Abstract
High-pressure assisted extraction was employed to obtain fig by-product derived extracts and its impact was evaluated on antioxidant activity and total phenolic, tannin, and flavonoid. A Box–Behnken design was applied to evaluate the effects of pressure, extraction time and ethanol concentration on extractions and optimal conditions were estimated by response surface methodology. The correlation analysis of the mathematical-regression model indicated that a quadratic polynomial model could be employed to optimize the high pressure extraction of compounds. Only the models developed for total antioxidant activity by DPPH· and for total flavonoids presented coefficient determinations lower than 0.95. From response surface plots, pressure, extraction time and ethanol concentration showed independent and interactive effects. The optimal conditions included 600 MPa, an extraction time between 18 and 29 min, depending on the parameter analyzed and a low ethanol concentration (<15%) except for flavonoids (48%). High pressure led to an increase of 8–13% of antioxidant activity and an increase of 8–11% of total phenolics, flavonoids and tannins content when compared to extracts performed at 0.1 MPa. Analysis of variance indicated a high goodness of fit of the models used and the adequacy of response surface methodology for optimizing high pressure extraction.
Keywords: Optimum extraction conditions, Extraction yields, Response surface methodology, Phenolic compounds, Antioxidant activity
Introduction
Fig (Ficus carica L.) is an important crop worldwide being consumed in Mediterranean diets. This fruit is an excellent source of crude fiber, minerals and vitamins; is free of fat, sodium and cholesterol; contains at least 17 different amino acids; and has high antioxidant capacity due its high content in several bioactive compounds such as polyphenols, anthocyanins and carotenoids (Oliveira et al. 2009). Figs have been conventionally used for its medicinal benefits as laxative, cardiovascular, respiratory, antispasmodic and anti-inflammatory activities (Guarrera 2005). This fruit is usually consumed fresh or dried, but when processed to produce juice, puree, jam or other derivate products, substantial remaining material is generated, which still contains high levels of bioactive compounds. Those by-products are frequently used to feed livestock, or sent to sanitary landfill (Soltana et al. 2016).
Depending on the availability of an adequate extraction technology, the industrial by-products might be valorized into commercial products suitable for other purposes such as, raw materials for secondary processes (intermediate foods ingredients), operating supplies, ingredients for new products or nutraceuticals, taking advantage of the great quantity that is usually produced with potentially beneficial compounds (Viuda-Martos et al. 2015). However, when the extractions are performed using conventional methods the active components are often extracted incompletely, the extraction time is long, and the efficiency is low. In addition, the high temperature usually used is likely detrimental to the bioactivity of the extracts due heat promoted reactions (Azmir et al. 2013; Huang et al. 2013).
The use of high-pressure assisted extraction (HPE) in order to obtain bioactive compounds from raw materials and/or by-products is recent but has high potential for extraction of bioactive compounds. These compounds can be extracted in shorter time, the process may be performed at room temperature (avoiding thermal degradation of heat labile components), and results in higher extraction yields (Huang et al. 2013). The process is energetically efficient and, by using different solvents can differentially extract compounds with different polarities. The extraction of new compounds is another possibility that may be achieved by HPE. The application of high-pressure leads to disruption of plant tissues, cellular walls, membrane and organelles, enhancing the mass transfer of solvents into materials and their soluble constituents into solvents (Grunovaité et al. 2016; Prasad et al. 2009a; Xi et al. 2009). Moreover, and since HPE does not use heat, no thermal degradation is observed when compared to the conventional temperature-based extraction methods (Huang et al. 2013; Santos et al. 2013). Recently, some studies have endorsed the use of HPE to obtain bioactive compounds derived from food and medicinal plant matrices (Casquete et al. 2015; Huang et al. 2013; M’hiri et al. 2015; Strati et al. 2015). Research in the field of HPE is taking the first steps and deeper studies are needed to ascertain the full potential of HPE.
The main objective of the present research work was to study the potential of high-pressure on the extraction of bioactive compounds from a fermented fig by-product obtained during the industrial production of fig vinegar. High-pressure assisted extraction impact was evaluated on total antioxidant activity by ABTS, DPPH· and FRAP methods, and on total phenolic, tannin, and flavonoid. Extraction yields were also determined and extraction conditions were optimized by a response surface methodology approach.
Materials and methods
Chemical materials
DPPH· (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), TPTZ (2,4,6-tripyridyl-S-triazine), aluminium chloride, quercetin, catechin and Trolox were acquired from Sigma Chemical Company (St. Louis, MO, USA). Folin–Ciocalteu reagent, gallic acid, ammonium iron (II) sulfate, potassium chloride, sodium acetate and sodium carbonate were purchased from PanReac AppliChem (Barcelona, Spain) and potassium persulfate and vanillin were from Acros Organics (Geel, Belgium). All reagents were of analytical grade.
Biological samples
Fermented fig by-product was obtained from a Portuguese processing industry producing fig vinegar (Mendes Gonçalves, Golegã). A batch of 5 kg was collected after 24 h of vinegar production, transported (2 h) under refrigeration, dried at 40 °C during 6 days, grounded and stored at −20 °C until used.
Extraction conditions
The extractions were carried out according to a full 33 factorial experiment design. Independent variables were pressure (0.1, 300 and 600 MPa), the extraction time (5, 17.5 and 30 min) and the ethanol concentration (0, 40 and 80%). The different ethanol concentrations were obtained using water. For each extraction, 0.5 g of ground sample were mixed with 30 ml of solvent (0, 40 and 80% of ethanol) in a plastic bag, which was heat-sealed after air removal. The experiments were carried out on an industrial-scale high-pressure equipment (Model 55, Hyperbaric, Burgos, Spain) with a pressure vessel of 55 l. The samples were placed inside the equipment, which is connected to a refrigeration unit (RMA KH 40 LT, Ferroli, San Bonifacio, Italy) that allows to control the temperature of the input water used as a pressurizing fluid. Control samples extracted at atmospheric pressure (0.1 MPa) were treated alike, except for the HPP treatment. After extraction the mixture was centrifuged at 15,000 rpm for 10 min at 4 °C and the supernatant was filtered and collected. All extracts were stored at −80 °C until used for the analyses. All quantifications were performed in triplicate.
Extraction yields
The extraction yield was calculated according to the method described by Zhang et al. (2007). Briefly, the solvent extracts were evaporated and the extraction yields calculated per g of dried by-product (DW) according the Eq. (1).
| 1 |
where m is the mass of the dried extract and m0 is the mass of the initial extract.
Free radical DPPH· scavenging capacity
The reduction of DPPH· was evaluated according the methodology described by Herald et al. (2012) with some modifications. Briefly, 180 µL of DPPH· solution (150 µM DPPH· in ethanol–water (80:20, (v/v)) and 20 µL of diluted extract were mixed in a 96-wells plate. After 40 min, optical density was read at 515 nm in the microplate reader (Multiskan GO Microplate Spectrophotometer, Thermo Scientific, Thermo Fisher Scientific Inc., USA). Trolox standards were prepared in different concentrations (0–500 µM) and analyzed alike to obtain the calibration curve. The percentage of inhibition was determined and the extracts antioxidant activity was expressed as mg Trolox equivalents (Eq.)/g dry weight (DW).
Radical cation ABTS•+ scavenging activity
The radical scavenging ability of the extracts for the ABTS•+ was performed according to the methodology described by Re et al. (1999). To prepare ABTS•+ radical cation, 7 mM of ABTS reacted with 2.45 mM potassium persulfate at least for 16 h in the dark at room temperature. Then ABTS•+ radical was diluted with ethanol to an absorbance of 0.80 ± 0.02 at 734 nm, and 200 µL were allowed to react with 20 µL of diluted extract for 6 min. Then the optical density was measured at 734 nm using the same microplate reader described above. Trolox standards (0–700 µM) were prepared alike to obtain the calibration curve. The percentage of inhibition was determined and the extracts antioxidant activity was expressed as mg Trolox equivalents (Eq.)/g dry weight (DW).
Ferric reducing antioxidant power (FRAP) assay
The reducing capacity of extracts was evaluated according to the method described by Benzie and Strain (1996). FRAP working solution consisted in a mixture of 10 mL of acetate buffer (300 mM, pH 3.6), 1 mL of TPTZ (40 mM dissolved with 40 mM HCl) and 1 mL of ferric chloride (20 mM in water) warmed at 37 °C. 280 µL of this working solution were mixed with 20 µL of extract and then incubated at 37 °C for 30 min in the dark and read at 595 nm using the microplate reader. Ammonium iron (II) sulfate (AIS) was used as standard (0-1 mM) to obtain the calibration curve. Extracts antioxidant activity was expressed as mg AIS equivalents (Eq.)/g dry weight (DW).
Quantification of total phenolics
Total phenolic compounds from extracts were analyzed by the Folin–Ciocalteu method (Singleton et al. 1999). Folin–Ciocalteu reagent was diluted (1:4) and 100 µL was mixed with 20 µL of extract. After 4 min 75 µL of Na2CO3 solution (100 g/L) were added and the solutions were allowed to react for 2 h in the dark, at room temperature. Finally, the optical density was recorded at 750 nm. Gallic acid (GA) was used as standard (0–200 mg/mL) and the results were expressed as mg Gallic acid equivalents (Eq.)/g dry weight (DW).
Quantification of total condensed tannin
The condensed tannin content of extracts solutions was determined by the vanillin method (Naczk et al. 2000). This method consists in the reaction of 50 µL of extract with 150 µL of vanillin (1% in 7 M H2SO4), which were mixed in an ice bath, incubated in the dark, at room temperature, for 15 min and then optical density reads were taken at 500 nm using a microplate reader. Catechin was used as standard (0–0.08 mg/mL) and the results were expressed as mg catechin equivalents (Eq.)/g dry weight (DW).
Quantification of total flavonoids
The total flavonoid content was determined according to the method described by Cruz et al. (2014). The reaction mixture consisted of 150 µL of 2% of AlCl3 with 150 µL of each extract solution reacting during 10 min in the dark and then read at 415 nm. Blank samples were prepared alike but AlCl3 was replaced by methanol. Standard solutions of quercetin were prepared at different concentrations (0–25 µg/mL) to obtain the calibration curve and the results were expressed as mg Quercetin (Eq.)/g dry weight (DW).
Response surface methodology and statistical analysis
The response surface methodology (RSM) was used to analyze the relationship between the measured responses and the individual and combined effects, as well as to find the optimum extraction conditions. The extraction process was developed following a Box–Behnken design (Box and Behnken 1960) formed by a full 33 design. The impact of three independent variables: pressure, extraction time and ethanol concentration on eighth dependent variables: the extraction yields, antioxidant activity by ABTS, DPPH· and FRAP, total phenolic, total tannins and total flavonoids was studied. Experiments (27) were randomized, to minimize the effects of unexplained variability in the observed responses, due to extraneous factors and temperature (25 °C) was kept constant. Error assessment was based on replication of the central point as suggested in the Box–Behnken design.
The mathematical relationship between the three significant independent variables was formulated by the following general quadratic polynomial.
| 2 |
where Y is the response, β0 is constant, β1 (EtOH), β2 (P) and β3 (t) are the linear, β11, β22 and β33 are the quadratic coefficients and β12, β13 and β23 are the crossed coefficients. The coefficients of determination, R2 and their adjusted values were used to evaluate the goodness of fit of the regression models. Significant factors and interactions were accessed through analyses of variance.
Analysis of phenolic acids by uHPLC
uHPLC analysis of phenolic acids was performed on Dionex Ultimate 3000 (Thermo Scientific; USA) equipped with a BDS Hypersil 150 × 4.6 mm i.d. (particle size 5 µm) reversed phase C18 column (Thermo Scientific; USA). Detection was carried out at 280 nm using a diode array detector. The solvents were (A) H2O/CH3COOH (99:1; v/v) and (B) H2O/CH3CN/CH3COOH (79:20:1; v/v/v) with the gradient 80–20% A over 55 min, 20–10% A from 55 to 70 min and 10–0% A from 70 to 90 min, at a flow rate of 0.3 mL/min. The sample injection volume was 20 μL.
LC-DAD/ESI–MS analysis of phenolic acids
The phenolic acids was evaluated by LC-DAD/MS. An Accela series liquid chromatograph, equipped with a 150 × 4.6 mm i.d., 5 μm, LicroCART reversed-phase C18 column was used, and detection was carried out 280 nm using an Accela PDA detector. The mass detection was performed using an LTQ Orbitrap XL mass spectrometer (Thermo Fischer Scientific, Bremen, Germany) controlled by LTQ Tune Plus 2.5.5 and Xcalibur 2.1.0. Solvents were (A) H2O/CH3COOH (99:1) and (B) H2O/CH3CN/CH3COOH (79:20:1). The gradient was performed using an Accela 600 Pump and consisted of 80–20% A for 55 min at a flow rate of 0.3 mL/min. The capillary voltage of the electrospray ionization (ESI) was set to 3100 V, and the capillary temperature was 275 °C. The sheath gas flow rate (nitrogen) was set to 5 (arbitrary units as provided by the software settings). The capillary voltage was 49 V and the tube lens voltage 250 V. Spectra were recorded in positive-ion mode between m/z 50 and 2000. The mass spectrometer was programmed to do a series of three scans: a full scan mass, a zoom scan of the most intense ion in the first scan (selected ion monitoring, SIM), and a MS–MS of the most intense ion using relative collision energies of 30 and 60 V.
Analysis of proanthocyanidins by HPLC–DAD
The samples were analyzed by HPLC (UNICAM) using two columns reverse-phase C18 (250 mm × 4.6 mm i.d.) (Merck, Darmstadt, Germany). The detection was carried out at 280 nm using a diode array detector (Knauer, K-2800). An HPLC pump Knauer K-1001 was used together with a Kauer K-3800 auto-sampler. The solvents were A, H2O/CH3COOH (97.5:2.5 v/v), and B, CH3CN/solvent A (80:20), and the elution occurred with flow rate of 1.0 mL min−1.
Statistical analysis
The analysis of data was performed by two-away analysis of variance (ANOVA) using the GraphPad Prism 5 program. The differences between compounds were estimated using the Bonferroni test at p < 0.05.
Results and discussion
General aspects of models
Experimental design was formulated to develop empirical models thereby examining the interaction of the different associated parameters responsible for extraction of different bioactive compounds determined and related activities from an industrial fig by-product. The extraction conditions were optimized using a multivariable system, in order to evaluate the fitness of response function in experimental set up. Linear and quadratic effects of independent variables (pressure, extraction time and ethanol concentration) as well as their interactions were analyzed for regression coefficients in RSM study.
Extraction yields
The response surface of pressure vs extraction time was plotted for an ethanol concentration of 0% and is shown in Fig. 1a). In general, high pressure extraction allowed an increase of extraction of more 15 and 18% (300 and 600 MPa, respectively) in relation to samples extracted at 0.1 MPa. Higher pressures and/or higher extraction times led to increase in extraction yield until 22 min of extraction, with no significant further improvement thereafter. In terms of the solvent concentration, the highest extraction yield was obtained with 100% of water. When water was used as solvent during 30 min, the impact of pressure in extraction yields was significant. The extraction yield increased from 5.32 obtained at 0.1 MPa to 6.61 and 7.13 at 300 and 600 MPa, which represent an increase of 25 and 34%, respectively. Thus, a pressure of 600 MPa, an extraction time of 22 min and a solvent of 100% water should be used to maximize the extraction yield to 7.13, representing an increase of 34% in relation samples extracted with water at 0.1 MPa during 30 min.
Fig. 1.
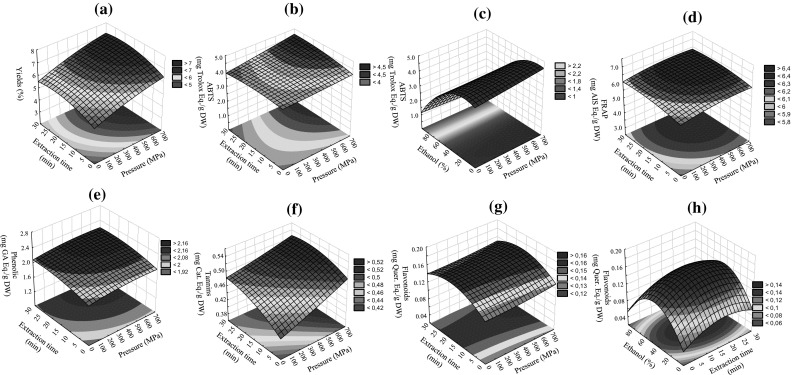
Representative response surfaces observed for a total yields, b and c antioxidant activity by ABTS method, d antioxidant activity by FRAP method, e total phenolic, f, total tannins and g and h total flavonoids for an ethanol concentration of 0% (extraction time vs pressure) or for an pressure of 600 MPa (ethanol concentration vs extraction time)
In fact, all independent variables showed a significant impact (p < 0.05) on extraction yield (Table 1). The p values of the model were lower than 0.05, which indicated that the model fitness was significant. Only crossed effects of pressure with extraction time, and extraction time with ethanol concentration had not significant impact on extraction yield. The ethanol concentration effect was by far the most significant effect observed presenting F values of 1657 and 71.58 (linear and quadratic effects, respectively). High pressure was the second most significant factor presenting F values of 129 and 5 (linear and quadratic effects, respectively). Prasad et al. (2009b) studied the effect of high-pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. These authors observed that extraction yield was influenced by high-pressure treatment, increasing 3% when compared to conventional extraction, and required shorter extraction time. According to the mass transfer theory, where the rate of mass transfer is equal to pressure/resistance, the pressurized cells show increased permeability. With the increase of the pressure, more solvent can enter into the cell and consequently more compounds can permeate the cell membrane. The difference between the pressure inside the cell and outside is so large that it will lead to a rapid permeation of the compounds attaining equilibrium in a shorter time. Additionally, the disruption of cellular walls and hydrophobic bonds in the cell membrane can increase the rate of mass transfer and enhance solvent penetration into the cells (Shouqin et al. 2004; Xi and Luo 2016).
Table 1.
Regression coefficients of the quadratic model, their significance at 5% significant level, and the determination coefficients for the full model
| Regression coefficients | Total yields | ABTS | DPPH | FRAP | Total phenolics | Total tannins | Total flavonoids | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| P (L) | 129.41 | 0.00 | 7.03 | 0.01 | 8.89 | 0.00 | 19.38 | 0.00 | 16.30 | 0.00 | 32.92 | 0.00 | 1.50 | 0.22 |
| P (Q) | 5.13 | 0.03 | 0.05 | 0.82 | 1.94 | 0.17 | 3.18 | 0.08 | 1.96 | 0.17 | 0.05 | 0.83 | 0.10 | 0.75 |
| t (L) | 13.79 | 0.00 | 0.57 | 0.45 | 1.49 | 0.23 | 3.22 | 0.08 | 18.28 | 0.00 | 8.22 | 0.01 | 0.16 | 0.69 |
| t (Q) | 24.96 | 0.00 | 2.40 | 0.13 | 2.50 | 0.12 | 3.29 | 0.07 | 4.13 | 0.05 | 2.88 | 0.09 | 12.31 | 0.00 |
| E (L) | 1657.17 | 0.00 | 1342.39 | 0.00 | 151.18 | 0.00 | 1457.08 | 0.00 | 1333.21 | 0.00 | 52.66 | 0.00 | 19.13 | 0.00 |
| E (Q) | 71.58 | 0.00 | 275.56 | 0.00 | 48.11 | 0.00 | 355.06 | 0.00 | 251.76 | 0.00 | 6.76 | 0.01 | 124.39 | 0.00 |
| P(L) by t (L) | 0.43 | 0.51 | 6.38 | 0.01 | 4.61 | 0.03 | 0.02 | 0.88 | 0.01 | 0.91 | 1.88 | 0.17 | 1.19 | 0.28 |
| P (L) by E (L) | 55.15 | 0.00 | 11.46 | 0.00 | 0.03 | 0.85 | 7.17 | 0.01 | 1.91 | 0.17 | 0.45 | 0.51 | 4.89 | 0.03 |
| t (L) by E (L) | 1.78 | 0.19 | 0.00 | 0.99 | 1.30 | 0.26 | 0.06 | 0.81 | 2.12 | 0.15 | 1.81 | 0.18 | 0.10 | 0.75 |
| R2 | 0.961 | 0.954 | 0.735 | 0.959 | 0.954 | 0.966 | 0.695 | |||||||
| R2 adj. | 0.957 | 0.949 | 0.706 | 0.955 | 0.949 | 0.962 | 0.661 | |||||||
P, t and E meaning Pressure, extraction time and Ethanol concentration, respectively
L and Q meaning Linear and Quadratic, respectively
The significant coefficients in each case are written in bold
By analysis of variance, the R2 value of this model was 0.961 and the R2 adj. value did not differ significantly from R2 value, which showed that the regression model fitted well the true behavior of the system representing the experimental data well (Table 1). Only 3.9% of the total variations were not explained by the model. Another parameter that made evident the adequacy of the model was the agreement level of the predictive values with the experimental data. The extraction yields obtained experimentally and predicted by the model for each extraction condition analyzed are presented in Table 2. In general, the experimental results and the predicted values by the model are in good agreement, presenting a variation among them, lower than 6%, except for the samples P0.1/T5/S80, P0.1/T30/S40 and P0.1/T30/S80, where the variation was lower than 13%.
Table 2.
Results obtained experimentally and estimated by the models for total yields and total antioxidant activity evaluated by ABTS, DPPH and FRAP assays for each extraction condition
| Sample | Total yields (%) | ABTS (mg Trolox Eq./g DW) | DPPH (mg Trolox Eq./g DW) |
FRAP (mg AIS Eq./g DW) |
||||
|---|---|---|---|---|---|---|---|---|
| Observed | Predicted (%variation)1 | Observed | Predicted (%variation)1 | Observed | Predicted (%variation)1 |
Observed | Predicted (%variation)1 |
|
| P0.1/T5/S0 | 5.38 ± 0.29 | 5.17 (3.61) | 4.22 ± 0.09 | 4.02 (4.77) | 2.41 ± 0.27 | 2.46 (−2.83) | 6.22 ± 0.13 | 6.05 (2.82) |
| P0.1/T5/S40 | 4.54 ± 0.18 | 4.59 (−1.26) | 3.68 ± 0.11 | 3.83 (−4.08) | 2.30 ± 0.13 | 2.48 (−8.15) | 5.47 ± 0.07 | 5.90 (−7.63) |
| P0.1/T5/S80 | 2.84 ± 0.20 | 3.05 (−7.44) | 2.00 ± 0.12 | 1.89 (5.26) | 1.82 ± 0.20 | 1.63 (9.78) | 3.73 ± 0.20 | 3.48 (6.14) |
| P0.1/T17.5/S0 | 5.59 ± 0.13 | 5.62 (−0.64) | 4.32 ± 0.04 | 4.02 (6.93) | 2.50 ± 0.12 | 2.56 (−2.37) | 6.38 ± 0.11 | 6.24 (2.23) |
| P0.1/T17.5/S40 | 5.11 ± 0.06 | 4.98 (2.48) | 3.72 ± 0.23 | 3.84 (−3.51) | 2.44 ± 0.02 | 2.52 (−3.45) | 5.89 ± 0.20 | 6.07 (−3.10) |
| P0.1/T17.5/S80 | 3.46 ± 0.14 | 3.38 (2.33) | 1.80 ± 0.12 | 1.90 (−5.88) | 1.34 ± 0.09 | 1.62 (−21.37) | 3.77 ± 0.23 | 3.63 (2.68) |
| P0.1/T30/S0 | 5.32 ± 0.07 | 5.49 (−3.36) | 3.42 ± 0.12 | 3.86 (−12.96) | 2.56 ± 0.22 | 2.46 (3.77) | 6.19 ± 0.07 | 6.21 (−0.37) |
| P0.1/T30/S40 | 4.36 ± 0.05 | 4.80 (−10.07) | 3.69 ± 0.12 | 3.68 (0.33) | 2.37 ± 0.14 | 2.37 (−0.22) | 5.72 ± 0.12 | 6.01 (−5.66) |
| P0.1/T30/S80 | 3.62 ± 0.25 | 3.14 (13.21) | 1.80 ± 0.07 | 1.74 (9.42) | 1.34 ± 0.29 | 1.41 (18.38) | 3.77 ± 0.13 | 3.56 (6.92) |
| P300/T5/S0 | 5.99 ± 0.12 | 6.00 (−0.10) | 3.59 ± 0.20 | 4.13 (−15.27) | 2.47 ± 0.20 | 2.39 (2.74) | 6.42 ± 0.06 | 6.44 (−0.25) |
| P300/T5/S40 | 5.00 ± 0.16 | 5.10 (−1.97) | 3.80 ± 0.15 | 3.81 (−0.23) | 2.63 ± 0.23 | 2.40 (8.23) | 6.01 ± 0.13 | 6.17 (−2.62) |
| P300/T5/S80 | 3.10 ± 0.26 | 3.23 (−4.53) | 1.64 ± 0.11 | 1.73 (−6.01) | 1.63 ± 0.15 | 1.54 (4.53) | 3.60 ± 0.21 | 3.65 (−1.50) |
| P300/T17.5/S0 | 6.64 ± 0.14 | 6.48 (2.48) | 4.57 ± 0.13 | 4.23 (7.32) | 2.73 ± 0.30 | 2.59 (4.13) | 6.76 ± 0.19 | 6.63 (2.01) |
| P300/T17.5/S40 | 5.48 ± 0.07 | 5.52 (−0.63) | 4.07 ± 0.12 | 3.91 (3.73) | 2.50 ± 0.08 | 2.55 (−2.14) | 6.76 ± 0.22 | 6.35 (6.11) |
| P300/T17.5/S80 | 3.71 ± 0.20 | 3.59 (3.03) | 1.85 ± 0.10 | 1.84 (0.71) | 1.60 ± 0.15 | 1.64 (−2.87) | 3.76 ± 0.12 | 3.80 (−1.32) |
| P300/T30/S0 | 6.61 ± 0.10 | 6.38 (3.52) | 4.38 ± 0.24 | 4.17 (4.64) | 2.51 ± 0.33 | 2.59 (−4.76) | 6.72 ± 0.27 | 6.60 (1.77) |
| P300/T30/S40 | 5.41 ± 0.23 | 5.36 (0.79) | 4.00 ± 0.12 | 3.86 (3.48) | 2.42 ± 0.17 | 2.50 (−3.54) | 6.39 ± 0.11 | 6.29 (1.38) |
| P300/T30/S80 | 3.02 ± 0.06 | 3.38 (−11.65) | 1.60 ± 0.14 | 1.78 (−11.50) | 1.10 ± 0.09 | 1.53 (−39.64) | 3.46 ± 0.27 | 3.73 (−9.11) |
| P600/T5/S0 | 6.37 ± 0.17 | 6.57 (3.19) | 4.45 ± 0.09 | 4.26 (4.31) | 2.65 ± 0.19 | 2.50 (5.15) | 6.54 ± 0.09 | 6.60 (−1.24) |
| P600/T5/S40 | 5.63 ± 0.17 | 5.34 (5.09) | 3.90 ± 0.01 | 3.80 (2.53) | 2.36 ± 0.24 | 2.51 (−6.71) | 6.57 ± 0.11 | 6.23 (5.16) |
| P600/T5/S80 | 3.34 ± 0.17 | 3.15 (5.39) | 1.77 ± 0.13 | 1.59 (9.93) | 1.28 ± 0.13 | 1.64 (−28.70) | 3.55 ± 0.21 | 3.59 (−1.32) |
| P600/T17.5/S0 | 6.75 ± 0.12 | 7.07 (−4.78) | 4.31 ± 0.13 | 4.47 (−3.71) | 2.37 ± 0.16 | 2.81 (−18.89) | 6.51 ± 0.19 | 6.80 (−4.46) |
| P600/T17.5/S40 | 5.75 ± 0.26 | 5.79 (−0.90) | 3.75 ± 0.07 | 4.01 (−6.98) | 2.94 ± 0.26 | 2.76 (5.80) | 6.52 ± 0.26 | 6.40 (1.74) |
| P600/T17.5/S80 | 3.43 ± 0.25 | 3.54 (-3.50) | 1.68 ± 0.10 | 1.80 (−7.48) | 2.30 ± 0.28 | 1.83 (19.57) | 3.53 ± 0.32 | 3.75 (−6.56) |
| P600/T30/S0 | 7.13 ± 0.25 | 7.00 (1.77) | 4.40 ± 0.09 | 4.51 (−2.45) | 3.08 ± 0.23 | 2.91 (5.05) | 6.58 ± 0.08 | 6.77 (−2.75) |
| P600/T30/S40 | 5.82 ± 0.21 | 5.66 (2.59) | 4.23 ± 0.08 | 4.06 (4.08) | 2.77 ± 0.36 | 2.81 (−2.53) | 6.66 ± 0.35 | 6.35 (4.55) |
| P600/T30/S80 | 3.26 ± 0.23 | 3.35 (−3.11) | 1.84 ± 0.09 | 1.84 (−0.22) | 1.84 ± 0.10 | 1.83 (0.51) | 3.71 ± 0.13 | 3.68 (0.48) |
| P300/T17.5/S40 | 5.64 ± 0.15 | 5.52 (2.12) | 3.86 ± 0.02 | 3.91 (−1.26) | 2.63 ± 0.02 | 2.55 (2.89) | 6.27 ± 0.17 | 6.35 (−1.25) |
| P300/T17.5/S40 | 5.55 ± 0.15 | 5.52 (0.53) | 3.80 ± 0.24 | 3.91 (−3.32) | 2.33 ± 0.17 | 2.55 (−9.88) | 6.35 ± 0.24 | 6.35 (0.01) |
| P300/T17.5/S40 | 5.41 ± 0.12 | 5.52 (−2.10) | 4.03 ± 0.11 | 3.91 (2.86) | 2.86 ± 0.22 | 2.55 (10.40) | 6.20 ± 0.13 | 6.35 (−2.30) |
| Sample | Total phenolic (mg GA Eq./g DW) |
Total tannins (mg catechin Eq./g DW) |
Total flavonoids (mg quercetin Eq./g DW) |
|||
|---|---|---|---|---|---|---|
| Observed | Predicted (%variation)1 |
Observed | Predicted (%variation)1 |
Observed | Predicted (%variation)1 |
|
| P0.1/T5/S0 | 2.050 ± 0.109 | 2.054 (−0.42) | 0.442 ± 0.026 | 0.436 (1.00) | 0.049 ± 0.007 | 0.082 (−67.98) |
| P0.1/T5/S40 | 1.862 ± 0.116 | 1.939 (−4.44) | 0.396 ± 0.015 | 0.403 (−1.92) | 0.150 ± 0.010 | 0.140 (6.14) |
| P0.1/T5/S80 | 1.084 ± 0.060 | 1.015 (6.17) | 0.415 ± 0.011 | 0.401 (3.39) | 0.101 ± 0.006 | 0.089 (11.46) |
| P0.1/T17.5/S0 | 2.206 ± 0.038 | 2.199 (0.28) | 0.481 ± 0.035 | 0.469 (2.23) | 0.105 ± 0.003 | 0.095 (43.89) |
| P0.1/T17.5/S40 | 1.949 ± 0.039 | 2.056 (−5.49) | 0.420 ± 0.006 | 0.430 (−2.26) | 0.159 ± 0.007 | 0.155 (−5.04) |
| P0.1/T17.5/S80 | 1.106 ± 0.046 | 1.103 (0.12) | 0.414 ± 0.012 | 0.422 (−2.04) | 0.148 ± 0.004 | 0.105 (0.43) |
| P0.1/T30/S0 | 2.231 ± 0.082 | 2.241 (−0.54) | 0.459 ± 0.013 | 0.482 (−5.04) | 0.049 ± 0.004 | 0.073 (−51.81) |
| P0.1/T30/S40 | 2.062 ± 0.034 | 2.069 (−0.35) | 0.429 ± 0.021 | 0.436 (−1.84) | 0.110 ± 0.004 | 0.135 (−22.66) |
| P0.1/T30/S80 | 1.106 ± 0.021 | 1.089 (10.48) | 0.445 ± 0.022 | 0.422 (−4.95) | 0.079 ± 0.004 | 0.086 (−8.87) |
| P300/T5/S0 | 2.306 ± 0.071 | 2.178 (5.50) | 0.479 ± 0.013 | 0.465 (2.84) | 0.059 ± 0.003 | 0.071 (−22.20) |
| P300/T5/S40 | 2.008 ± 0.129 | 2.036 (−1.67) | 0.402 ± 0.015 | 0.428 (-6.74) | 0.140 ± 0.006 | 0.139 (0.57) |
| P300/T5/S80 | 1.049 ± 0.017 | 1.085 (−3.49) | 0.419 ± 0.009 | 0.424 (−1.10) | 0.085 ± 0.004 | 0.096 (−13.23) |
| P300/T17.5/S0 | 2.375 ± 0.047 | 2.325 (2.05) | 0.513 ± 0.011 | 0.492 (4.07) | 0.056 ± 0.004 | 0.089 (-59.91) |
| P300/T17.5/S40 | 2.306 ± 0.129 | 2.155 (6.34) | 0.481 ± 0.011 | 0.449 (6.55) | 0.155 ± 0.006 | 0.157 (−1.59) |
| P300/T17.5/S80 | 1.095 ± 0.048 | 1.176 (−7.51) | 0.441 ± 0.014 | 0.438 (0.45) | 0.109 ± 0.003 | 0.115 (−5.66) |
| P300/T30/S0 | 2.406 ± 0.048 | 2.369 (1.53) | 0.529 ± 0.023 | 0.498 (5.79) | 0.089 ± 0.007 | 0.071 (19.66) |
| P300/T30/S40 | 2.032 ± 0.107 | 2.171 (−7.03) | 0.440 ± 0.030 | 0.449 (−2.54) | 0.139 ± 0.006 | 0.141 (21.00) |
| P300/T30/S80 | 1.081 ± 0.041 | 1.163 (−7.78) | 0.434 ± 0.012 | 0.432 (0.13) | 0.113 ± 0.006 | 0.100 (10.86) |
| P600/T5/S0 | 2.076 ± 0.231 | 2.230 (−8.28) | 0.478 ± 0.025 | 0.497 (−4.04) | 0.091 ± 0.005 | 0.064 (28.75) |
| P600/T5/S40 | 2.125 ± 0.116 | 2.062 (2.76) | 0.476 ± 0.029 | 0.457 (3.73) | 0.136 ± 0.007 | 0.140 (−3.05) |
| P600/T5/S80 | 1.124 ± 0.039 | 1.084 (3.51) | 0.454 ± 0.015 | 0.449 (0.88) | 0.117 ± 0.002 | 0.105 (10.01) |
| P600/T17.5/S0 | 2.397 ± 0.185 | 2.380 (0.30) | 0.499 ± 0.002 | 0.517 (−3.72) | 0.084 ± 0.002 | 0.086 (−1.65) |
| P600/T17.5/S40 | 2.199 ± 0.066 | 2.183 (0.66) | 0.506 ± 0.035 | 0.472 (6.51) | 0.159 ± 0.002 | 0.162 (−2.27) |
| P600/T17.5/S80 | 1.122 ± 0.052 | 1.177 (−5.11) | 0.452 ± 0.006 | 0.458 (−1.27) | 0.111 ± 0.006 | 0.129 (−16.00) |
| P600/T30/S0 | 2.356 ± 0.064 | 2.426 (−3.01) | 0.493 ± 0.020 | 0.517 (−5.03) | 0.059 ± 0.002 | 0.073 (−23.59) |
| P600/T30/S40 | 2.328 ± 0.071 | 2.201 (5.40) | 0.501 ± 0.014 | 0.465 (6.98) | 0.148 ± 0.005 | 0.150 (−1.99) |
| P600/T30/S80 | 1.183 ± 0.071 | 1.167 (1.12) | 0.420 ± 0.022 | 0.446 (−6.40) | 0.123 ± 0.006 | 0.118 (4.03) |
| P300/T17.5/S40 | 2.121 ± 0.129 | 2.155 (−1.85) | 0.436 ± 0.013 | 0.449 (−3.09) | 0.158 ± 0.002 | 0.157 (0.81) |
| P300/T17.5/S40 | 2.148 ± 0.089 | 2.155 (−0.45) | 0.434 ± 0.036 | 0.449 (−4.04) | 0.153 ± 0.004 | 0.157 (−2.43) |
| P300/T17.5/S40 | 2.198 ± 0.160 | 2.155 (1.59) | 0.418 ± 0.022 | 0.449 (−7.69) | 0.156 ± 0.005 | 0.157 (0.78) |
All results were expressed as mg of standard equivalent (Eq.)/g of dried weight (DW)
Mean values ± standard deviation
P, T and S meaning pressure, extraction time and solvent concentration, respectively
1
The regression coefficients and their significance were calculated and are presented in Table 3 to write the polynomial equation. The regression coefficients had a significant (p < 0.05) impact on the extraction yields, except for β3, β12 and β23.
Table 3.
Regression coefficients of the quadratic polynomial equation for each dependent variable
| Factor | Total Yields | ABTS | DPPH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Regre. coeff. | Standard error | p | Regre. coeff. | Standard error | p | Regre. coeff. | Standard error | p | |
| β0 | 4.83 | 0.14 | 0.00 | 3.97 | 0.13 | 0.00 | 2.36 | 0.16 | 0.00 |
| β1 | 3.15 × 10−3 | 0.00 | 0.00 | 1.90 × 10−4 | 0.00 | 0.66 | −6.47 × 10−4 | 0.00 | 0.22 |
| β11 | −1.45 × 10−6 | 0.00 | 0.03 | 1.31 × 10−7 | 0.00 | 0.82 | 9.76 × 10−7 | 0.00 | 0.17 |
| β2 | 7.72 × 10−2 | 0.01 | 0.00 | 1.22 × 10−2 | 0.01 | 0.35 | 2.23 × 10−2 | 0.02 | 0.15 |
| β22 | −1.84 × 10−3 | 0.00 | 0.00 | −5.25 × 10−4 | 0.00 | 0.13 | −6.38 × 10−4 | 0.00 | 0.12 |
| β3 | −1.69 × 10−3 | 0.00 | 0.63 | 1.74 × 10−2 | 0.00 | 0.00 | 1.20 × 10−2 | 0.00 | 0.00 |
| β33 | −3.04 × 10−4 | 0.00 | 0.00 | −5.49 × 10−4 | 0.00 | 0.00 | −2.73 × 10−4 | 0.00 | 0.00 |
| β12 | 7.60 × 10−6 | 0.00 | 0.51 | 2.70 × 10−5 | 0.00 | 0.01 | 2.73 × 10−5 | 0.00 | 0.03 |
| β13 | −2.69 × 10−5 | 0.00 | 0.00 | −1.13 × 10−5 | 0.00 | 0.00 | −7.38 × 10−7 | 0.00 | 0.85 |
| β23 | −1.16 × 10−4 | 0.00 | 0.19 | 1.37 × 10−6 | 0.00 | 0.99 | −1.09 × 10−4 | 0.00 | 0.26 |
| FRAP | Total phenolics | Total flavonoids | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Regre. coeff. | Standard error | p | Regre. coeff. | Standard error | p | Regre. coeff. | Standard error | p | |
| β0 | 5.90 | 0.15 | 0.00 | 1.97 | 0.06 | 0.00 | 0.07 | 0.01 | 0.00 |
| β1 | 1.69 × 10−3 | 0.00 | 0.00 | 5.28 × 10−4 | 0.00 | 0.01 | −4.45 × 10−5 | 0.00 | 0.28 |
| β11 | −1.18 × 10−6 | 0.00 | 0.08 | −3.97 × 10−7 | 0.00 | 0.17 | 1.73 × 10−8 | 0.00 | 0.75 |
| β2 | 3.10 × 10−2 | 0.01 | 0.04 | 1.91 × 10−2 | 0.01 | 0.00 | 3.56 × 10−3 | 0.00 | 0.00 |
| β22 | −6.94 × 10−4 | 0.00 | 0.07 | −3.32 × 10−4 | 0.00 | 0.05 | −1.11 × 10−4 | 0.00 | 0.00 |
| β3 | 2.45 × 10−2 | 0.00 | 0.00 | 7.51 × 10−3 | 0.00 | 0.00 | 2.84 × 10−3 | 0.00 | 0.00 |
| β33 | −7.03 × 10−4 | 0.00 | 0.00 | −2.53 × 10−4 | 0.00 | 0.00 | −3.44 × 10−5 | 0.00 | 0.00 |
| β12 | −1.79 × 10−6 | 0.00 | 0.88 | 6.05 × 10−7 | 0.00 | 0.91 | 1.09 × 10−6 | 0.00 | 0.28 |
| β13 | −1.01 × 10−5 | 0.00 | 0.01 | −2.22 × 10−6 | 0.00 | 0.17 | 6.88 × 10−7 | 0.00 | 0.03 |
| β23 | −2.17 × 10−5 | 0.00 | 0.81 | −5.62 × 10−5 | 0.00 | 0.15 | 2.40 × 10−6 | 0.00 | 0.75 |
| Total tannins | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Regre. coeff. | Standard error | p | |||||||
| β0 | 4.17 × 10−1 | 0.01 | 0.00 | ||||||
| β1 | 1.00 × 10−4 | 0.00 | 0.04 | ||||||
| β11 | 1.43 × 10−8 | 0.00 | 0.83 | ||||||
| β2 | 4.08 × 10−3 | 0.00 | 0.01 | ||||||
| β22 | −6.46 × 10−5 | 0.00 | 0.09 | ||||||
| β3 | −1.15 × 10−3 | 0.00 | 0.00 | ||||||
| β33 | 9.66 × 10−6 | 0.00 | 0.01 | ||||||
| β12 | −1.65 × 10−6 | 0.00 | 0.17 | ||||||
| β13 | −2.50 × 10−7 | 0.00 | 0.51 | ||||||
| β23 | −1.21 × 10−5 | 0.00 | 0.18 | ||||||
In regression coefficients, 0 means constant, 1 pressure, 2 extraction time and 3 ethanol concentration. The significant coefficients in each case are written in bold
Antioxidant activity
The responses surfaces obtained for ABTS (Fig. 1b and c) and DPPH· methods were very similar. The highest antioxidant activities were obtained for the highest pressure as well as for the highest extraction time. However, for FRAP method a profile slightly different (Fig. 1d) from those was observed, since increasing extraction time above 21 min slightly decreased the antioxidant activity. When ethanol concentration decreased the antioxidant activity increased. For example, for an extraction time of 30 min and an ethanol concentration of 40%, the total antioxidant activity increases 8 and 15% (300 and 600 MPa), 2 and 17% (300 and 600 MPa) and 12 and 16% (300 and 600 MPa) using ABTS, DPPH· and FRAP methods, respectively. The estimated optimum extraction conditions were very similar for antioxidant activity determined by ABTS, DPPH·, and FRAP methods. To maximize the antioxidant activity measured by ABTS (4.57 mg Trolox Eq./g dw) the optimum conditions were 600 MPa, 27 min and 10% of ethanol and for DPPH· (3.00 mg Trolox Eq./g dw) were 600 MPa, 29 min and 15% of ethanol. With the FRAP method, the maximum value would be of 6.93 mg AIS Eq./g dw, obtained at 600 MPa, 22 min and using 13% of ethanol. For similar extraction conditions of 30 min and 0% of ethanol but at 0.1 MPa, the optimized conditions allow an increase of 34, 17 and 12% (ABTS, DPPH· and FRAP methods, respectively) of the antioxidant activity. Usually, high pressure of 300 and 600 MPa led to an increase in antioxidant activity between 8 and 9% and between 6 and 13%, respectively when compared with samples extracted at 0.1 MPa.
Ethanol concentration was the variable that presented the highest impact on antioxidant activity value (Table 1). F values calculated were of 1342, 151 and 1457 for linear effects and of 276, 48 and 355 for quadratic effects, respectively for ABTS, DPPH· and FRAP methods. On the other hand, the extraction time was not significant. Corrales et al. (2009) reported that red grape skin extracts obtained at an ethanol concentration of 50%, at 70 °C and 600 MPa possessed the highest antioxidant capacity. Prasad et al. (2010) also quantified total antioxidant activity in extracts of longan fruit pericarp by high-pressure-assisted and verified that extractions at 500 MPa, the highest pressure used, showed the highest antioxidant activities. In our work, the conditions that led to a higher extraction of total antioxidants were equal in terms of pressure (600 MPa) applied but the better ethanol concentration found was 10%. However, should be highlighted that our food matrix is very different, moreover is a by-product.
The R2 values (Table 1) of the models for ABTS and FRAP are relatively high (0.954 and 0.959, respectively), which means that only 4.6 and 4.1% of the total variations were not explained by the ABTS and FRAP models, respectively. Moreover, the . values did not differ considerably from R2 value, which showed that the regression model defined well the true behavior of the system representing the experimental data well. The model developed for DPPH· did not fit so well and consequently the determination coefficient was not so high (0.735). The experimental and predict values (Table 2) obtained through ABTS and FRAP methods are very similar while the values predicted with FRAP model are slightly different from the experimental results. For ABTS and FRAP, the variation between observed and predicted values was lower than 10%, except for P0.1/T30/S0, P300/T5/S0 and P300/T30/S80 analyzed by ABTS, which also showed that the regression models defined well the true behavior of the experimental system. For FRAP, 23% of samples showed a variation between observed and predicted values higher than 10%.
The regression coefficients to express the total antioxidant activity as a function of the pressure, extraction time and ethanol concentration, and their significance (0.05 level) were determined and are presented in Table 3. β0, β3 and β33 regression coefficients were significantly for the three models.
Total phenolic content
According to the model profile (Fig. 1e), increase in pressure and extraction time resulted in a higher extraction of total phenolic compounds. In general, when high-pressure extraction was used the phenolics content increased around 10% in relation to samples extracted at 0.1 MPa. Low ethanol concentration improved the extraction of phenolic compounds. In fact, ethanol concentration was also by far the variable that showed the major impact on phenolic extraction (F values of 1333 and 252 for linear and quadratic effects, respectively). However, pressure and extraction time were also significant (p < 0.05). When water was used as solvent during 17.5 min, the total phenolics content increased from 2.21 mg GA Eq./g DW obtained at 0.1 MPa to 2.38 and 2.40 mg GA Eq./g DW obtained at 300 and 600 MPa, which represent an increase of 8 and 9%, respectively. The optimal conditions to obtain the highest extraction of phenolic compounds (2.45 mg GA Eq./g DW) were 600 MPa, 29 min and 9% of ethanol, which should represent an increase of 10% in relation to extractions preformed during 30 min using water as solvent but at 0.1 MPa. Prasad et al. (2009b) also reported a positive effect of high pressure on total phenolics extraction of longan fruit pericarp. In this study, total phenolic content increased from 14.6 (conventional extraction; 12 h at 30º and 0.1 MPa) to 21 mg/g DW (30 min, 500 MPa and 30 °C), representing an increase of 40%. This extraction method can cause deprotonation of charged groups, and break of salt bridges and hydrophobic bonds, resulting in conformational changes and denaturation of proteins and then rendering phenolic compounds (many times associated with proteins) more available to extraction (Prasad et al. 2009a; Zhu et al. 2016). Moreover, may provide the possibility of inactivating degrading enzymes, which may account for higher extraction yield and antioxidant activity compared to other methods (Prasad et al. 2009a; Zhu et al. 2016).
The fitness and adequacy of the model was high since the R2 obtained for the model was 0.954, which means that only 4.6% of the total variation was not explained by the model. Additionally, . (0.949) did not differ significantly from R2. Moreover, predicted values and experimental results varied in maximum 10.48% (P0.1/T30/S80), but in the most part of samples this variation was much lower (Table 2).
Equation 4 expresses the mathematical model used, representing the total phenolics (TP) content as a function of the independent variables within the region under investigation. The regression coefficients that presented a significant (p < 0.05) effect on the extraction of total phenolic were only β0, β1, β2, β3 and β33 (Table 3).
Total condensed tannin content
Model fitness was significant (p < 0.05), i.e., all the independent variables had a significant effect on tannins extraction. The response surface obtained for pressure vs extraction time (Fig. 1f) reveled that when pressure and/or extraction time increased a higher extraction of tannins was obtained. In general, high-pressure extraction allowed an extraction yield of more 4 and 10% (300 and 600 MPa, respectively) in relation to samples extracted at 0.1 MPa. A significant effect of ethanol concentration was also observed, but lower than the effects observed for the other models, since the use of a lower ethanol concentration led to higher values of extracted compounds. Considering an ethanol concentration of 40%, it was obtained 0.43 mg catechin Eq./g DW after 30 min of extraction at 0.1 MPa. However, for same ethanol concentration and extraction time was obtained more 3 and 17% at 300 and 600 MPa, respectively. The optimal extraction conditions to obtain the maximum of 0.52 mg catechin Eq./g DW were 600 MPa, 24 min and 0% of ethanol. This value represent more 13% in relation to extractions performed at 0.1 MPa, during 30 min and using water as solvent. The variable with higher impact was the ethanol concentration presenting F values of 53 and 7 for linear and quadratic interactions, respectively. However, pressure and extraction times were also significant (Table 2). The fitness and adequacy of the model was relatively high since the R2 obtained for the model was 0.966. Thus, the model did not explain only 3.4% of the total variation. The . (0.962) was close to the value founded for R2 and did not differ significantly. Moreover, the obtained and predictive values did not differed more than 7% (Table 2).
In Table 3 the regression coefficients and their significance (0.05 level) determined for the polynomial equation are presented, which express the mathematical model for total condensed tannins extraction as a function of each independent variable. The regression coefficients that presented a significant (p < 0.05) effect on the extraction of total condensed tannins were β0, β1, β2, β3 and β33.
Total flavonoids content
The highest extraction of total flavonoids (Fig. 1g and h) was obtained for the highest pressure used (600 MPa) and when higher extraction times and/or ethanol concentration were used, but only until certain values (18 min and 47%), with no significant further improvement thereafter. When 40% of ethanol was used as solvent during 30 min, the total flavonoids content increased from 0.11 mg quercetin Eq./g DW (0.1 MPa) to 0.14 and 0.15 mg quercetin Eq./g DW obtained at 300 and 600 MPa, which represent an increase of 26 and 35%, respectively. The estimated highest content of total flavonoids is 0.16 mg quercetin Eq./g DW for extraction performed under the optimal conditions (600 MPa, 18 min and 47% of ethanol), representing an increase of 45% in relation to extractions preformed during 30 min using 40% of ethanol but at 0.1 MPa. In general can be extracted more 10% of tannins when high-pressure is used, in relation to samples extracted at 0.1 MPa. The ethanol concentration was the most important effect to achieve higher extraction yields, which presented F values of 124 and 19 (quadratic and linear effects, respectively). In this model, pressure was not significant (p > 0.05). Contrary to our results, Shouqin et al. (2005) studied the high hydrostatic pressure extraction of flavonoids from propolis and verified a significant effect of high pressure on flavonoids extraction. These authors results showed that the durations of heat reflux extraction and extraction at room temperature were respectively about 240 and 10 080 times more than when preformed at 600 MPa. Prasad et al. (2009a) also observed that high pressure extraction (400 MPa) from litchi fruit pericarp increased the flavonoid extraction yield up to 2.6 times in comparison with conventional extraction. Results reported for extracts obtained from some products (Cardoso et al. 2013) showed some different profile behavior when compared with fig by-product extracts. In relation to solvent concentration, Shouqin et al. (2005) observed that when the volume of ethanol in the solvent was lower than 75% (v/v), the extraction yield was increased with the increase of ethanol concentration. In our work we observed the same effect but for a lower ethanol concentration (48%). These results may be due to the fact that the solubility of flavonoids in ethanol increases with the ethanol concentrations and additionally this effect may differ quantitatively for individual flavonoids, with the ethanol concentration thus also affecting the relative composition on individual flavonoids (Shouqin et al. 2005).
The fitness and adequacy of this model was not very good when compared with the other models, since the R2 obtained was 0.695, which means that 30.5% of total variations are not explained by the model. The variation between observed and predicted values was the highest observed among all models, once only 50% of the samples presented a variation between the values lower than 10% (Table 2). The regression coefficients and their significance were calculated to obtain the polynomial equation and only β0, β1, β2, β3 and β33 had a significant impact on the models.
Individual compounds
Individual compounds were analyzed by uHPLC and LC-DAD/ESI–MS analysis for phenolic acids detection and proanthocyanidins were analyzed by HPLC–DAD. The samples were selected based on optimum extraction conditions and compared with a sample extracted in similar extraction conditions but at room pressure (Table 4). The chromatograms revealed that all samples were poor in phenolic and proanthocyanidins compounds being detected only 3 and 2 peaks, probably because the fig residue was previously fermented. It was possible to identify only one compound, the hydroxymethylfurfural, however this compound may result from sugar caramelization during dehydration of samples at 40 °C. For individual compounds, only the 3rd peak obtained for the conditions P600/T29/S9 showed a significant higher area when compared with the extract performed at 0.1 MPa. The amount of total phenolics obtained was low but quantifiable.
Table 4.
Relative area of individual compounds by HPLC using the method frequently used to identify phenolic compounds
| Compound (% relative area) |
P600/T29/S9 | P600/T29/S15 | P600/T22/S13 | P600/T18/S47 | P600/T24/S0 | P0.1/T29/S9 |
|---|---|---|---|---|---|---|
| 1—unknown | 1.78 ± 0.19a | 1.56 ± 0.06b | 1.65 ± 0.01ab | 0.58 ± 0.08c | 1.82 ± 0.02a | 1.67 ± 0.01ab |
| 2 Hydroxy-methylfurfural | 96.78 ± 0.25a | 97.19 ± 0.03b | 97.08 ± 0.06bc | 98.21 ± 0.08d | 96.84 ± 0.08a | 97.13 ± 0.09be |
| 3—unknown | 1.44 ± 0.07a | 1.25 ± 0.04b | 1.27 ± 0.06ab | 1.22 ± 0.01bc | 1.35 ± 0.07ab | 1.21 ± 0.08bd |
Each number corresponds to one compound, identified or not (1-unknown; 2- Hydroxymethylfurfural; 3- unknown)
Significant differences between values in the same line are indicated by different letters (p < 0.05)
Correlation between dependent variables
In the present study, the bioactive compounds extracted (and related activities) by the eight different methods and prepared from 27 different extraction conditions were evaluated and the responses varied depending on extraction conditions. Therefore, correlations between each response were investigated and R2 are presented in Table 5. Almost all correlations between the responses of all dependent variables were significant. For total extraction yields, their correlation with antioxidant activities performed by ABTS, DPPH· and FRAP and with total phenolic were high (R2 of 0.844, 0.622, 0.855 and 0.853, respectively) when compared to flavonoids and tannins (R2 of 0.004 and 0.364, respectively). However, the highest correlations were observed between ABTS and FRAP, ABTS and total phenolic and FRAP and total phenolics. These correlations presented R2 very high of 0.934, 0.903 and 0.942, respectively. A much reduced correlation was observed for total flavonoids (R2 < 0.01) in relation to the other compounds extractions (Table 5).
Table 5.
Correlations between the responses of all dependent variables
| R2 | ABTS | DPPH | FRAP | Total phenolic | Total flavonoids | Total tannins | Total yields |
|---|---|---|---|---|---|---|---|
| ABTS | 1 | ||||||
| DPPH | 0.663 | 1 | |||||
| FRAP | 0.934 | 0.668 | 1 | ||||
| Total phenolics | 0.903 | 0.660 | 0.942 | 1 | |||
| Total flavonoids | 0.012 | 0.009 | 0.013 | 0.004 | 1 | ||
| Total tannins | 0.206 | 0.156 | 0.239 | 0.255 | 0.044 | 1 | |
| Total yields | 0.844 | 0.622 | 0.855 | 0.853 | 0.004 | 0.364 | 1 |
Bold correlations were significant at p < 0.05
Conclusion
The exploitation of high pressure for extracting bioactive compounds from an industrial fig by-product for their application in food is a promising field. The results of this study indicate that the amount of almost all bioactive compounds extracted from fig processing waste can be increased by the use of high pressure conjointly with an optimized ethanol concentration and extraction time. The high correlation of the mathematical models indicated that a quadratic polynomial model could be employed to optimize extraction yields from fig by-product by high-pressure. The fitness and adequacy of models were high almost for all models (R2 > 0.95).
Pressure, extraction time and ethanol concentration significantly influenced the total extractions, independently and interactively. Ethanol concentration was the variable that showed a higher impact in all extractions. However, the pressure used also affect significantly the amount of total compounds extracted, with the exception of total flavonoids. In general, high-pressure can be used to increase the extraction yields in average until to 18% in relation to samples extracted at 0.1 MPa. Extraction time had a lower effect on the extractions of all bioactive compounds.
The optimum extraction conditions were obtained for a pressure of 600 MPa. The optimum extraction time vary according to the model, while optimum solvent concentration was in general water or low ethanol concentrations (<15%).
Independently of ethanol concentration and extraction time, high pressure extraction resulted always in improved extraction, up to a maximum of 35% for total flavonoids at 600 MPa, 40% ethanol and 30 min of extraction time. The optimized conditions obtained in this work are important from a perspective of by-products valorization since high-pressure increased antioxidant activity and the content of all total compounds.
Acknowledgements
This work was supported by National Funds from FCT–Fundação para a Ciência e a Tecnologia through project PEst-OE/EQB/LA0016/2013 And by FCT/MEC by the financial support to the QOPNA research Unit (FCT UID/QUI/00062/2013), through national funds and where applicable co-financed by the FEDER, within the PT2020 Partnership Agreement. The authors also acknowledge FCT for the financial support to UID/AGR/00115/2013 to ICAAM. Author Elisabete Maria Cruz Alexandre also is grateful for the financial support of this work from “Fundação para a Ciência e Tecnologia - FCT” through the Post-doctoral Grant SFRH/BPD/95795/2013.
Footnotes
Two more authors (Paula Araújo and Victor de Freitas) were added to the list of authors, since information about individual compounds present in the extracts (analyzed by HPLC-MS) was added to the MS with the collaboration of these 2 authors.
References
- Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117(4):426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the frap assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Box GEP, Behnken DW. Three level design for the study of quantitative variables. Technometrics. 1960;2:455–475. doi: 10.1080/00401706.1960.10489912. [DOI] [Google Scholar]
- Cardoso LC, Serrano CM, Quintero ET, López CP, Antezana RM, Martínez de la Ossa E. High pressure extraction of antioxidants from Solanum stenotomun peel. Molecules. 2013;18(3):3137–3151. doi: 10.3390/molecules18033137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casquete R, Castro SM, Martín A, Ruiz-Moyano S, Saraiva JA, Córdoba MG, Teixeira P. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov Food Sci Emerg. 2015;31:37–44. doi: 10.1016/j.ifset.2015.07.005. [DOI] [Google Scholar]
- Corrales M, García AF, Butz P, Tauscher B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J Food Eng. 2009;90(4):415–421. doi: 10.1016/j.jfoodeng.2008.07.003. [DOI] [Google Scholar]
- Cruz LC, Batista JES, Zemolin APP, Nunes MEM, Lippert DB, Royes LFF, Soares FA, Pereira AB, Posser T, Franco JL. A study on the quality and identity of Brazilian pampa biome honey: Evidences for its beneficial effects against oxidative stress and hyperglycemia. Int J Food Sci. 2014;2014(3):1–11. doi: 10.1155/2014/470214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunovaité L, Pukalskiené M, Pukalskas A, Venskutonis PR. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J Funct Foods. 2016;24:85–96. doi: 10.1016/j.jff.2016.03.018. [DOI] [Google Scholar]
- Guarrera PM. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium) Fitoterapia. 2005;76(1):1–25. doi: 10.1016/j.fitote.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Herald TJ, Gadgil P, Tilley M. High-throughput micro-plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J Sci Food Agric. 2012;92:2326–2331. doi: 10.1002/jsfa.5633. [DOI] [PubMed] [Google Scholar]
- Huang HW, Hsu CP, Yang BB, Wang CY. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci Technol. 2013;33(1):54–62. doi: 10.1016/j.tifs.2013.07.001. [DOI] [Google Scholar]
- M’hiri N, Ioannou I, Mihoubi Boudhrioua N, Ghoul M. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod Process. 2015;96:161–170. doi: 10.1016/j.fbp.2015.07.010. [DOI] [Google Scholar]
- Naczk M, Amarowicz R, Pink D, Shahidi F. Insoluble condensed tannins of canola/rapeseed. J Agri Food Chem. 2000;48(5):1758–1762. doi: 10.1021/jf9908401. [DOI] [PubMed] [Google Scholar]
- Oliveira AP, Valentão P, Pereira JA, Silva BM, Tavares F, Andrade PB. Ficus carica L.: metabolic and biological screening. Food Chem Toxicol. 2009;47(11):2841–2846. doi: 10.1016/j.fct.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Yang BAO, Zhao M, Ruenroengklin N, Jiang Y. Application of ultrasonication or high-pressure extraction of flavonoids from litchi fruit pericarp. J Food Process Eng. 2009;32(6):828–843. doi: 10.1111/j.1745-4530.2008.00247.x. [DOI] [Google Scholar]
- Prasad KN, Yang E, Yi C, Zhao M, Jiang Y. Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. Innov Food Sci Emerg Technol. 2009;10(2):155–159. doi: 10.1016/j.ifset.2008.11.007. [DOI] [Google Scholar]
- Prasad KN, Yang B, Shi J, Yu CY, Zhao MM, Xue S, Jiang YM. Enhanced antioxidant and antityrosinase activities of longan fruit pericarp by ultra-high-pressure-assisted extraction. J Pharm Biomed Anal. 2010;51(2):471–477. doi: 10.1016/j.jpba.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Santos MC, Salvador ÂC, Domingues FM, Cruz JM, Saraiva JA. Use of high hydrostatic pressure to increase the content of xanthohumol in beer wort. Food Bioprocess Technol. 2013;6(9):2478–2485. doi: 10.1007/s11947-012-0952-0. [DOI] [Google Scholar]
- Shouqin Z, Junjie Z, Changzhen W. Novel high pressure extraction technology. Int J Pharm. 2004;278(2):471–474. doi: 10.1016/j.ijpharm.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Shouqin Z, Jun X, Changzheng W. High hydrostatic pressure extraction of flavonoids from propolis. J Chem Technol Biotechnol. 2005;80(1):50–54. doi: 10.1002/jctb.1153. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin–ciocalteu reagent. Method Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Soltana H, Tekaya M, Amri Z, El-Gharbi S, Nakbi A, Harzallah A, Mechri B, Hammami M. Characterization of fig achenes’ oil of Ficus carica grown in Tunisia. Food Chem. 2016;196:1125–1130. doi: 10.1016/j.foodchem.2015.10.053. [DOI] [PubMed] [Google Scholar]
- Strati IF, Gogou E, Oreopoulou V. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod Process. 2015;94:668–674. doi: 10.1016/j.fbp.2014.09.012. [DOI] [Google Scholar]
- Viuda-Martos M, Barber X, Pérez-Álvarez JA, Fernández-López J. Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Ind Crops Prod. 2015;69:472–479. doi: 10.1016/j.indcrop.2015.03.005. [DOI] [Google Scholar]
- Xi J, Luo S. The mechanism for enhancing extraction of ferulic acid from Radix Angelica sinensis by high hydrostatic pressure. Sep Purif Technol. 2016;165(13):208–213. doi: 10.1016/j.seppur.2016.04.011. [DOI] [Google Scholar]
- Xi J, Shen D, Zhao S, Lu B, Li Y, Zhang R. Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction. Int J Pharm. 2009;382(1–2):139–143. doi: 10.1016/j.ijpharm.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Zhu X, Cheng Y, Chen P, Peng P, Liu S, Li D, Ruan R. Effect of alkaline and high-pressure homogenization on the extraction of phenolic acids from potato peels. Innov Food Sci Emerg Technol. 2016;37((Part A)):91–97. doi: 10.1016/j.ifset.2016.08.006. [DOI] [Google Scholar]
- Zhang SQ, Bi HM, Liu CJ. Extraction of bio-active components from Rhodiola sachalinensis under ultrahigh hydrostatic pressure. Sep Purif Technol. 2007;57(2):277–282. doi: 10.1016/j.seppur.2007.04.022. [DOI] [Google Scholar]


