Abstract
Defatted soy flour is a potential source of food protein, amino acids, ash and isoflavones. The supercritical carbon dioxide (SC-CO2) and a traditional organic solvent extraction methods were used to remove fat from soy flour, and the quality characteristics of a control soy flour (CSF), defatted soy flour by SC-CO2 (DSFSC-CO2) and defatted soy flour by an organic solvent (DSF-OS) were compared. The SC-CO2 process was carried out at a constant temperature of 45 °C, and a pressure of 40 MPa for 3 h with a CO2 flow rate of 30 g/min. The DSFSC-CO2 had significantly higher protein, ash, and amino acids content than CSF and DSF-OS. The sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis demonstrated that CSF and DSFSC-CO2 had protein bands of similar intensity and area that indicated no denaturation of protein, whereas DSF-OS showed diffuse bands or no bands due to protein denaturation. In addition to higher nutritional value and protein contents, DSFSC-CO2 showed superior functional properties in terms of total soluble solids content, water and oil absorption, emulsifying and foaming capacity. The SC-CO2 method offers a nutritionally and environmentally friendly alternative extraction processing approach for the removal of oil from high-protein food sources. It has a great potential for producing high-protein fat-free, and low-calorie content diet than the traditional organic solvent extraction method.
Keywords: Defatted soy flour, Quality characteristics, Supercritical carbon dioxide, Organic solvent
Introduction
Soybean is a very versatile food and a rich source of essential nutrients, including protein, fat, dietary fiber, vitamins, minerals, soy saponins and isoflavones (Riaz 2006; Singh et al. 2008). Soy protein is nutritionally equivalent to animal proteins from egg, milk, fish and beef (Agostoni et al. 2006). The high fat content of soy flour may limit the extraction of proteins, amino acids and encourage the oxidation reaction that leads to rancidification, and produces off flavors with volatile compounds, makes them undesirable to consumers as well as shorter the shelf-life (Christianson et al. 1984). After removing fat from soybeans, the remaining material is referred to as defatted soy flour (DSF) that can use to produce high-protein, low-fat diet food and a promising protein source for the future. When used in food production, DSF has a range of attractive functional properties such as being solubility, water and oil absorption capacity (WAC and OAC respectively), emulsifying, swelling, gelling and foaming properties. In addition to high nutritional value, DSF is widely available, highly digestible and inexpensive (Singh et al. 2008). Research has suggested that soy protein provides protection against heart disease, cancer, cardiovascular disease, diabetes, osteoporosis and bowel and kidney diseases (Faraj and Vasanthan 2004). Soy protein is also a good basis for the special formulas used for infants feeding, geriatric nutrition, postoperative diet, and other therapeutic diets that provide the complete nutrition (Agostoni et al. 2006).
Conventional mechanical and solvent methods of oil extraction have several limitations such as being time-consuming, expensive and potentially hazardous to human health and the environment (Russin et al. 2011). Degradation and decomposition of thermolabile compounds are unavoidable in these conventional methods while extracting lipids using organic solvents causes protein denaturation and loss of functional properties (Russin et al. 2011; Ali-Nehari et al. 2012). It was reported that aqueous extracts of hexane-defatted soy flour have undesirable off-flavors that diminish their usefulness in food materials and that protein-lipid complexes resist the defatting treatment with n-hexane (Riaz 2006).
Supercritical carbon dioxide (SC-CO2) offers an efficient alternative approach for the extraction of natural substances from foods (Sun and Temelli 2006). It was reported that the extraction using CO2 at room temperature produces less denaturation of seed proteins than methods using hot organic solvents (Stahl et al. 1980). SC-CO2 has many advantages over conventional organic solvent methods such as being easily controllable, nontoxic, nonflammable, noncorrosive and inexpensive. Minimal post-extraction manipulation is required, the operating temperature is low and materials can be extracted in large quantities with high purity (Raynie 1997; Sihvonen et al. 1999; Russin et al. 2011). The modern SC-CO2 system offers shorter extraction times, potentially higher selectivity and increased sample throughput than conventional solvent extraction techniques (Turner et al. 2001).
The objective of this study was to compare the major quality characteristics such as proximate composition, the amino acids profile, the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) pattern and functional properties in terms of solubility, WAC and OAC, emulsifying and foaming properties of control soy flour (CSF), defatted soy flour by SC-CO2 (DSFSC-CO2) and defatted soy flour by organic solvent (DSF-OS).
Materials and methods
Materials
Soy flour was purchased from a local market in Jinju, Gyeongsangnam-do Province, Korea and stored at 4 °C in an air-tight glass container until used. Hexane and all other chemicals used in the study were purchased from Sigma Aldrich (Seoul, Korea) and all of them were of high purity analytical grade.
Defatting soy flour by supercritical carbon dioxide
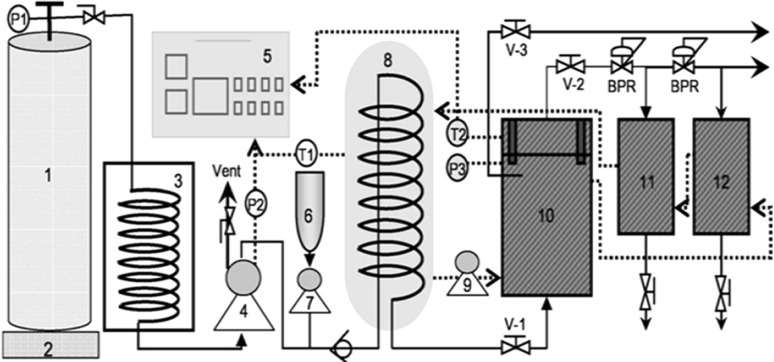
A laboratory scale supercritical CO2 unit (Ilshin Autoclave Co., Daejeon, Korea) was used for defatting the soy flour. Liquid CO2 (purity ≥ 99.99%) contained in the siphoned cylinder was cooled by the chiller. Soy flour (300 g) was loaded into the extraction vessel and the head of the extraction vessel of the supercritical CO2 unit was tightly sealed. CO2 was pumped through the vessel at a flow rate of 30 g/min, a constant temperature of 45 °C and a pressure of 40 MPa for an extraction period of 3 h. Preliminary studies indicated these conditions gave the greatest extraction of oil without denaturing the proteins. After completion, the vessel was gradually depressurized. DSF was maintained at 4 °C in an air-tight glass container prior to analysis. A schematic of the SC-CO2 extraction process is shown in Fig. 1.
Fig. 1.
Schematic diagram of supercritical carbon dioxide (SC-CO2) treatment system. (1) CO2 cylinder, (2) electronic balance, (3) chiller, (4) CO2 pump, (5) controller, (6) co-solvent reservoir, (7) co-solvent pump, (8) heating bath, (9) circulation pump, (10) extractor, (11) separator-1, and (12) separator-2. V-1: valve-1, V-2: valve-2, P1: pressure gauge (PG)-1, P2: PG-2, P3: PG-3, T1, T2: temperature and BPR: Back pressure regulator. Dotted lines indicate the water lines; solid lines indicate the CO2 lines
Defatting soy flour by organic solvent
Oil was also extracted from soy flour using an organic solvent. The soy flour (100 g) was placed in a Soxhlet apparatus and extracted with hexane for 12–16 h. After the extraction was complete, the supernatant including the oil was drained from the flour. Residual solvent was removed from the defatted meal by air drying under a fume hood overnight, followed by oven (JSOF-150, JS Research Inc, Korea) drying at 45 °C for 2 h until no hexane odor was discernible. Samples were then stored at 4 °C in an air-tight glass container until further use.
Proximate composition of soy flour
The proximate composition of CSF, DSFSC-CO2 and DSF-OS were determined by AOAC (2000) methods and included moisture, ash, lipid, carbohydrate and protein (N × 6.25).
SDS-PAGE analysis
Soy flour proteins were separated using SDS-PAGE following the method of Laemmli (1970). Soy flour (25 mg) was mixed with 1.8 mL of distilled water for 1 h in a water bath, then centrifuged (Supra 22 K High speed centrifuge, Hanil, Korea) at 13,000 rpm for 20 min to remove undissolved material. For electrophoresis, 10 μL of supernatant was mixed with a sample buffer containing 60 mmol/L Tris–HCl (pH = 6.8), 20 mL/100 mL of glycerol (v/v), 2 g/100 mL of sodium dodecylsulfate (w/v), 0.1 g/100 mL of bromophenol blue (w/v), and 5 mL/100 mL of 2-mercaptoethanol (v/v). The samples were incubated for 5 min at 100 °C to dissociate the proteins and 5 μL of the supernatant was used as the final sample for loading. The 1.5 mm thick PAGE gel system consisted of 4 g/100 mL stacking gel and 12.5 g/100 mL separating gel (w/v). The running buffer (pH 8.3) contained 192 mmol/L glycine, 0.1 g/100 mL SDS and 25 mmol/L tris (hydroxymethyl) aminomethane. The process was performed for 20–30 min at 80 V, followed by 60–70 min at 120 V. The gradient PAGE gel was 1 mm thick, comprising 4–20 g/100 mL precast Tris–HCl gel (w/v) (Bio-Rad) and the running buffer. Following the run, the PAGE gel was stained with coomassie brilliant blue R-250 (1 g/L) in methanol, acetic acid and water (25:10:65, v/v/v) and destained in the same solution without dye. The quantification of protein subunits was performed using a scanning densitometer (UMAX PowerLook 1100, Taiwan) and Total Lab software (Phloretix International LTD, England).
Amino acids analysis
Amino acids analysis was conducted following the method of Jeong and Shim (2004) with minor modifications. Samples of 0.1 g were placed in heatproof screw cap test tubes and dissolved in 5 mL of 6 N HCl with vortexing (TSS2, IKA, Korea) for 30 s. The tubes were flushed with N2 gas for 1 min and then sealed. The samples were subsequently incubated in a dry oven (JSOF-150, JS Research Inc, Korea) at 110 °C for 24 h. When the reaction was completed, the supernatant was removed using a glass filter. To remove HCl, the supernatant was evaporated in a vacuum rotary evaporator (HS-2005S, Jisico Co, Ltd, Korea) and the concentrate dissolved in 3 mL of sodium citrate buffer (pH 2.2). Finally, the solution was filtered with 0.45 μm PTFE filters (Sigma-Aldrich, Seoul, South Korea) and analyzed using an auto aminoacid analyzer (Biochrom 30, Biochrom, Sweden) at 570 nm.
Water and oil absorption capacity
Water absorption capacity (WAC) was determined using a modification of the method of Jyothirmayi et al. (2006). Samples of 1 g were dispersed in 10 mL water, thoroughly stirred in a vortex (TSS2, IKA, Korea) and then heated at 70 °C for 15 min in a water bath (BS-21, Jeio Tech, Korea). The mixture was placed in an orbital shaker (NB-303, N-Biotek Inc, Korea) at 300 rpm for 1 h at room temperature and subsequently cooled to 4 °C in the refrigerator (GC-114HDMP, LG, Korea) for 1 h. The slurry was centrifuged (FLETA 5, Hanil Scientific Industrial Co, Ltd, Korea) at 4000 rpm for 30 min and the supernatant was removed and the pellets were drained for 30 min. The per unit weight gain was reported as WAC (g/g). Oil absorption capacity (OAC) was determined using the same method but with soybean oil in place of water.
Total soluble solids content
Total soluble solids (TSS) were determined following the method of Eastman and Moore (1984) with some modifications. Samples of 1 g were dispersed in 100 mL of deionized water and dissolved using a magnetic stirrer (HSD 150-03 P, Misung Scientific Co, Ltd, Korea) for 3–4 h. The slurry was heated to 80 °C in a water bath (BS-21, Jeio Tech, Korea) at 185 rpm for 30 min. After heating was complete the slurry was allowed to stand for 30 min at room temperature and then centrifuged (FLETA 5, Hanil Scientific Industrial Co, Ltd, Korea) at 4000 rpm for 30 min. An aliquot of 10 mL of the supernatant was transferred to the pre-weighed aluminum weighing dishes and dried in a laboratory oven (JSOF-150, JS Research Inc, Korea) at 105 °C for 3 h. TSS (%) was calculated with the Eq. 1;
| 1 |
Foaming properties
Foaming capacity (FC) and foaming stability (FS) were measured following the methods of Ogunwolu et al. (2009) and Adebowale et al. (2005), respectively, with minor modifications. Soy flour solutions of 1 g/100 mL were prepared in a 50 mL graduated cylinder and blended (D-500 homogenizer, Wiggen Hauser, Berlin, Germany) at 30,000 rpm for 2 min. For FC, the volume was determined before (V L) and immediately after (V o) the high-speed blending. FC was expressed as the increase in volume due to foaming. To determine FS, the graduated cylinder was allowed to stand and the foam volume (V t) was recorded at 20, 40, 60, 80 and 100 min. FC and FS were calculated with the Eq. 2 and 3 respectively;
| 2 |
| 3 |
Emulsifying properties
Emulsifying activity (EA) and emulsion stability (ES) were determined following the method of Mao and Hua (2014) with some modifications. A sample of 1 g was dispersed in 12.5 mL of deionized water at room temperature and 12.5 mL of refined soybean oil was added to it. The mixture was homogenized (D-500 homogenizer; Wiggen Hauser, Berlin, Germany) at 10,000 rpm for 1 min and subsequently centrifuged (FLETA 5, Hanil Scientific Industrial Co, Ltd, Korea) at 1600 rpm for 5 min. The volume was recorded before and after centrifugation and EA calculated by using the Eq. 4;
| 4 |
Where V T is the total volume before centrifugation, and V e is the volume of the emulsified layer after centrifugation. The emulsion stability (ES) was calculated by determining the volume of the emulsified layer (V 1h) after maintaining samples at 80 °C for 1 h with the Eq. 5;
| 5 |
Statistical analysis
All data were presented as mean values ± standard deviations. The data were analyzed using the SAS® program (version 9.1; SAS Institute Inc., Cary, NC, USA). Analysis of variance (ANOVA) was performed and Duncan’s multiple range tests were used to determine the difference of means, with p < 0.05 for determining the statistical significance.
Results and discussion
Composition
Table 1 shows the proximate composition of all the flour samples used in this study, and indicates the levels of major macronutrients. It was found that the DSFSC-CO2 had a significantly higher (p < 0.05) protein and ash content than those of CSF and DSF-OS. Because of the removal of fat, DSFSC-CO2 and DSF-OS had higher protein, carbohydrate and ash content as well as significantly lower fat content than that of CSF. As compared to DSF-OS, DSFSC-CO2 had lower residual fat and higher protein and ash content. Similarly, Singh et al. (2008) reported that defatting of soy flour or soy grits increased the protein, carbohydrate, and ash content as the level of fat was reduced. Bader et al. (2011) reported that defatting increased the concentration of lupin (Lupinus angustifolius L.) protein due to decreased levels of fat. Jitngarmkusol et al. (2008) showed that defatted macadamia flours were higher in protein, carbohydrate, and ash content than partially defatted macadamia flours. The results of this study were consistent with those reported by Sparks et al. (2006), who showed that SC-CO2 defatted rice bran had more protein and ash than rice bran defatted with organic solvents. It was noted that the maximum recovery of soy protein was achieved using a pressurized CO2 extraction process (Khorshid et al. 2007). The SC-CO2 extraction processing had no deteriorating effect on the nutritional value of the oil seeds (Stahl et al. 1980).
Table 1.
Proximate composition of control soy flour (CSF), defatted soy flour by SC-CO2 (DSFSC-CO2), and defatted soy flour by organic solvent (DSF-OS)
| Components (%) | CSF | DSFSC-CO2 | DSF-OS |
|---|---|---|---|
| Moisture | 7.23 ± 0.29a | 5.69 ± 0.18b | 6.10 ± 0.30b |
| Protein | 35.07 ± 1.44c | 49.38 ± 1.18a | 43.75 ± 0.64b |
| Fat | 18.78 ± 0.15a | 1.64 ± 0.13c | 2.81 ± 0.65b |
| Ash | 4.82 ± 0.02c | 6.07 ± 0.16a | 5.71 ± 0.10b |
| Carbohydrate | 34.09 ± 1.03c | 37.22 ± 1.15b | 42.62 ± 0.60a |
All values are mean ± SD (n = 3). Different letters indicate that means are significantly different (p < 0.05) by Duncan’s test
SDS-PAGE
SDS-PAGE is a common analytical technique for separating and characterizing proteins in terms of factors such as molecular weight (Mw), distribution pattern, polarity, structure and purity. Therefore, the protein patterns of CSF, DSFSC-CO2 and DSF-OS were examined under reducing condition using SDS-PAGE analysis. The area and staining intensity of the protein bands of DSFSC-CO2 were identical to those of untreated CSF (Fig. 2, lanes 4 and 2 respectively). In contrast, protein bands of the DSF-OS (Fig. 2, lane 3) were quite diffuse. While the CSF and DSFSC-CO2 samples had > 16 distinct bands, the DSF-OS samples had only 4 or 5 clearly distinct bands. Furthermore, the protein Mw of the DSF-OS had diminished content in the 10–37 kDa region, with increased density in the 50–75 kDa region (Fig. 2, lane 3). This might be due to denaturation of protein in DSF-OS due to the very low polarity hexane used during lipid extraction. In contrast, in the SC-CO2 method the CO2 acts as a mild solvent to remove oil from DSFSC-CO2. As a result, there was no protein denaturation in the DSFSC-CO2 sample, as seen by the similar protein banding intensity as compared with the untreated CSF. Bader et al. (2011) reported that exposure to nonpolar organic solvents can cause protein denaturation by disrupting the intramolecular hydrogen bonding of protein side chains. It has also been found that SC-CO2 extraction had no deteriorating effects on seed proteins, whereas extraction of oil with organic solvents such as hexane, diethyl ether, chloroform or acetone leads to the denaturation of seed proteins (Stahl et al. 1980). Our results are also similar to those of Asaduzzaman and Chun (2015), who found that PAGE protein bands were more prominent in SC-CO2 treated mackerel residues than in n-hexane-treated samples, and attributed this to the lack of protein denaturation in SC-CO2 treated samples. It was also demonstrated that there was no protein denaturation in the residues of mackerel viscera, squid viscera (Todarodes pacificus) and krill (Euphausia superba) after oil extraction using the SC-CO2 method, and that the intensity of electrophoretic patterns between untreated control and SC-CO2 treated samples were identical (Park et al. 2008; Uddin et al. 2009; Ali-Nehari et al. 2012).
Fig. 2.
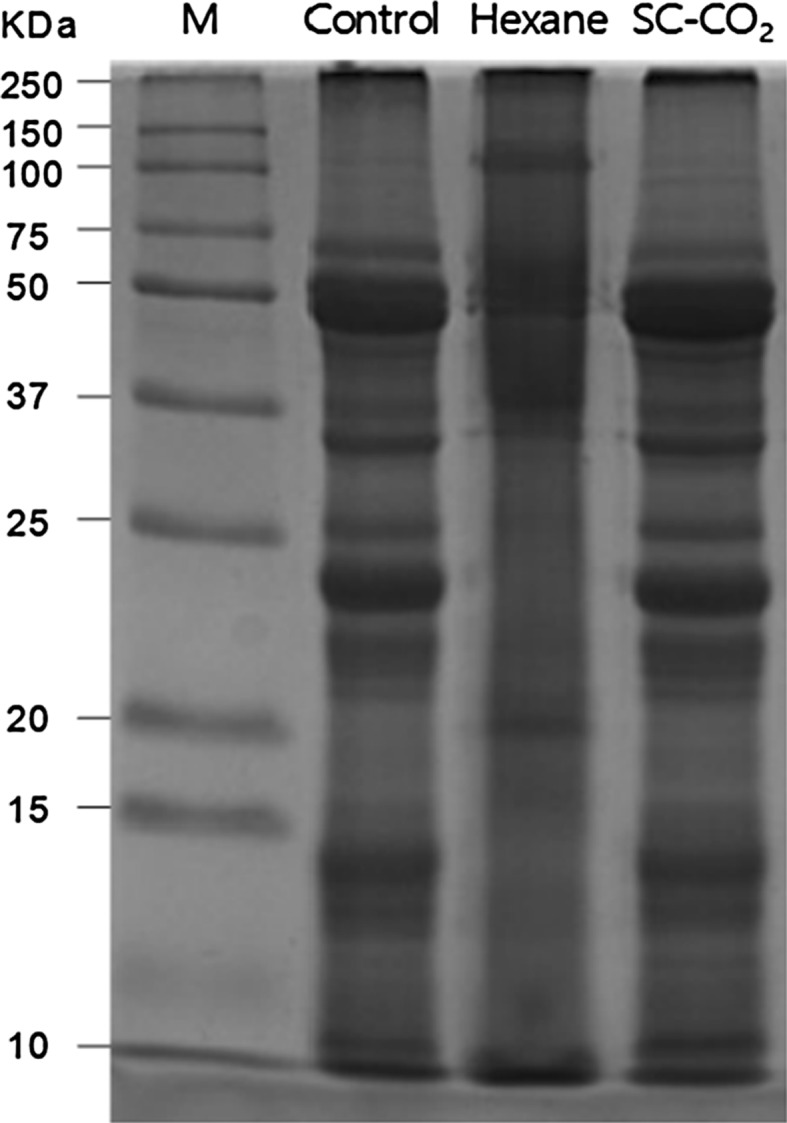
The sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) pattern of different soy flour samples. Lane 1: molecular weight standard (M), Lane 2: control soy flour (CSF), Lane 3: defatted soy flour by an organic solvent (DSF-OS), and Lane 4: defatted soy flour by SC-CO2 (DSFSC-CO2)
Amino acid analysis
Amino acids are essential nutrients involved in the growth of infants and the development of immunity. Soy protein has been shown to be comparable to milk and beef protein in terms of quality (Singh et al. 2008). The amino acid composition of the different soy flours used in this study is listed in Table 2. A total of nine essential and eight nonessential amino acids were identified, whereas, glutamic acid was present in the highest amount in all samples, followed by aspartic acid, arginine, lysine, leucine, proline, valine, isoleucine, glycine etc. The sulfur-containing amino acid cysteine was present in the lowest amount, particularly in the hexane-extracted samples. The total amino acid content including essential and non-essential amino acids of DSFSC-CO2 (39.16%) was significantly higher (p < 0.05) than those of DSF-OS (33.04%) and CSF (31.68%). The high content of lipid in CSF resulted in lower levels of protein and amino acids as compared to that in DSF-OS and DSFSC-CO2. Likewise, fat levels in DSFSC-CO2 were lower than in DSF-OS resulting in higher protein and amino acid content. Similar observations were reported by Christianson et al. (1984), who showed that SC-CO2 extracted dry-milled corn germ flour had greater amino acid content than that extracted with hexane. Kang et al. (2017) reported that the amino acid content of SC-CO2 treated bovine liver was greater than that treated with organic solvents, again due to the relatively higher levels of lipid remaining in the latter. Park et al. (2008) found that the free, essential and total amino acid content of mackerel viscera after the removal of oil by SC-CO2 were higher than those of untreated control samples. The results of the current study were closely matched the reported results of other protein-rich materials. Furthermore, DSFSC-CO2 has all the essential and non-essential amino acids needed for producing high value diets for lactation, infant growth, mal-nourished children and at-risk adults (Agostoni et al. 2006; Singh et al. 2008). In addition, the balances of amino acids in DSFSC-CO2 were close to standard values issued by the FAO/WHO for children (Ge et al. 2000).
Table 2.
Amino acid composition of control soy flour (CSF), defatted soy flour by SC-CO2 (DSFSC-CO2), and defatted soy flour by organic solvent (DSF-OS) (Unit: g/100 g)
| Amino acids | CSF | DSFSC-CO2 | DSF-OS |
|---|---|---|---|
| Total essential amino acids | 14.89 ± 0.18b | 18.63 ± 0.49a | 15.68 ± 2.04b |
| Threonine | 1.25 ± 0.04b | 1.57 ± 0.06a | 1.30 ± 0.22b |
| Valine | 1.59 ± 0.01b | 1.93 ± 0.05a | 1.67 ± 0.18ab |
| Methionine | 0.38 ± 0.06b | 0.54 ± 0.04a | 0.44 ± 0.10ab |
| Isoleucine | 1.57 ± 0.04b | 1.98 ± 0.05a | 1.68 ± 0.25ab |
| Leucine | 2.39 ± 0.04b | 2.99 ± 0.07a | 2.53 ± 0.34b |
| Phenylalanine | 1.71 ± 0.02b | 2.15 ± 0.06a | 1.76 ± 0.22b |
| Histidine | 0.91 ± 0.01b | 1.17 ± 0.03a | 0.97 ± 0.14b |
| Lysine | 2.55 ± 0.04b | 3.02 ± 0.11a | 2.63 ± 0.25ab |
| Arginine | 2.55 ± 0.03b | 3.28 ± 0.10a | 2.69 ± 0.34b |
| Total nonessential amino acids | 16.79 ± 0.12b | 20.53 ± 0.36a | 17.36 ± 2.24b |
| Aspartic acid | 3.65 ± 0.05b | 4.45 ± 0.18a | 3.82 ± 0.40b |
| Serine | 1.44 ± 0.07b | 1.82 ± 0.06a | 1.50 ± 0.21b |
| Glutamic acid | 5.62 ± 0.02b | 6.69 ± 0.16a | 5.83 ± 0.59b |
| Proline | 2.01 ± 0.10a | 2.24 ± 0.35a | 1.99 ± 0.47a |
| Glycine | 1.48 ± 0.01b | 1.86 ± 0.07a | 1.55 ± 0.20b |
| Alanine | 1.39 ± 0.01b | 1.79 ± 0.06a | 1.46 ± 0.19b |
| Cysteine | 0.11 ± 0.02a | 0.30 ± 0.17a | 0.10 ± 0.04a |
| Tyrosine | 1.09 ± 0.03b | 1.37 ± 0.05a | 1.12 ± 0.15b |
| Total | 31.68 ± 0.28b | 39.16 ± 0.85a | 33.04 ± 4.28b |
All values are mean ± SD (n = 3). Different letters indicate that means are significantly different (p < 0.05) by Duncan’s test
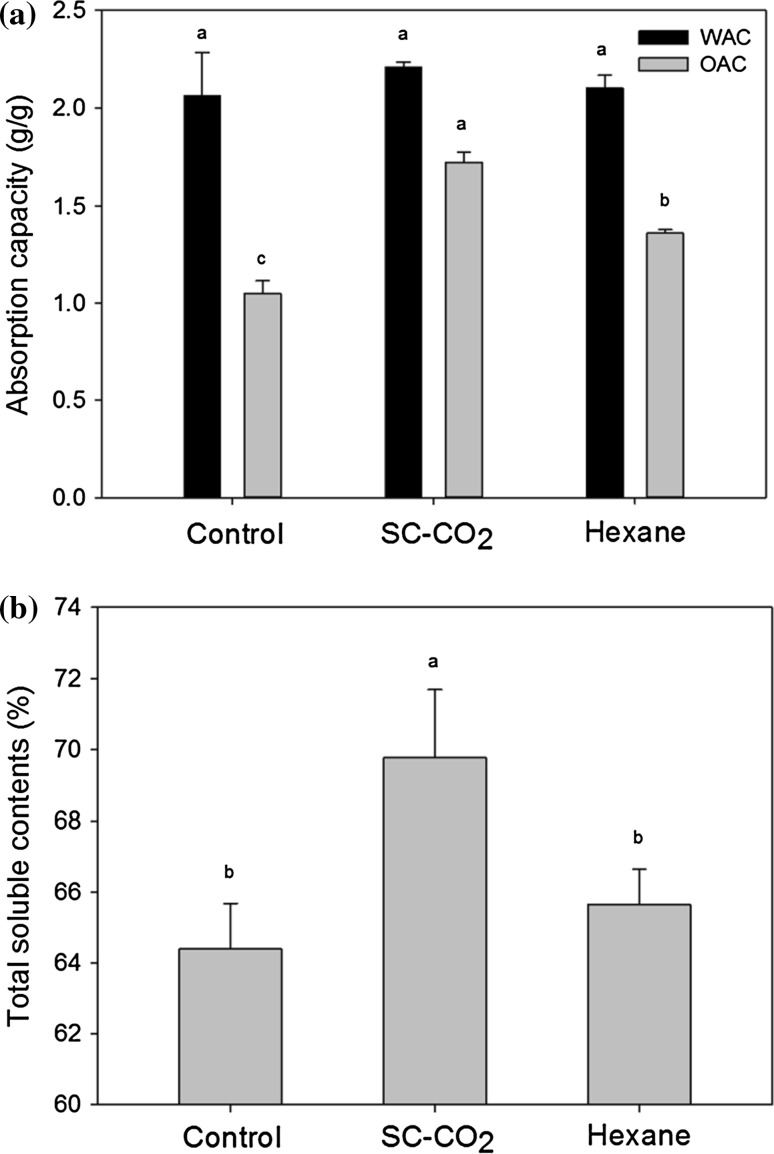
Water and oil absorption capacity
Liquid retention is an index of the ability of proteins to absorb and retain oil and water. The WAC and OAC of the control and defatted soy flours are shown in Fig. 3a. Values of WAC were 2.21 gH2O/g for DSFSC-CO2, 2.10 gH2O/g for DSF-OS and 2.06 gH2O/g for CSF, however the differences were not statistically significant. It might be expected that the higher levels of lipid in CSF might serve to hinder binding of water. In contrast, DSFSC-CO2 had lower fat levels and greater total protein and amino acid levels as compared to DSF-OS, both factors which would encourage greater WAC in the DSFSC-CO2. Other researchers have found that WAC increases with protein content, as these present more amino acids with exposed polar groups that can bind water (Zayas 1997; Moure et al. 2006). Kang et al. (2017) reported that the WAC of SC-CO2 treated bovine liver was significantly higher than that of bovine liver treated with organic solvents. WAC is an important quality factor for textural properties of a new product development and the viscous foods, such as soups, require WAC values ranging from 1.49–4.72 g/g (Chandi and Sogi 2007).
Fig. 3.
a Water absorption capacity (WAC) and oil absorption capacity (OAC) of different soy flour samples (g/g); b TSS (%) of control soy flour (CSF), defatted soy flour by SC-CO2 (DSFSC-CO2), and defatted soy flour by organic solvent (DSF-OS). Different letters of bars indicate significant differences (p < 0.05) among different samples
OAC is another important functional property of foods, playing a role in the enhancement of mouthfeel and retention of flavor. High oil absorption is essential in foods such as sausage, cake batter, mayonnaise and salad dressing. As seen in Fig. 3a, the OAC of DSFSC-CO2 (1.72 g/g) was greater than that of DSF-OS (1.36 g/g) and CSF (1.05 g/g). The OAC of DSFSC-CO2 and DSF-OS may be greater than that of CSF due to the lower fat levels and higher protein content. That is, proteins can serve as emulsifiers and higher levels may be beneficial to emulsifying properties, and CSF already has more lipid that is competing with any added oil. Moure et al. (2006) reported that defatting of soy flour increased the hydrophobic domains in proteins that bind hydrocarbon chains, thus resulting in increased OAC. In addition, the denaturation of soy protein may reduce the fat binding capacity due to the destruction of hydrophobic domains (Zayas 1997). Related studies on commercial defatted soy flour reported water absorption of 1.70–2.07 kg/kg and oil absorption of 110–120 ml/100 g (Bass et al. 1997). It has also been shown that the OAC of cashew nut and soy protein products increase proportionately with the protein content (Ogunwolu et al. 2009). Similarly, it has been demonstrated that proteins and amino acids are the main component affecting OAC and that defatted soy flour had a higher OAC due to its higher protein and amino acids content (Jitngarmkusol et al. 2008).
Total soluble solids contents
Solubility is the most important functional property, as it affects most other functional properties of food. It also supports the incorporation of protein into food. Solubility was declined as the protein became denatured, limiting the potential uses of soy flour as a protein source and highly soluble proteins are most promising in food applications (Moure et al. 2006; Adebowale et al. 2005). The total soluble matter depends on multiple factors, including the origin of the species, chemical composition and processing parameters in terms of the method for removing the fat, time of heating, temperature, pH, the ionic strength of the aqueous solvent, the speed of blending as well as the degree of agitation. It was demonstrated by Konak et al. (2014) that the solubility of CO2-oat protein was significantly higher under alkaline conditions than acidic conditions because of the increase in net protein charge. The current study was conducted at neutral pH and it was found that DSFSC-CO2 had a significantly greater (p < 0.05) TSS content (69.77%) than DSF-OS (65.65%) and CSF (64.39%), as shown in Fig. 3b. Again, this is likely due to the higher levels of protein and lower levels of fat in the DSFSC-CO2, as well as the greater amount of undenatured protein in DSFSC-CO2 as compared to DSF-OS. Moreover, total solubility is closely related to the WAC, so it would be expected that DSFSC-CO2 samples had the most soluble solids and WAC. It has been shown that higher lipid content produces increased hydrophobic interactions in foods and interferes with hydrophilic characteristics that decrease water solubility (Heywood et al. 2002; Moure et al. 2006). It has also been observed that water solubility is positively correlated with protein concentration, as the protein provides more polar groups for binding of water, and negatively correlated with protein denaturation (Zayas 1997). Moreover, Moure et al. (2006) stated that organic solvents such as alcohol used to extract oil can also remove soluble sugars that losses total soluble matter. Konak et al. (2014) showed that the defatted oat flours defatted using SC-CO2 had more soluble protein than the oat flours prepared by other processing methods. Similarly, it was also reported that the SC-CO2 extracted mackerel residues contained more soluble extracts including protein than the hexane extracted de-oiled mackerel residues (Asaduzzaman and Chun 2015).
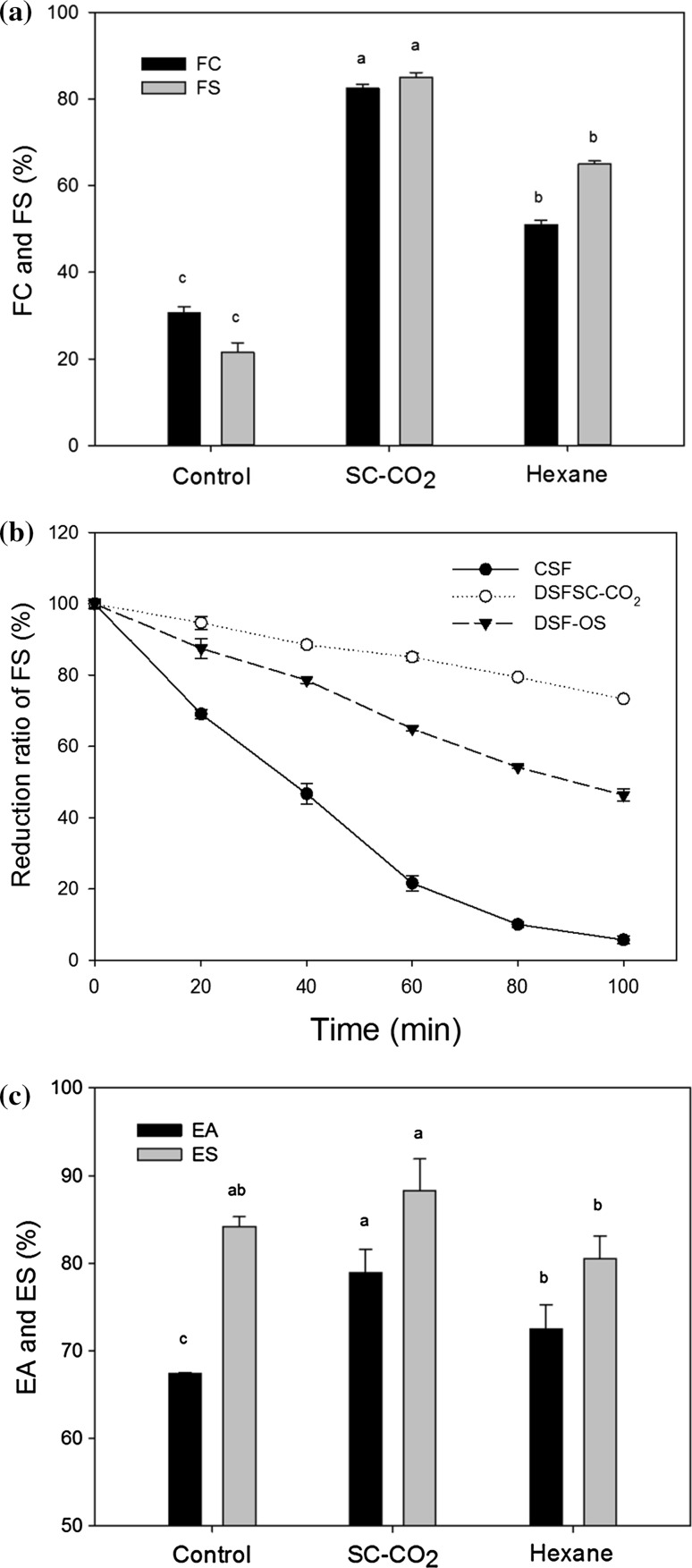
Foaming properties
Foaming properties are another important group of functionalities in the food uses of protein products. A greater concentration of soluble proteins in the aqueous phase enhances foam formation (Sun et al. 2008). In the current study, the FC of CSF, DSFSC-CO2 and DSF-OS were found to be 30.68, 82.40 and 51.01%, respectively right after homogenization, and after 1 h of homogenization, FS was found to be 21.60, 85.04 and 64.99% respectively (Fig. 4a). These results indicated that the FC and FS of DSFSC-CO2 were significantly higher (p < 0.05) than those of CSF and DSF-OS. In the current study, FS was also continuously recorded at room temperature after 20, 40, 60, 80 and 100 min, and it was observed that the FS of CSF and DSF-OS decreased more rapidly than that of DSFSC-CO2 samples (Fig. 4b). Greater foaming capacity and stability by the DSFSC-CO2 is likely due to the higher concentration of protein in DSFSC-CO2 compared to CSF and DSF-OS. Many proteins can serve as surface-active agents, as their fraction of hydrophobic and hydrophilic side-chains can bridge the gap between the air and aqueous phases, and allow creation of a large interfacial area (Moure et al. 2006; Konak et al. 2014). In addition, the proteins can interact at the interfacial film layers through intermolecular interactions to stabilize the foam (Moure et al. 2006; Zayas 1997). Sun et al. (2008) reported that the SC-CO2 extracted canola meal resulted in greater foam volume and stability than the hexane-extracted canola and hexane-extracted canola-pressed meals. Similarly, it was demonstrated by Konak et al. (2014) that the extracts from oat endosperm flour and oat fine flour had a poorer FC than the extracts of CO2 extracted oat flour.
Fig. 4.
Foaming and emulsifying properties of control soy flour (CSF), defatted soy flour by SC-CO2 (DSFSC-CO2) and defatted soy flour by an organic solvent (DSF-OS). a Foaming capacity (FC) following mixing and foaming stability (FS) after 1 h; b Reduction of foam stability over time; c Emulsifying activity (EA) and emulsion stability (ES) of different samples (%). Different letters of bars indicate significant differences (p < 0.05) among different sample
Emulsifying properties
Emulsifying properties are important in the development of new plant protein products for use as foods. These properties reflect the presence of chemicals such as proteins and polysaccharide (Jitngarmkusol et al. 2008). In this study, it was found that the emulsifying activity of DSFSC-CO2 (78.95%) was significantly higher (p < 0.05) than those of CSF (67.41%) and DSF-OS (72.50%). After the heat treatment for 1 h, it was also found that the emulsion stability of DSFSC-CO2 (88.24%) was significantly higher (p < 0.05) than that of DSF-OS (80.57%), but not significantly different (p > 0.05) than CSF (84.22%), as shown in Fig. 4c. The superior emulsifying properties of DSFSC-CO2 could be due to the higher protein content, compared to the protein content of DSF-OS and CSF. In food products, various proteins can act as emulsifiers or surfactants, and as noted for foaming properties, this is related to the array of hydrophobic and hydrophilic side chains. This helps reduce the oil–water interfacial tension, making it easier to create the interface between these phases, and helping to maintain the stability of the interface over time (Jitngarmkusol et al. 2008; Moure et al. 2006; Riaz 2006). Zayas (1997) showed that a high concentration of protein leads multiple layers that stabilize the emulsion, while low protein concentration results in a thin film with low emulsion strength. It has been found that the emulsifying capacity of SC-CO2 defatted canola meal (65%) was higher than that of the hexane-extracted canola-pressed meal (59%), and that SC-CO2 extraction has the potential for enhancing the emulsifying properties of ingredients used for food applications (Sun et al. 2008). It has also been shown that the SC-CO2 defatted wheat germ protein had strong emulsifying properties, making it a good functional protein additive for use in emulsified foods (Ge et al. 2000). This indicated that the higher protein content and better-structured protein and amino acids in DSFSC-CO2 influenced the good functionality including emulsifying properties. Similar results were demonstrated by Konak et al. (2014) that the higher protein content of the extracts prepared from CO2 oats showed superior foaming and emulsification properties compared to other oat materials. Therefore, the finding of this study confirm with those from previous studies.
Conclusion
Supercritical CO2 extraction was found to be an effective means of defatting soy flours, resulting in a product with good nutritional properties, higher protein and improved functional properties related to water binding, emulsification and foaming. In addition, SC-CO2 is a cost-effective unit operation and is generally environmentally friendly, in that there are no process effluents and the primary solvent is non-toxic. In comparison with conventional organic solvent methods, SC-CO2 can produce a product that is higher in protein and amino acids, and with properties that make it attractive for use in a variety of low-fat or enhanced-nutrition products. SC-CO2 treated soy flour had better functional properties in terms of solubility, WAC, OAC, foaming and emulsification properties. In addition, the denaturation of protein and loss of labile nutrients is more likely with soy extracted with organic solvents.
Acknowledgements
This research (Grants No. 2016-0591) was supported by Business for Cooperative R&D between Industry, Academy, and Research Institute funded Korea Small and Medium Business Administration in 2017.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
References
- Adebowale YA, Deyemi IAA, Oshodi AA. Functional and physicochemical properties of flours of six Mucuna species. Afr J Biotechnol. 2005;4(12):1461–1468. [Google Scholar]
- Agostoni C, Axelsson I, Goulet O, Koletzko B, Michaelsen KF, Puntis J, Rieu D, Rigo J, Shamir R, Szajewska H, Turck D. Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2006;42(4):352–361. doi: 10.1097/01.mpg.0000189358.38427.cd. [DOI] [PubMed] [Google Scholar]
- Ali-Nehari A, Kim SB, Lee YB, Chun BS. Digestive enzymes characterization of krill (Euphausia superba) residues deoiled by supercritical carbon dioxide and organic solvents. J Ind Eng Chem. 2012;18:1314–1319. doi: 10.1016/j.jiec.2012.01.026. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of the association of analytical chemists. Washington: AOAC; 2000. [Google Scholar]
- Asaduzzaman AKM, Chun BS. Characterization of digestive enzymes from de-oiled mackerel (Scomber japonicus) muscle obtained by supercritical carbon dioxide and n-hexane extraction as a comparative study. J Food Sci Technol. 2015;52(6):3494–3503. doi: 10.1007/s13197-014-1408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader S, Oviedo JP, Pickardt C, Eisner P. Influence of different organic solvents on the functional and sensory properties of lupin (Lupinus angustifolius L.) proteins. Food Sci Technol. 2011;44(6):1396–1404. [Google Scholar]
- Bass N, Singh G, Tanwar IK. Functional characteristics of commercial defatted soy flours. Beverage Food World. 1997;24:27–28. [Google Scholar]
- Chandi GK, Sogi DS. Functional properties of rice bran protein concentrates. J Food Eng. 2007;79:592–597. doi: 10.1016/j.jfoodeng.2006.02.018. [DOI] [Google Scholar]
- Christianson DD, Friedrich JP, List GR, Warner K, Bagley EB, Stringfellow AC, Inglett GE. Supercritical fluid extraction of dry milled corn germ with carbon dioxide. J Food Sci. 1984;49:229–232. doi: 10.1111/j.1365-2621.1984.tb13714.x. [DOI] [Google Scholar]
- Eastman JE, Moore CO (1984) Cold water soluble granular starch for gelled food composition. US Patent no 4,465,702
- Faraj A, Vasanthan T. Soybean isoflavones: effects of processing and health benefits. Food Rev Int. 2004;20(1):51–75. doi: 10.1081/FRI-120028830. [DOI] [Google Scholar]
- Ge Y, Sun A, Ni Y, Cai T. Some nutritional and functional properties of defatted wheat germ protein. J Agric Food Chem. 2000;48:6215–6218. doi: 10.1021/jf000478m. [DOI] [PubMed] [Google Scholar]
- Heywood AA, Myers DJ, Bailey TB, Johnson LA. Functional properties of low-fat soy flour produced by an extrusion-expelling system. J Am Oil Chem Soc. 2002;79(12):1249–1253. doi: 10.1007/s11746-002-0635-y. [DOI] [Google Scholar]
- Jeong CH, Shim KH. Quality characteristics of sponge cakes with addition of Pleurotus eryngii mushroom powders. J Korean Soc Food Sci Nutr. 2004;33:716–722. doi: 10.3746/jkfn.2004.33.4.716. [DOI] [Google Scholar]
- Jitngarmkusol S, Hongsuwankul J, Tananuwong K. Chemical compositions, functional properties, and microstructure of defatted macadamia flours. Food Chem. 2008;110:23–30. doi: 10.1016/j.foodchem.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Jyothirmayi T, Rao PG, Walde SG. Nitrogen extractability and functional properties of defatted Erythrina variegata flour. Food Chem. 2006;96:242–247. doi: 10.1016/j.foodchem.2005.02.023. [DOI] [Google Scholar]
- Kang SW, Kim HM, Rahman MS, Kim AN, Yang HS, Choi SG. Nutritional quality and physicochemical characteristics of defatted bovine liver treated by supercritical carbon dioxide and organic solvent. Korean J Food Sci Anim. 2017;37(1):29–37. doi: 10.5851/kosfa.2017.37.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorshid N, Hossain MM, Farid MM. Precipitation of food protein using high pressure carbon dioxide. J Food Eng. 2007;79(4):1214–1220. doi: 10.1016/j.jfoodeng.2006.04.037. [DOI] [Google Scholar]
- Konak UI, Ercili-Cura D, Sibakov J, Sontag-Strohm T, Certel M, Loponen J. CO2-defatted oats: solubility, emulsification and foaming properties. J Cereal Sci. 2014;60:37–41. doi: 10.1016/j.jcs.2014.01.013. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mao XY, Hua YF. Chemical composition, molecular weight distribution, secondary structure and effect of NaCl on functional properties of walnut (Juglans regia L.) protein isolates and concentrates. J Food Sci Technol. 2014;51(8):1473–1482. doi: 10.1007/s13197-012-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moure A, Sineiro J, Domínguez H, Parajó JC. Functionality of oilseed protein products: a review. Food Res Int. 2006;39(9):945–963. doi: 10.1016/j.foodres.2006.07.002. [DOI] [Google Scholar]
- Ogunwolu SO, Henshaw FO, Mock HP, Santros A, Awonorin SO. Functional properties of protein concentrates and isolates produced from cashewnut (Anacardium occidentale L.) Food Chem. 2009;115(3):852–858. doi: 10.1016/j.foodchem.2009.01.011. [DOI] [Google Scholar]
- Park JY, Back SS, Chun BS. Protein properties of mackerel viscera extracted by supercritical carbon dioxide. J Environ Biol. 2008;29(4):443–448. [PubMed] [Google Scholar]
- Raynie DE (1997) Meeting the natural products challenge with supercritical fluids. In: Supercritical fluids extraction and pollution prevention, ACS Symposium Series 670, Washington DC, pp 68–75
- Riaz MN (2006) Soy applications in foods. CRC Press, Taylor and Francis Group, pp 19, 68–69, 211, 227–247
- Russin TA, Boye JI, Arcand Y, Rajamohamed SH. Alternative techniques for defatting soy: a practical review. Food Bioprocess Technol. 2011;4:200–223. doi: 10.1007/s11947-010-0367-8. [DOI] [Google Scholar]
- Sihvonen M, Jarvenpaa E, Hietaniemi V, Huopalahti R. Advances in supercritical carbon dioxide technologies. Trends Food Sci Technol. 1999;10:217–222. doi: 10.1016/S0924-2244(99)00049-7. [DOI] [Google Scholar]
- Singh P, Kumar R, Sabapathy SN, Bawa AS. Functional and edible uses of soy protein products. Compr Rev Food Sci Food Saf. 2008;7:14–28. doi: 10.1111/j.1541-4337.2007.00025.x. [DOI] [Google Scholar]
- Sparks D, Hernandez R, Zappi M, Blackwell D, Flaming T. Extraction of rice bran oil using supercritical carbon dioxide and propane. J Am Oil Chem Soc. 2006;83:885–891. doi: 10.1007/s11746-006-5042-x. [DOI] [Google Scholar]
- Stahl E, Schutz E, Mangold HK. Extraction of seed oils with liquid and supercritical carbon dioxide. J Agric Food Chem. 1980;28:1153–1157. doi: 10.1021/jf60232a023. [DOI] [Google Scholar]
- Sun M, Temelli F. Supercritical carbon dioxide extractions of carotenoids from carrot using canola oil as a continuous cosolvent. J Supercrit Fluids. 2006;37:397–408. doi: 10.1016/j.supflu.2006.01.008. [DOI] [Google Scholar]
- Sun M, Xu L, Saldana MDA, Temelli F. Comparison of canola meals obtained with conventional methods and supercritical CO2 with and without ethanol. J Am Oil Chem Soc. 2008;85:667–675. doi: 10.1007/s11746-008-1239-5. [DOI] [Google Scholar]
- Turner C, King JW, Mathiasson L. Supercritical fluid extraction and chromatography for fat-soluble vitamin analysis. J Chromatogr A. 2001;936:215–237. doi: 10.1016/S0021-9673(01)01082-2. [DOI] [PubMed] [Google Scholar]
- Uddin MS, Ahn HM, Kishimura H, Chun BS. Comparative study of digestive enzymes of squid (Todarodes pacificus) viscera after supercritical carbon dioxide and organic solvent extraction. Biotechnol Bioprocess Eng. 2009;14:338–344. doi: 10.1007/s12257-008-0271-5. [DOI] [Google Scholar]
- Zayas FJ (1997) Functionality of proteins in foods. Springer, Berlin, Heidelberg, New York, pp 7, 34, 79, 244, 272