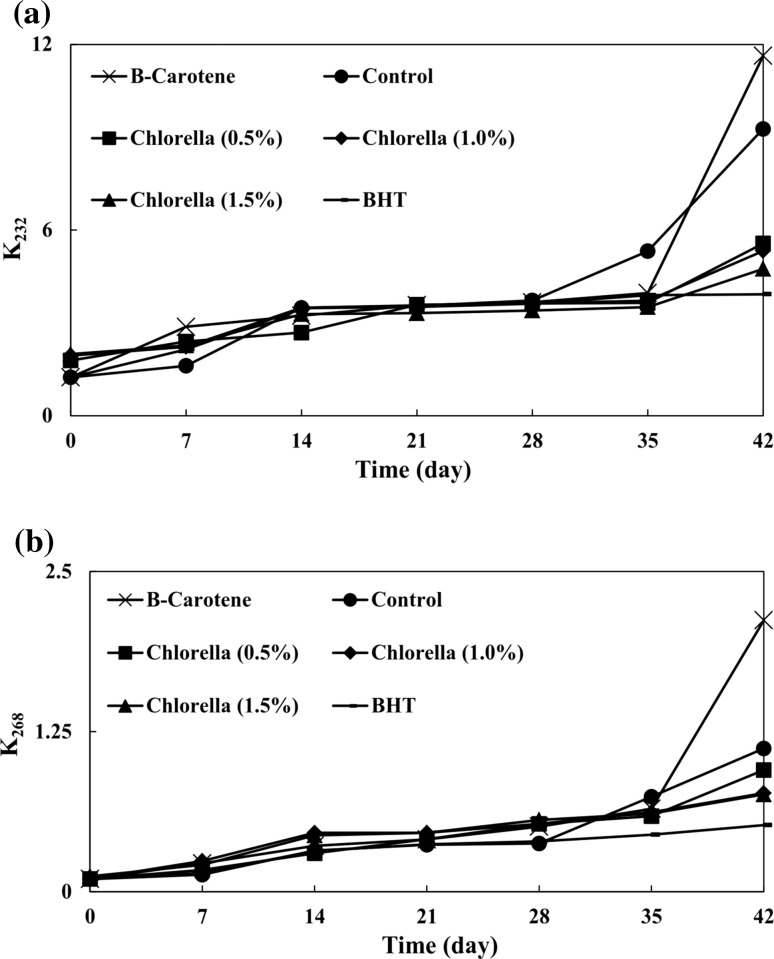
K232 and K268
Oxidation degree is related to oil absorption at 232 nm (K232) and 268 nm (K268) due to the presence of conjugated diene and triene compounds, respectively (AOCS 1998). Initial K232 values of all samples were lower than the VOO legal limit (K232 ≤ 2.60) established by the International Olive Council (IOC 2015). The K232 increased by prolonging the storage period (Fig. 3a). In comparison with the control, K232 values of Chlorella samples were significantly lower at the end of storage. Therefore, Chlorella is efficient in inhibiting the formation of compounds with conjugated double bonds in VOO. As the concentration of Chlorella increased, the K232 values significantly decreased until the end of storage. The K232 value of β-carotene sample was significantly higher than those of Chlorella samples, but nonetheless the K232 value of BHT sample was significantly lower than all other samples.
Fig. 3.
Effects of different concentrations of Chlorella on a K232 and b K268 of virgin olive oil
Initial K268 values of all samples were lower than the VOO legal limit (K268 ≤ 0.25) established by the International Olive Council (IOC 2015). K268 values were increased during the storage period (Fig. 3b). At the end of the storage period, K268 values of samples containing 0.5, 1.0, and 1.5% Chlorella came to be 0.95, 0.77, and 0.76, respectively, which were significantly lower than that of the control (which measured 1.12). This phenomenon indicates that different concentrations of Chlorella can significantly retard the formation of triene compounds in comparison with the control. There were no significant differences between K268 values of samples containing 1.0 and 1.5% Chlorella, but their K268 values were significantly higher than that of the 0.5% Chlorella. At the end of the storage period, the β-carotene sample exhibited the highest K268 value. Also, the BHT sample had the lowest K268 value in comparison with all other samples.
Conclusion
The protective effects of Chlorella at different concentrations against VOO oxidation were demonstrated. Chlorella addition to the VOO significantly increased in its stability. By comparison, the synthetic antioxidant BHT proved to be more efficient than Chlorella, while β-carotene exhibited pro-oxidant effect. Since synthetic antioxidants are not entirely permitted to be added into VOO, Chlorella can serve as an alternative source that can safely offer natural antioxidants, contributing to the stabilization of VOO.
Acknowledgements
This research Project was financially supported by Shiraz University. We would like to thank the Edible Oil Industries Group of Etka Organization for providing the VOO. We also thank the Persian editor, Mohsen Hamedpour-Darabi, for natively editing the English of the paper.
References
- Antolovich M, Prenzler PD, Patsalides E, Mcdonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis of AOAC international. Washington DC: Association of Official Analytical Chemists; 1997. [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemist’s Society. Illinois: AOCS Press; 1998. [Google Scholar]
- Apak R, Güçlü K, Özyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Ayadi MA, Grati-Kamoun N, Attia H. Physico-chemical change and heat stability of extra virgin olive oils flavoured by selected Tunisian aromatic plants. Food Chem Toxicol. 2009;47:2613–2619. doi: 10.1016/j.fct.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Cha KH, Kang SW, Kim CY, Um BH, Na YR, Pan CH. Effect of pressurized liquids on extraction of antioxidants from Chlorella vulgaris. J Agric Food Chem. 2010;58:4756–4761. doi: 10.1021/jf100062m. [DOI] [PubMed] [Google Scholar]
- Codex (2001) Codex Alimentarius Commission. Codex standard for olive oil, virgin and refined, and for refined olive–pomace oil–Codex Stan 33–1981 (Rev. 1–1981)
- Dere S, Güneş T, Sivaci R. Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot. 1998;22:13–18. [Google Scholar]
- Di Marco G, Gismondi A, Canuti L, Scimeca M, Volpe A, Canini A. Tetracycline accumulates in Iberis sempervirens L. through apoplastic transport inducing oxidative stress and growth inhibition. Plant Biol. 2014;16:792–800. doi: 10.1111/plb.12102. [DOI] [PubMed] [Google Scholar]
- FAOSTAT (2013) Food and Agriculture Organization of the United Nations. http://www.faostat3.fao.org/download/Q/QD/E. Accessed Dec 2015
- Frankel EN. Chemistry of extra virgin olive oil: adulteration, oxidative stability, and antioxidants. J Agric Food Chem. 2010;58:5991–6006. doi: 10.1021/jf1007677. [DOI] [PubMed] [Google Scholar]
- Golmakani MT, Rezaei K, Mazidi S, Razavi SH. Effect of alternative C2 carbon sources on the growth, lipid, and γ-linolenic acid production of spirulina (Arthrospira platensis) Food Sci Biotechnol. 2012;21:355–363. doi: 10.1007/s10068-012-0047-8. [DOI] [Google Scholar]
- Golmakani MT, Rezaei K, Mazidi S, Razavi SH. γ-Linolenic acid production by Arthrospira platensis using different carbon sources. Eur J Lipid Sci Technol. 2012;114:306–314. doi: 10.1002/ejlt.201100264. [DOI] [Google Scholar]
- Gouveia L, Raymundo A, Batista AP, Sousa I, Empis J. Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. Eur Food Res Technol. 2006;222:362–367. doi: 10.1007/s00217-005-0105-z. [DOI] [Google Scholar]
- Gouveia L, Nobre BP, Marcelo FM, Mrejen S, Cardoso MT, Palavra AF, Mendes RL. Functional food oil coloured by pigments extracted from microalgae with supercritical CO2. Food Chem. 2007;101(2):717–723. doi: 10.1016/j.foodchem.2006.02.027. [DOI] [Google Scholar]
- Gutiérrez-Rosales F, Garrido-Fernández J, Gallardo-Guerrero L, Gandul-Rojas B, Minguez-Mosquera MI. Action of chlorophylls on the stability of virgin olive oil. J Am Oil Chem Soc. 1992;69:866–871. doi: 10.1007/BF02636334. [DOI] [Google Scholar]
- Habibi M, Golmakani MT, Farahnaky A, Mesbahi G, Majzoobi M. NaOH-free debittering of table olives using power ultrasound. Food Chem. 2016;192:775–781. doi: 10.1016/j.foodchem.2015.07.086. [DOI] [PubMed] [Google Scholar]
- Hajimahmoodi M, Faramarzi MA, Mohammadi N, Soltani N, Oveisi MR, Nafissi-Varcheh N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J Appl Phycol. 2010;22:43–50. doi: 10.1007/s10811-009-9424-y. [DOI] [Google Scholar]
- IOC (2015) International Olive Council. Trade standard applying to olive oils and olive–pomace oils. Decision COI/T.15/NC No 3/Rev. 8. Madrid
- Lee YC, Kim DH. Effects of β-carotene on the stability of soybean oil subject to autoxidation and photosensitized oxidation. Food Sci Biotechnol. 1992;1:1–7. [Google Scholar]
- Lima Araújo KG, Domingues JR, Sabaa Srur AUO, da Silva AJR. Production of antioxidants by anabaena PCC 7119 and evaluation of their protecting activity against oxidation of soybean oil. Food Biotechnol. 2006;20:65–77. doi: 10.1080/08905430500524200. [DOI] [Google Scholar]
- Limón P, Malheiro R, Casal S, Acién-Fernández FG, Fernández-Sevilla JM, Rodrigues N, Cruz R, Bermejo R, Pereira JA. Improvement of stability and carotenoids fraction of virgin olive oils by addition of microalgae Scenedesmus almeriensis extracts. Food Chem. 2015;175:203–211. doi: 10.1016/j.foodchem.2014.10.150. [DOI] [PubMed] [Google Scholar]
- Minguez-Mosquera MI, Rejano-Navarro L, Gandul-Rojas B, Sanchezgomez AH, Garrido-Fernandez J. Color-pigment correlation in virgin olive oil. J Am Oil Chem Soc. 1991;68:332–336. doi: 10.1007/BF02657688. [DOI] [Google Scholar]
- Mohd Azamai ES, Sulaiman S, Mohd Habib SH, Looi ML, Abdul Hamid NA, Ngah WZW, Yusof YAM. Chlorella vulgaris triggers apoptosis in hepatocarcinogenesis-induced rats. J Zhejiang Univ Sci B. 2009;10:14–21. doi: 10.1631/jzus.B0820168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales MT, Przybylski R. Olive oil oxidation. In: Aparicio R, Harwood J, editors. Handbook of olive oil: analysis and properties. New York: Springer; 2013. p. 482. [Google Scholar]
- Namiki M. Antioxidants/antimutagens in food. Crit Rev Food Sci Nutr. 1990;29:273–300. doi: 10.1080/10408399009527528. [DOI] [PubMed] [Google Scholar]
- Plaza M, Santoyo S, Jaime L, Avalo B, Cifuentes A, Reglero G, García-Blairsy Reina G, Señoráns FJ, Ibáñez E. Comprehensive characterization of the functional activities of pressurized liquid and ultrasound-assisted extracts from Chlorella vulgaris. LWT-Food Sci Technol. 2012;46:245–253. doi: 10.1016/j.lwt.2011.09.024. [DOI] [Google Scholar]
- Polavarapu S, Oliver CM, Ajlouni S, Augustin MA. Physicochemical characterisation and oxidative stability of fish oil and fish oil-extra virgin olive oil microencapsulated by sugar beet pectin. Food Chem. 2011;127:1694–1705. doi: 10.1016/j.foodchem.2011.02.044. [DOI] [Google Scholar]
- Rao AR, Sarada R, Ravishankar GA. Stabilization of astaxanthin in edible oils and its use as an antioxidant. J Sci Food Agric. 2007;87:957–965. doi: 10.1002/jsfa.2766. [DOI] [Google Scholar]
- Safi C, Zebib B, Merah O, Pontalier PY, Vaca-Garcia C. Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sustain Energy Rev. 2014;35:265–278. doi: 10.1016/j.rser.2014.04.007. [DOI] [Google Scholar]
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects–a review. J Funct Foods. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Shalaby EA, Shanab SM. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J Geo Mar Sci. 2013;42:556–564. [Google Scholar]
- Silva FAM, Borges F, Ferreira MA. Effects of phenolic propyl esters on the oxidative stability of refined sunflower oil. J Agric Food Chem. 2001;49:3936–3941. doi: 10.1021/jf010193p. [DOI] [PubMed] [Google Scholar]
- Vijayavel K, Anbuselvam C, Balasubramanian MP. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol Cell Biochem. 2007;303:39–44. doi: 10.1007/s11010-007-9453-2. [DOI] [PubMed] [Google Scholar]
- Wanasundara PKJPD, Shahidi F. Antioxidants: science, technology, and applications. In: Shahidi F, editor. Bailey’s industrial oil and fat products. New York: Wiley; 2005. p. 434. [Google Scholar]
- Wang HM, Pan JL, Chen CY, Chiu CC, Yang MH, Chang HW, Chang JS. Identification of anti-lung cancer extract from Chlorella vulgaris C–C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010;45:1865–1872. doi: 10.1016/j.procbio.2010.05.023. [DOI] [Google Scholar]
- Wang L, Yang B, Yan B, Yao X. Supercritical fluid extraction of astaxanthin from Haematococcus pluvialis and its antioxidant potential in sunflower oil. Innov Food Sci Emerg Technol. 2012;13:120–127. doi: 10.1016/j.ifset.2011.09.004. [DOI] [Google Scholar]
- Wu LC, Ho JAA, Shieh MC, Lu IW. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J Agric Food Chem. 2005;53:4207–4212. doi: 10.1021/jf0479517. [DOI] [PubMed] [Google Scholar]
- Yanishlieva NV, Marinova EM. Stabilisation of edible oils with natural antioxidants. Lipid Sci Technol. 2001;103:752–767. doi: 10.1002/1438-9312(200111)103:11<752::AID-EJLT752>3.0.CO;2-0. [DOI] [Google Scholar]