Abstract
The objective of this study was to determine the effects of powdered leaves of lotus (LP), shepherd’s purse (SP) and goldenrod (GP) on oxidation stability and quality characteristics of cooked duck/pork patties. Fresh duck tenderloin (M. pectoralis) and pork meat (M. biceps femoris, semitendinosus, and semimembranosus) were ground, formulated with LP, SP, GP or butylated hydroxytoluene (BHT), and mixed with other non-meat ingredients. The manufactured patties were cooked, packaged, and stored at 3 °C for 4 weeks. The patties containing 1% of LP, SP and GP had significantly lower values in redness, thiobarbituric acid reactive substances, conjugated dienes and total volatile basic nitrogen compared to control. No significant differences in sensory tenderness between the control and treated samples were observed. Addition of LP had a similar warmed-over flavor extent compared to patties with BHT. These results indicate that incorporation of the natural leaves could effectively inhibit oxidation and maintain freshness of cooked patties without any detrimental effects on sensory attributes during storage.
Keywords: Plant powder, Antioxidant property, Lipid oxidation, Restructured duck/pork patty
Introduction
There has been a growing interest in manufacturing duck meat-based processed meat products mainly due to its nutritional value and unique organoleptic characteristics (Liao et al. 2010). Especially, restructured duck/pork patties are sold in demand market in Asian country. In general, duck meat contains more than 70% of unsaturated fatty acids (Park et al. 1986) and essential amino acids, such as lysine (2.19%), valine (1.29%), isoleucine (1.22%), and methionine (0.56%) (Genchev et al. 2008), however the proximate composition can be changed depending on age, sex, feed, and so on (Poole et al. 1999). However, the high contents of unsaturated fatty acids could result in oxidative quality defects of the duck meat products during further processing and/or storage periods (Park, et al. 1986). A study found that ground duck meat had five times higher extent of lipid oxidation than that of ground chicken meat when thermally treated at 80 °C (Hoac et al. 2006).
Lipid oxidation of meat products induced by a thermal treatment and its subsequent adverse impacts on flavor and nutritive value have been well documented (Serpen et al. 2012). The addition of antioxidants to meat products could prevent or delay the oxidation process and production of toxic compounds during processing, such as cooking and storage (Cosio et al. 2006). Synthetic antioxidants such as butylhydroxytoluene (BHT), buthylhydroxyanisole (BHA) and tert-butylhydroquinone (TBHQ) have been used in food industry for many years. However, due to potential health problems associated with using synthetic antioxidants (Armitage et al. 2002), the application of natural antioxidants (mostly originated from plant, grains or fruits) in meat products has been extensively studied (Shah et al. 2014) and practiced in the meat industry.
It has been reported that the lotus leaf powder (LP), one of the widely cultivated plants in Asia (Kim and Park 2008) contains natural potent antioxidants, including phenolic acids and flavonoids, whilst exhibiting functional properties, such as anticancer and antimicrobial activities (Lee and Lee 2006; Jeong et al. 2010). Furthermore, the leaf powders of shepherd’s purse (Capsella bursa-pastoris, SP) and goldenrod (Solidago virgaurea, GP) were found to have some antioxidants, such as polyphenols and vitamin A (Hong et al. 1994). Although the antioxidant properties of LP in various meat products, including both fresh and cooked products, have been previously studied (Choe et al. 2011; Huang et al. 2011), there has been very little research reported on antioxidant activities of GP and SP in the processed meat application. Given the known general antioxidant properties of GP and SP, it would be reasonable to hypothesize that the addition of GP and/or SP into meat products could minimize oxidation related quality defects of the meat products (particularly products containing high unsaturated fatty acids, like duck meat) during further processing and/or storage periods. Therefore, the objective of the present study was to evaluate the antioxidant effects of the LP, SP and GP on cooked restructured duck and pork patties prepared according to commercial practices and stored refrigerated.
Materials and methods
Preparation of powdered leaves of lotus, shepherd’s purse, and goldenrod
The LP, SP and GP (free-radical scavenging activity: 92.8, 85 and 88% at concentration of 5 mg/ml, respectively) were purchased from a local market (Seoul, Korea). The leaves were thoroughly washed with water, chopped, and dried in a hot air dryer (Enex-Co-600, Enex, Koyang, Korea) at 50 °C for 15 h. They were pulverized with a blender (KA-2610, Jworld Tech, Ansan, Korea) for 30 s, ground with a screen to pass through a 35-mesh sieve, and stored at −20 °C until use.
Preparation of duck/pork patties
Fresh duck tenderloin (4.5 kg) (M. pectoralis minor) and pork meat (3.5 kg) (M. biceps femoris, M. semitendinosus, and M. semimembranosus) at 48 h postmortem were purchased from a local meat purveyor. All subcutaneous, intramuscular fat and visible connective tissues were removed from the fresh pork. The duck and pork restructured cooked patties were produced to the following formulation: 55% duck meat, 35% pork meat, 10% ice, 5.8% soy sauce, 1% isolated soybean protein, 1% sugar, 0.5% nitrite pickled salt (salt:nitrite = 99.4:0.6), 0.5% onion powder and 0.1% black pepper. The duck tenderloin and lean pork meat were ground through a 3 mm plate, and then mixed with ice and salt. The lotus leaf powder (LP), shepherd’s purse (SP) and goldenrod leaf powder (GP) were added at 1%, and butylated hydroxytoluene (BHT) at 0.02% into the ground duck/pork meat (LPP, SPP, GPP and BHTP, respectively). These percentages were based on the weight of duck/pork restructured meat without antioxidant powder. Samples were mixed using Kneader mixer 1305 (Thermometer, Mainca, Barcelona, Spain) for 5 min. Then, the mixed meat was weighed (120 ± 1 g) and formed (diameter: 100 mm, thickness: 20 mm) using a patty mold (PM 10/13 ground press, AB Services Food Machinery, Coventry, England). The formed mixed meat were placed in a mold and cooked at 170 °C in a convection oven (Convotherm OES 6.06 mini, HRS, Eglfing, Germany) until the internal temperature of meat samples reached 75 ± 0.5 °C for approximately 30 min, as measured by a temperature probe (RS-232/Data logger 1305 Thermometer, TES, Taishi, Taiwan). The cooked restructured patties on each treatment (control, LPP, SPP, GPP and BHTP) were cooled at room temperature and three patties assigned to each storage sampling day. Then meat samples were placed into PE/nylon film bags and sealed and stored in the dark at 3 ± 1 °C for 4 weeks.
Determination of pH values
Following the blending of 5 g of the cooked meat samples with 20 ml of distilled water for 60 s in a homogenizer (Ultra-Turrax SK15, Janke & Kunkel, Staufen, Germany), pH values of the samples were determined using a pH meter (Model 340, Mettler-Toledo GmbH, Schwerzenbach, Switzerland) on a weekly basis for 4 weeks.
Evaluation of instrumental color
The color values (CIE L*, a*, and b*) of cooked patties were measured using a colorimeter (Chroma meter CR-210, Minolta Co., Osaka, Japan; illuminate C, 10° standard observer, calibrated with a white standard plate CIE L* = +97.83, CIE a* = −0.43, CIE b* = +1.98, consisting of a measuring area with a 8 mm diameter and an illumination area with a 50 mm diameter). Each patty surface was scanned in two different locations and averaged for further data analysis.
Lipid oxidation
Determination of conjugated dienes (CD)
Lipids were extracted using the method described by Folch et al. (1957). Conjugated dienes (CD) were determined as outlined by Prasetyo et al. (2008). Briefly, 15 mg of the extracted lipid samples were placed in a 25 ml volumetric flask and brought to volume with isooctane. Subsequently, the absorbance of the samples was measured against a blank (isooctane) at 234 nm using a UV–Visible spectrophotometer (Libra S22, Biochrom Ltd., Cambridge, England). The concentration of CD was calculated using a molar extinction coefficient of 25,200 M−1 cm−1 and the results were expressed as μmol/mg meat lipid sample.
Determination of thiobarbituric acid reactive substances (TBARS)
Lipid oxidation was assessed using the direct-distillation method as described by Tarladgis et al. (1960), with minor modifications. Each sample was analyzed in triplicate. Briefly, 10 g of the sample was blended with 50 ml of distilled water prior to homogenization (AM-7, Nihonseiki Kaisha Ltd., Japan) at 10,000 rpm for 2 min and transferred to a distillation flask. The cup used for blending was washed with an additional 47.5 ml of distilled water, which was subsequently added to the same distillation flask containing 2.5 ml of 4 N HCl and a few drops of an anti-foaming agent, silicone o/w (KMK-73, Shin-Etsu Silicone Co., Ltd., Seoul, Korea). Then, the mixture was distilled and 50 ml of distillate was collected. Five ml of 0.02 M 2-thiobarbituric acid in 90% acetic acid (TBA reagent) was added to a vial containing 5 ml of the distillate and mixed. The vials were capped and heated in a boiling water bath for 30 min to develop the chromogen and cooled to room temperature. The absorbance was measured at 538 nm (Libra S22, Biochrom Ltd., Cambridge, England) against a blank prepared with 5 ml distilled water and 5 ml TBA reagent. The K value was determined using 1,1,3,3-tetraethoxypropane (Sigma) as the standard and TBARS values were calculated by multiplying the absorbance values by the corresponding K value.
Determination of total volatile basic nitrogen (TVBN)
Total volatile basic nitrogen was determined by the Conway micro diffusion method (PSQ 1980) and the results were expressed as mg N/100 g of sample using following steps: 10 g of meat sample was homogenized with 15 ml of distilled water (DW) for 1 min and then DW was added to bring the volume up to the final 50 ml followed by filtering through Whatman paper no. 1. One milliliter of filtrate was placed in outer well of Conway unit, and 1 ml of 0.01 N H3BO3 and 200 μl of Conway reagent (0.066% methyl red in ethanol: 0.066% bromocresol green in ethanol = 1:1) were added in inner well of Conway unit. The Conway unit was sealed immediately after adding 1 ml of 50% K2CO3 to the outer well. The sealed Conway unit was rotated slowly and incubated at 37 °C for 2 h. Then, 0.02 N H2SO4 was added to inner well till the blue color changes to pink for titration.
Determination of shear force
The shear force values of the cooked meat samples were obtained using a texture analyzer (TA-XT2i, Stable Micro System Ltd., Surrey, UK) fitted with a Warner–Bratzler shear attachment. The shear force values were analyzed to obtain the maximum force required to shear through each sample and the results were expressed in Newton (N).
Sensory evaluations
The sensory evaluation was performed by a 24 semi-trained panelists. The training of panelists was performed according to Meilgaard, Civille, and Carr (1999). They were trained using commercial patties in five batches to familiarize them with the general product characteristics to be evaluated. Panelists were instructed to cleanse their palates with water between the samples. The color, tenderness, and warmed-over flavor (1 = extremely undesirable, 5 = moderate, 10 = extremely desirable) of the samples were evaluated using a 10-point descriptive category scale.
Statistical analyses
The experimental design of the current study was a complete randomized block design, where each non-meat ingredient addition was randomly assigned to raw duck/pork meat mixture processed from a single batch, and the storage time effect was tested within the treatment. The interaction effect between treatment and storage time was considered for pH, CD, TBARS, VBN and shear force values. The experiment was replicated in three different times. Analysis of variance was performed on all the variables measured using the general linear model (GLM) procedure of the SAS statistical package (SAS 2010). Differences in means between treatments were determined using Duncan’s multiple range tests (P < 0.05).
Results and discussion
Change in pH values
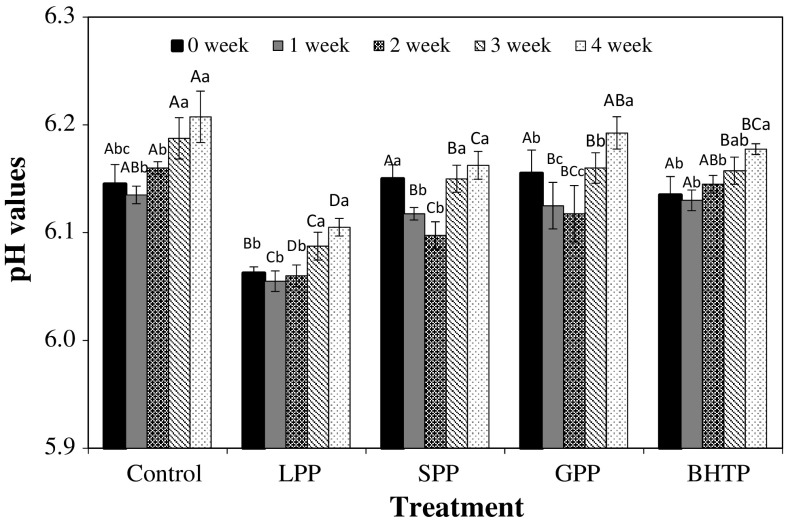
The pH values of cooked duck/pork patties during the refrigerated storage were ranged from 6.06 to 6.21 (Fig. 1). There was no significant interaction effect between treatment and storage time, but significant main effects (leaf powder addition and storage time) on pH values were found. The pH values of the LP treated meat samples were significantly lower compared to other treatments, which could be due to the presence of organic acids in LP during the entire storage period (Yang et al. 2007). The pH values of the SPP and BHTP treated samples were decreased until 2 weeks and then increased until the end of storage (P < 0.05). Similar trends were reported in a previous study, where pH values increased in cooked meat products with/without BHA or tocopherol with increasing storage time (Aksu and Kaya 2005).
Fig. 1.
Change of pH values of cooked duck/pork patties with adding of various leaf powders and BHT during refrigerated storage. Control: duck/pork patties without antioxidant, LPP: duck/pork patties with 1% lotus leaf powder, SPP: duck/pork patties with 1% shepherd’s purse leaf powder, The error bars indicate standard deviation, n = 6. GPP: duck/pork patties with 1% goldenrod leaf powder, BHTP: duck/pork patty with 0.02% BHT. Different superscript letters (A–D) within each treatment indicate significant differences between storage times (P < 0.05). Different superscript letters (a–c) within each storage time indicate significant differences between treatments (P < 0.05)
Change in color evaluation
The color of meat, both fresh and cooked, is an important quality attribute that influences a consumer’s purchasing choice. Meat discoloration observed in cooked meat occurs due to the denaturation of protein (mainly myoglobin) by heat (King and Whyte 2006). Significant main effects (leaf powder addition and storage time) on the color of cooked duck/pork patties were found (Table 1; Fig. 2). The addition of leaf powders significantly decreased L* (lightness) values in freshly cooked patties. In general, L* values increased with increasing storage time, except for control (P < 0.05). Choe et al. (2011) reported similar results where the L* values of cooked ground pork meat samples treated with lotus leaf powder were significantly increased with increasing storage time at 4 °C.
Table 1.
Change of L* (lightness) and b* (yellowness) values of cooked duck/pork patties with addition of various leaf powders and BHT during refrigerated storage
| Treatment1 | Storage time (weeks) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| L* | Control | 57.29 ± 1.28Aa | 56.21 ± 1.05ABa | 55.16 ± 1.28BCa | 54.91 ± 0.93BCa | 53.75 ± 1.30Cb |
| LPP | 39.73 ± 0.72De | 43.49 ± 1.05Cd | 44.16 ± 1.45BCd | 45.27 ± 0.85Bd | 48.70 ± 0.81Ac | |
| SPP | 48.83 ± 1.32Cc | 50.01 ± 0.79BCc | 50.60 ± 1.40Bb | 52.40 ± 0.51Ab | 53.22 ± 0.89Ab | |
| GPP | 41.29 ± 1.50Dd | 44.47 ± 0.88Cd | 45.97 ± 1.23BCc | 46.67 ± 1.36Bc | 48.72 ± 1.35Ac | |
| BHTP | 53.36 ± 1.19Db | 54.69 ± 0.61Cb | 55.14 ± 0.95BCa | 55.76 ± 0.56ABa | 56.31 ± 0.68Aa | |
| b* | Control | 18.70 ± 0.24Bb | 18.43 ± 0.63Bb | 19.03 ± 0.55Ba | 18.73 ± 0.49Bbc | 20.10 ± 0.69Aa |
| LPP | 16.60 ± 0.39Bc | 16.80 ± 0.36Bc | 17.13 ± 0.38Bb | 17.88 ± 0.52Acd | 18.21 ± 0.49Ac | |
| SPP | 19.75 ± 0.33a | 19.34 ± 0.88ab | 19.52 ± 0.69a | 19.08 ± 1.12ab | 18.98 ± 0.45b | |
| GPP | 16.38 ± 0.94Cc | 16.99 ± 1.14BCc | 17.07 ± 0.36BCb | 17.64 ± 0.79Bd | 18.77 ± 0.44Abc | |
| BHTP | 18.85 ± 0.62ABb | 19.93 ± 0.81ABa | 19.32 ± 0.81Ba | 19.64 ± 0.48ABa | 20.39 ± 0.69Aa | |
All values are mean ± SD, n = 9
A–DMeans within rows with different superscript letters are significantly different (P < 0.05)
a–dMeans within columns with different superscript letters are significantly different (P < 0.05)
1Control, duck/pork patties without antioxidant; LPP, duck/pork patties with 1% lotus leaf powder; SPP, duck/pork patties with 1% shepherd’s purse leaf powder; GPP, duck/pork patties with 1% goldenrod leaf powder; BHTP, duck/pork patty with 0.02% butylhydroxytoluene
Fig. 2.
Change of a* values of cooked duck/pork patties added with various leaf powders and BHT during refrigerated storage. (Filled squares) Control: duck/pork patties without antioxidant, (filled diagonals) LPP: duck/pork patties with 1% lotus leaf powder, (open squares) SPP: duck/pork patties with 1% shepherd’s purse leaf powder, (open diagonals) GPP: duck/pork patties with 1% goldenrod leaf powder, (open circles) BHTP: duck/pork patty with 0.02% BHT. The error bars indicate standard deviation, n = 12
Significant increase in b* (yellowness–blueness) values were observed at week 3 or week 4 for LPP and GPP and control and BHTP, respectively, compared with initial one of storage. No significant change in b* value was seen in SPP.
In general, a* values (redness) decreased with storage time (P < 0.05). The meat samples treated with various leaf powders have lower a* values (P < 0.05), when compared to control and those treated with BHT throughout the whole storage periods (Fig. 2). This might be attributed to the strong green pigment of various leaf powders. The highest a* value was found in the BHT and the lowest was found in the GP treated meat samples throughout the entire storage periods. However, the LP and GP showed lower extent of discoloration between initial and end of storage time compared to control. This result might be attributed to the fact that polyphenol of plant powder delay rate of ferrihemochrome formation, that has brown color, by inhibiting oxidation from ferrohemochrome (King and Whyte 2006; van Laack et al. 1996). Several studies showed that natural antioxidants, such as lotus leaf, grape dietary fiber and rosemary reduce discoloration and inhibit the oxidation of protein between initial and end of storage days on cooked ground meat (Han and Rhee 2005; Sáyago-Ayerdi et al. 2009; Choe et al. 2011). The results of the present study also showed a color stabilizing impact of the addition of LP, SP, and GP on duck/pork patties by minimizing the change of redness during the 4 weeks of storage time.
Change in conjugated dienes (CD) values
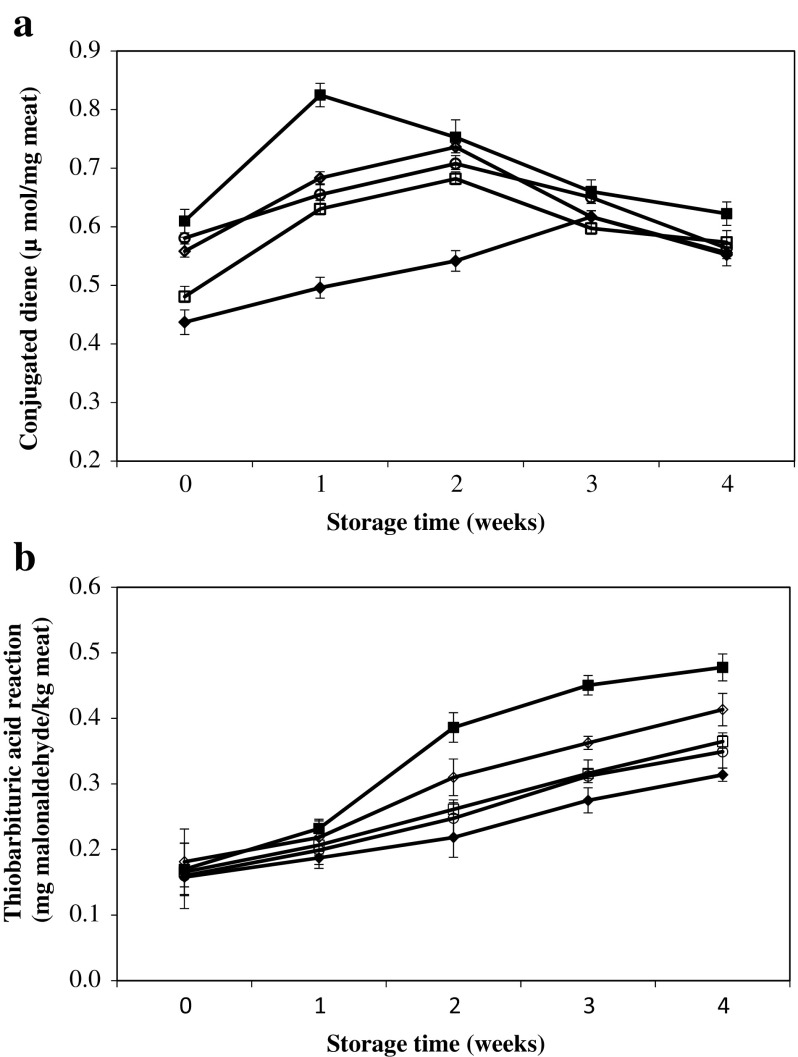
Duck meat products are prone to lipid oxidation due to the presence of unsaturated fatty acids (Park et al. 1986). Unsaturated fatty acids are liable to intramolecular rearrangements of double bonds, thus producing conjugated dienes (–C=C–C=C–), with low hydrogen donating capacity. Thus, CD concentration indicates the degree of primary lipid oxidation in meat and meat products. A significant interaction effect between leaf powder treatment and storage time on CD values was found as shown in Fig. 3a. At day 0, meat products mixed with LP and SP had significantly lower CD values compared to other treatments. After 1 week of storage, there was a sharp increase in CD of control samples, whereas the meat samples treated with LP maintained the lowest CD values among the treatments until week 2. The meat samples treated with GP, SP and BHT showed similar extent of CD accumulation during storage. Taken together, in general, the addition of LP, SP and GP delayed the accumulation rate of primary lipid oxidation products compared to control. Our findings are in accordance with a previous study by Sampaio et al. (2012). They reported that the addition of natural ingredients from plants effectively inhibited primary lipid oxidation in cooked meat samples, which were, in turn, associated with the lowest CD values. We also observed that the CD values initially peaked and subsequently declined with increasing storage time, which would likely indicate that the extent of decomposition of secondary products might be much higher than that of the primary products during lipid oxidation. However, no significant differences in CD values were observed between the treatments at week 4. Juntachote et al. (2006) found similar results that at end of storage time, when there was no significant difference in CD values of cooked ground pork added different leaf powders.
Fig. 3.
Change of a conjugated dienes (CD) values (μmol/mg meat) and b thiobarbituric acid reaction substance (TBARS) values (mg MDA/kg meat) of cooked duck/pork patties with adding of various leaf powders and BHT during refrigerated storage. (Filled squares) Control: duck/pork patties without antioxidant, (filled diagonals) LPP: duck/pork patties with 1% lotus leaf powder, (open squares) SPP: duck/pork patties with 1% shepherd’s purse leaf powder, (open diagonals) GPP: duck/pork patties with 1% goldenrod leaf powder, (open circles) BHTP: duck/pork patty with 0.02% BHT. The error bars indicate standard deviation, n = 12
Change in thiobarbituric acid reactive substances (TBARS) values
TBARS values have been extensively used to measure the concentration of secondary lipid oxidation products (malondialdehydes) produced by the decomposition of primary lipid oxidation products (Juntachote et al. 2006; Choe et al. 2011). In the present study, the TBARS values of the meat products significantly increased during the refrigerated storage (Fig. 3b). There was no significant interaction effect between treatment and storage time in TBARS values. At the initial storage time, no significant differences on TBARS values were observed between the control and treatments (P > 0.05). However, from week 2, the meat samples treated with LP, SP, and BHT showed significantly lower TBARS values compared to the control and those treated with GP. The rate of increase in TBARS values observed was the highest (39.9%) in control between week 1 and week 2 of refrigerated storage. These results could be associated with the decline in CD values as discussed above. Herein, the meat samples treated with various leaf powders and BHT showed significantly lower TBARS values compared to the control at week 4. Particularly, the addition of LP resulted in the lowest TBARS values among the treatment at week 4 of refrigerated storage. In this study, the addition of LP, SP, GP and BHT led to a decrease in TBARS values stating 83, 62, 54 and 63%, respectively, compared to TBARS values of control at week 4 of storage. These results indicate a strong antioxidant property of LP, SP, and GP against lipid oxidation of restructured duck/pork patties. The observed superior inhibition of LP against lipid oxidation could be due to its high phenolic contents comprised of hyperin, isoquercetin, astragalin, and kaempferol (Ohkoshi et al. 2007).
Change in total volatile basic nitrogen (TVBN) values
Proteins in duck meat could be prone to oxidative decomposition by microorganisms or enzymes during storage (Liu et al. 2009). As a result, ammonia, trimethylamine (TMA) and dimethylamine (DMA) are produced and can be assayed by VBN content, which could be an indicator determining freshness of meat products (He et al. 2013).
The TVBN values of duck/pork meat samples ranged from 124.2 to 172.8 mg N/100 g during refrigerated storage for 4 weeks (Table 2). There was no significant interaction between treatment and storage time, but significant main effects (leaf powder addition and storage time) on the TVBN values were observed. At the initial storage time, no differences in the TVBN was found among the treatment except for LPP that had lower TVBN content. However, from week 2–4, treated samples had significantly lower TVBN values than control. Most importantly, at week 4, the TVBN values of restructured duck/pork patties treated with LP and BHT were significantly reduced compared to the other treatments. In this study, these numerical or significant low TVBN values of meat samples with natural powder might be due to the antimicrobial activities of LP, SP and GP, which were reported by previous studies (Li and Xu 2008; Lim and Yum 2009; Starks et al. 2010). Further studies would be warranted to determine the antimicrobial activity of leaf powders and its subsequent impacts on protein composition of duck/pork restructured meat products.
Table 2.
Change of total volatile basic nitrogen (TVBN) (mg N/100 g) and shear force (N) of cooked duck/pork patties with addition of various leaf powders and BHT during refrigerated storage
| Trait | Treatment1 | Storage time (weeks) | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| VBN | Control | 134.5 ± 0.28Da | 153.1 ± 0.16Ca | 161.5 ± 0.56Ba | 166.2 ± 0.74Ba | 172.8 ± 0.16Aa |
| LPP | 124.2 ± 0.43Db | 133.5 ± 0.16Cc | 141.9 ± 0.16Bc | 148.5 ± 0.28Ac | 153.1 ± 0.43Ac | |
| SPP | 143.8 ± 0.28Da | 146.6 ± 0.16Cb | 156.9 ± 0.28Bb | 160.6 ± 0.16Bab | 166.2 ± 0.43Aab | |
| GPP | 137.3 ± 0.28Da | 148.5 ± 0.28Cb | 153.1 ± 0.16Cb | 161.5 ± 0.43Bab | 168.1 ± 0.28Aab | |
| BHTP | 134.5 ± 0.28Ca | 145.7 ± 0.28Bb | 154.1 ± 0.28Ab | 158.7 ± 0.43Ab | 160.6 ± 0.65Abc | |
| Shear force | Control | 201.9 ± 1.69Db | 211.5 ± 1.62CD | 223.2 ± 1.74BCa | 232.3 ± 1.43Ba | 253.4 ± 2.46Aa |
| LPP | 212.8 ± 1.24ABab | 228.0 ± 1.71A | 199.1 ± 1.55Bab | 209.5 ± 1.99ABb | 220.4 ± 1.35Ab | |
| SPP | 226.4 ± 1.51Aa | 228.2 ± 1.02A | 191.9 ± 2.77Bb | 212.8 ± 1.44ABb | 212.2 ± 2.42ABb | |
| GPP | 212.3 ± 1.66ABab | 219.4 ± 2.65AB | 188.9 ± 1.85Bb | 189.7 ± 1.62Bc | 229.7 ± 2.31Ab | |
| BHTP | 200.5 ± 1.65Bb | 228.8 ± 2.75AB | 207.5 ± 2.93Bab | 218.5 ± 2.12ABab | 237.4 ± 1.60Aab | |
All values are mean ± SD, n = 12
A–DMeans within rows with different superscript letters are significantly different (P < 0.05)
a–cMeans within columns with different superscript letters are significantly different (P < 0.05)
1Control, duck/pork patties without antioxidant; LPP, duck/pork patties with 1% lotus leaf powder; SPP, duck/pork patties with 1% shepherd’s purse leaf powder; GPP, duck/pork patties with 1% goldenrod leaf powder; BHTP, duck/pork patty with 0.02% butylhydroxytoluene
Change in shear force
The incorporation of various leaf powders affected the shear force values of duck/pork patties during the refrigerated storage for 4 weeks (P < 0.05; Table 2). The meat samples treated with LP, SP, and GP had higher shear force values compared to the control and BHT on day 0, prior to storage. Particularly, the SP addition resulted in the highest shear force value at day 0 (P < 0.05). The shear force values significantly increased in control as well as in duck/pork patties treated with BHT at week 4 compared to day 0. On the contrary, the LPP, SPP, and GPP treatments had no impact on the shear force of the samples during refrigerated storage period. At week 4, the shear force values of duck/pork patties treated with LP, SP, and GP were significantly lower compared to that of control. However, practically, the observed difference in shear force values between the samples should be considered as marginal (<3 N differences), and thus would not be practically meaningful to affect eating quality attributes (particularly tenderness) of cooked patties (Bickerstaffe et al. 2001), which were to be discussed in more detail in the following section.
Change in sensory qualities
The sensory properties were partially affected by addition of various leaf powders on duck/pork patties during the refrigerated storage for 4 weeks (P < 0.05; Table 3). No significant differences in color and tenderness were observed between control and various treatments at start and end of storage trials. Also there was no significant difference in tenderness between control and the treatments during the entire of storage time. This result would be likely due to the fact that the panel would not be able to distinguish the observed tenderness difference between 1 and 5 N of duck/pork patties (Bickerstaffe et al. 2001), as mentioned above section. The sensory panel found an increase in warmed-over flavor of the samples with an increase in storage periods. However, LPP and BHTP exhibited the lowest warmed-over flavor scores at the end of storage (P < 0.05). This result showed that the incorporation of LP and BHT could delay development of warm-over flavor caused by lipid oxidation, cooking and/or storage.
Table 3.
Sensory evaluation of cooked duck/pork patties with addition of various leaf powders and BHT during refrigerated storage
| Treatment1 | Storage time (weeks) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Color2 | Control | 8.20 ± 1.32A | 8.56 ± 0.53Aa | 8.11 ± 0.60Aab | 7.30 ± 0.82AB | 7.20 ± 0.42B |
| LPP | 7.70 ± 0.67 | 7.33 ± 1.00b | 7.56 ± 0.73ab | 7.60 ± 0.84 | 7.40 ± 0.97 | |
| SPP | 7.40 ± 1.17 | 7.89 ± 0.78ab | 8.11 ± 0.93ab | 7.90 ± 0.57 | 7.20 ± 0.94 | |
| GPP | 7.90 ± 0.99A | 7.22 ± 0.97ABb | 7.33 ± 1.22ABb | 7.60 ± 0.70AB | 6.70 ± 0.95B | |
| BHTP | 8.80 ± 1.14A | 8.67 ± 0.50Aa | 8.33 ± 0.71Aa | 8.30 ± 0.82A | 7.30 ± 0.67 | |
| Tenderness | Control | 8.20 ± 0.42 | 7.67 ± 0.71 | 8.00 ± 0.71 | 7.80 ± 0.42 | 7.70 ± 0.67 |
| LPP | 7.90 ± 0.32 | 7.67 ± 1.12 | 7.67 ± 0.50 | 7.50 ± 0.53 | 7.50 ± 0.71 | |
| SPP | 8.00 ± 0.82 | 7.56 ± 1.13 | 7.44 ± 1.42 | 7.50 ± 0.71 | 7.30 ± 0.82 | |
| GPP | 7.90 ± 0.99 | 7.78 ± 1.30 | 7.78 ± 0.97 | 7.70 ± 0.48 | 7.00 ± 0.67 | |
| BHTP | 8.20 ± 0.85 | 8.11 ± 1.05 | 7.89 ± 0.33 | 7.90 ± 0.88 | 7.70 ± 0.67 | |
| Warmed-over flavor | Control | 8.80 ± 0.92A | 8.33 ± 1.00AB | 8.44 ± 1.13AB | 7.90 ± 0.99AB | 6.30 ± 1.07Bb |
| LPP | 8.90 ± 0.99A | 7.22 ± 0.97AB | 8.56 ± 1.01A | 8.20 ± 0.92AB | 7.50 ± 0.18Ba | |
| SPP | 8.40 ± 1.07A | 7.56 ± 0.73A | 7.85 ± 1.69A | 7.20 ± 1.48AB | 6.20 ± 0.79Bb | |
| GPP | 8.40 ± 1.17A | 7.56 ± 0.73A | 7.90 ± 1.05A | 7.50 ± 0.85A | 6.70 ± 1.06Bb | |
| BHTP | 8.70 ± 1.06A | 8.22 ± 0.83AB | 8.56 ± 1.13A | 8.00 ± 1.05AB | 7.40 ± 0.70Ba | |
All values are mean ± SD, n = 48
A–BMeans within rows with different superscript letters are significantly different (P < 0.05)
a–bMeans within colums with different superscript letters are significantly different (P < 0.05)
1Control, duck/pork patties without antioxidant; LPP, duck/pork patties with 1% lotus leaf powder; SPP, duck/pork patties with 1% shepherd’s purse leaf powder; GPP, duck/pork patties with 1% goldenrod leaf powder; BHTP, duck/pork patty with 0.02% butylhydroxytoluene
2Color, tenderness, warmed-over flavor: 1 = extremely undesirable, 5 = moderate, 10 = extremely desirable
Conclusion
The results from the current study suggest that incorporation of different leaf powders (LP, SP, and GP) in cooked duck/pork patties, which are prone to oxidative deterioration, substantially inhibited or delayed lipid oxidation and deamination of protein, as indicated by the lower CD, TBARS and TVBN values compared to control. In particular, addition of LP into cooked duck/pork patties had considerably superior anti-oxidation stability based on the observed lower amounts of primary and secondary oxidation products compared to the meat samples treated with BHT. Furthermore, the addition of leaf powders to cooked duck/pork patties was not adversely affected sensory properties. Further studies to assess the underlying mechanism by which specific antioxidant compounds of LP, SP and GP inhibit lipid oxidation and extent of protein decomposition of meat during storage would be warranted.
Acknowledgements
The authors would like to acknowledge Eui-Joo Yeo and Fu-Yi He for sample and data collections.
References
- Aksu Mİ, Kaya M. The effect of α-tocopherol and butylated hydroxyanisole on the colour properties and lipid oxidation of kavurma, a cooked meat product. Meat Sci. 2005;71:277–283. doi: 10.1016/j.meatsci.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Armitage DB, Hettiarachchy NS, Monsoor MA. Natural antioxidants as a component of egg albumen film in the reduction of lipid oxidation in cooked and uncooked poultry. J Food Sci. 2002;67:631–634. doi: 10.1111/j.1365-2621.2002.tb10650.x. [DOI] [Google Scholar]
- Bickerstaffe R, Bekhit AED, Robertson LJ, Roberts N, Geesink GH. Impact of introducing specifications on the tenderness of retail meat. Meat Sci. 2001;59:303–315. doi: 10.1016/S0309-1740(01)00083-3. [DOI] [PubMed] [Google Scholar]
- Choe JH, Jang A, Lee ES, Choi JH, Choi YS, Han DJ, Kim HY, Lee MA, Shim SY, Kim CJ. Oxidative and color stability of cooked ground pork containing lotus leaf (Nelumbo nucifera) and barley leaf (Hordeum vulgare) powder during refrigerated storage. Meat Sci. 2011;87:12–18. doi: 10.1016/j.meatsci.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Cosio MS, Buratti S, Mannino S, Benedetti S. Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem. 2006;97:725–731. doi: 10.1016/j.foodchem.2005.05.043. [DOI] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Genchev A, Mihaylova G, Ribarski S, Pavlov A, Kabakchiev M. Meat quality and composition in Japanese quails. Trakia J Sci. 2008;6:72–82. [Google Scholar]
- Han J, Rhee KS. Antioxidant properties of selected oriental non-culinary/nutraceutical herb extracts as evaluated in raw and cooked meat. Meat Sci. 2005;70:25–33. doi: 10.1016/j.meatsci.2004.11.017. [DOI] [PubMed] [Google Scholar]
- He X, Liu R, Nirasawa S, Zheng D, Liu D. Effect of high voltage electrostatic field treatment on thawing characteristics and post-thawing quality of frozen pork tenderloin meat. J Food Eng. 2013;115:245–250. doi: 10.1016/j.jfoodeng.2012.10.023. [DOI] [Google Scholar]
- Hoac T, Daun C, Trafikowska U, Zackrisson J, Åkesson B. Influence of heat treatment on lipid oxidation and glutathione peroxidase activity in chicken and duck meat. Innov Food Sci Emerg Technol. 2006;7:88–93. doi: 10.1016/j.ifset.2005.10.001. [DOI] [Google Scholar]
- Hong JI, Ra KS, Yang HC. Free radical scavenging and antioxidative activities by ethanol extract from Capsella bursa-pastoris. J Korean Soc Food Sci Nutr. 1994;7:169–176. [Google Scholar]
- Huang B, He J, Ban X, Zeng H, Yao X, Wang Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011;87:46–53. doi: 10.1016/j.meatsci.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Jeong CH, Son KB, Kim JH, Kang SK, Park EY, Seo KI, Shim KH. Antioxidant and anticancer activities of lotus (Nelumbo nucifera) leaf and root. Korean J Food Preserv. 2010;17:131–138. [Google Scholar]
- Juntachote T, Berghofer E, Siebenhandl S, Bauer F. The antioxidative properties of Holy basil and Galangal in cooked ground pork. Meat Sci. 2006;72:446–456. doi: 10.1016/j.meatsci.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Kim GS, Park GS. Quality characteristics of cookies prepared with lotus leaf powder. Korean J Food Cook Sci. 2008;24:398–404. [Google Scholar]
- King NJ, Whyte R. Does it look cooked? A review of factors that influence cooked meat color. J Food Sci. 2006;71:31–40. doi: 10.1111/j.1750-3841.2006.00029.x. [DOI] [Google Scholar]
- Lee KS, Lee KY. Antimicrobial effect of the fractions extracted from a lotus (Nelumbo nucifera) leaf. J Korean Soc Food Sci Nutr. 2006;35:219–223. doi: 10.3746/jkfn.2006.35.2.219. [DOI] [Google Scholar]
- Li M, Xu Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch Pharmacal Res. 2008;31:640–644. doi: 10.1007/s12272-001-1206-5. [DOI] [PubMed] [Google Scholar]
- Liao GZ, Wang GY, Xu XL, Zhou GH. Effect of cooking methods on the formation of heterocyclic aromatic amines in chicken and duck breast. Meat Sci. 2010;85:149–154. doi: 10.1016/j.meatsci.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Lim HA, Yum SI. Antimicrobial activities of Capsella bursa-pastoris extracts. Korean J Food Preserv. 2009;16:562–566. [Google Scholar]
- Liu DC, Tsau RT, Lin YC, Jan SS, Tan FJ. Effect of various levels of rosemary or Chinese mahogany on the quality of fresh chicken sausage during refrigerated storage. Food Chem. 2009;117:106–113. doi: 10.1016/j.foodchem.2009.03.083. [DOI] [Google Scholar]
- Meilgaard M, Civille GV, Carr BT. Sensory evaluation techniques. 3. Boca Raton: CRC Press; 1999. p. 354. [Google Scholar]
- Ohkoshi E, Miyazaki H, Shindo K, Watanabe H, Yoshida A, Yajima H. Constituents from the leaves of Nelumbo nucifera stimulate lipolysis in the white adipose tissue of mice. Planta Med. 2007;73:1255–1259. doi: 10.1055/s-2007-990223. [DOI] [PubMed] [Google Scholar]
- Park GB, Chang PH, Kim YH. Changes in water holding capacity and fatty acid composition of duck meat during postmortem storage. J Inst Dev Livest Prod. 1986;13:1–7. [Google Scholar]
- Poole GH, Lyon CE, Buhr RJ, Young LL, Alley A, Hess JB, Bilgili SF, Northcutt JK. Evaluation of age, gender, strain, and diet on the cooked yield and shear values of broiler breast fillets. J Appl Poult Res. 1999;8:170–176. doi: 10.1093/japr/8.2.170. [DOI] [Google Scholar]
- Prasetyo M, Chia M, Hughey C, Were LM. Utilization of electron beam irradiated almond skin powder as a natural antioxidant in ground top round beef. J Food Sci. 2008;73:T1–T6. doi: 10.1111/j.1750-3841.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- PSQ . Standard methods of analysis for hygienic chemists with commentary. Tokyo: Pharmaceutical Society of Japan; 1980. [Google Scholar]
- Sampaio GR, Saldanha T, Soares RAM, Torres EAFS. Effect of natural antioxidant combinations on lipid oxidation in cooked chicken meat during refrigerated storage. Food Chem. 2012;135:1383–1390. doi: 10.1016/j.foodchem.2012.05.103. [DOI] [PubMed] [Google Scholar]
- SAS . SAS user’s guide: basic statistical analysis. Cary: Statistical Analysis Systems Institute; 2010. [Google Scholar]
- Sáyago-Ayerdi SG, Brenes A, Goñi I. Effect of grape antioxidant dietary fiber on the lipid oxidation of raw and cooked chicken hamburgers. Food Sci Technol. 2009;42:971–976. [Google Scholar]
- Serpen A, Gökmen V, Fogliano V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012;90:60–65. doi: 10.1016/j.meatsci.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Shah MA, Bosco SJD, Mir SA. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014;98:21–33. doi: 10.1016/j.meatsci.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Starks CM, Williams RB, Goering MG, O’Neil-Johnson M, Norman VL, Hu JF, Garo E, Hough GW, Rice SM, Eldridge GR. Antibacterial clerodane diterpenes from goldenrod (Solidago virgaurea) Phytochem. 2010;71:104–109. doi: 10.1016/j.phytochem.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Tarladgis B, Watts B, Younathan M, Dugan L., Jr A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc. 1960;37:44–48. doi: 10.1007/BF02630824. [DOI] [Google Scholar]
- van Laack Riette LJM, Berry BW, Solomon MB. Effect of precooking conditions on color of cooked beef patties. J Food Protect. 1996;59:976–983. doi: 10.4315/0362-028X-59.9.976. [DOI] [PubMed] [Google Scholar]
- Yang HC, Heo NC, Choi KC, Ahn YJ. Nutritional composition of white-flowered and pink-flowered lotus in different parts. Korean J Food Sci Technol. 2007;39:14–19. [Google Scholar]