Abstract
Rice accumulates 10-fold higher inorganic arsenic (i-As), an established human carcinogen, than other grains. This review summarizes epidemiologic studies that examined the association between rice consumption and biomarkers of arsenic exposure. After reviewing the literature we identified 20 studies, among them included 18 observational and 2 human experimental studies that reported on associations between rice consumption and an arsenic biomarker. Among individuals not exposed to contaminated water, rice is a source of i-As exposure — rice consumption has been consistently related to arsenic biomarkers, and the relationship has been clearly demonstrated in experimental studies. Early-life i-As exposure is of particular concern due to its association with lifelong adverse health outcomes. Maternal rice consumption during pregnancy also has been associated with infant toenail total arsenic concentrations indicating that dietary exposure during pregnancy results in fetal exposure. Thus, the collective evidence indicates that rice is an independent source of arsenic exposure in populations around the world and highlights the importance of investigating its affect on health.
Keywords: Arsenic metabolites, Bio monitoring, Dietary, Exposure, Rice, Urine, Toenails
Graphical abstract
1. Introduction
Arsenic is a ubiquitous metalloid and its presence in the environment is the result of both natural geologic processes and environmental pollution. Human exposure to arsenic has been associated with a broad range of adverse effects on health (IARC, 2004; Sharma et al., 2014; UN FAO/WHO, 2011). It is estimated that 140 million people worldwide are exposed to inorganic arsenic (i-As) primarily through the consumption of unregulated contaminated water — the known primary source of i-As human exposure (States et al., 2011). Adverse health effects associated with high levels of i-As exposure are well recognized. However, recent studies have reported an elevated risk of certain cancers, cardiovascular diseases, respiratory conditions, and diabetes associated with relatively low levels of i-As exposure (Amaral et al., 2012; EFSA, 2009; Ettinger et al., 2009; Karagas et al., 2004, 2001; Leonardi et al., 2012; Navas-Acien et al., 2008; Sohel et al., 2009). Such associations have been observed in populations who were known to consume water with concentrations of i-As well below the World Health Organization’s guideline of 10 μg/L.
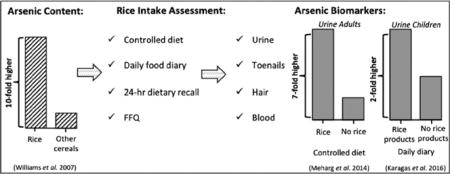
While seafood is a major dietary source of exposure due to high levels of arsenobetaine, a non-toxic organic form of arsenic, fruits, fruit juices, and grains are among the primary dietary sources of i-As exposure, especially in regions with access to water low in i-As (Meacher et al., 2002; Taylor et al., 2016; Xue et al., 2010; Yost et al., 2004). Rice, a crop that is grown in flooded plains, is of particular concern as the plant has been shown to bioaccumulate i-As at approximately 10-fold higher rate than other grains such as wheat and barley (Ma et al., 2008; Meharg et al., 2009; Mitani et al., 2009; Williams et al., 2007a, b; Williams et al., 2005). Nevertheless, wide variations exist in the amount and type of arsenic found in rice dependent on both where the plant was grown and the rice species (Bastias et al., 2010; Norton et al., 2012; Signes-Pastor et al., 2016a; Williams et al., 2007a, b; Williams et al., 2005). For instance, rice grown in the U.S. contains higher amounts of total arsenic (t-As) and dimethylarsinic acid (DMA) — the primary arsenic metabolite found in rice (Meharg et al., 2009; Williams et al., 2005). High concentrations of i-As have also been found in rice-based products, including those consumed by infants and young children, who are especially vulnerable to the adverse health effects of i-As exposure (Farzan et al., 2013; Jackson et al., 2012; Signes-Pastor et al., 2016b).
In the context of dietary i-As exposure assessment, it is important to differentiate between estimates of intake of contaminated food versus biological measures of internal dose. Numerous studies have explored the relationship between diet and arsenic biomarker concentrations; however, only a few accounted for arsenic intake from water by direct measurement of water samples (e.g., Gilbert-Diamond et al., 2011). The most common methods to measure dietary intake include the 24-hour food recall (administered via an interview or a food diary) and food frequency questionnaires (FFQ). The gold standard of dietary intake assessment is multiple 24-hour food diaries. However, both 24-hour food diaries and food frequency questionnaires rely on self-report, and thus are prone to measurement error (Orloff et al., 2009; Tao and Bolger, 1999).
For the present study, we performed a comprehensive literature review of published studies that examined the relationship between rice consumption and arsenic exposure using human biomarkers. Table 1 summarizes the relevant articles included in this review.
Table 1.
Summary of studies that examined associations between rice consumption and human exposure to arsenic.
| Order | References | Study design | Study population | Rice intake assessment | Rice arsenic level | Water arsenic level | Biomarkers | Results |
|---|---|---|---|---|---|---|---|---|
| 1 | He and Zheng, 2010 | Experimental | Female adults (n = 2). | Mass balance of arsenic metabolites. Wheat diet (5 days) vs rice-based diet (418 g of rice/day - 5 days) | 101 μg/kg of i-As, 25 μg/kg DMA, and 7.5 μg/kg other arsenic species. | ≤1 μg/kg | Mixture of two spot urine samples, the first in the morning and last in the evening. | Urinary t-As concentration doubled with rice diet in the two participants (from the mean value of 8.2 μg to 16.3 μg). DMA dominated urinary arsenic speciation. The mean percentage of rice-diet ingested arsenic excreted in urine was ~63%. |
| 2 | Meharg et al., 2014 | Experimental | Male adults (n = 9). | Mass balance of arsenic metabolites. 6 participants that followed a rice diet (300 g/day - 5 days) vs 3 participants that did not consume rice (5 days). | 99 μg/kg of i-As, 99 μg/kg of DMA, and 3 μg/kg MMA. | Distilled water | Spot urine, and 24-hour urine pass for participants consuming rice. | Urinary t-As was 7.3-fold higher after 4 days consuming rice (from 6.8 μg/L to 49.9 μg/L). Percentage of urinary DMA, and MMA increased 65%, and 6%, respectively after rice consumption. The percentage of urinary i-As decreased ~ 20% after rice consumption. The percentage of rice ingested arsenic excreted in urine was ~ 40%. |
| 3 | Lovreglio et al., 2012 | Prospective cohort study | Adults Caucasian males resident in a coastal area of southern Italy (n = 12). | Daily food diary starting 3 days before the start of the study and continuing throughout the study period. | - | Refers to regulation (<10 μg/kg) | Morning spot urine samples for 10 consecutive days. | Rice intake was not shown to influence either urinary excretion of i-As and its methylated species. Rice played a minor role in participants’ diet. |
| 4 | deCastro et al., 2014 | Cross-sectional study | Adults, adolescents, and children from NHANES (n = 8300). | 24-Hour dietary recall including rice, rice cakes/crackers, and rice beverage consumption. | - | - | Spot urine | Children consumption of rice and adolescents consumption of rice cakes/crackers was related to an increase in urinary DMA (positive regression slopes of 115 and 872, respectively). An increase in urinary MMA was also associated with an increase of rice beverages in adults, and an increase of rice cakes/crackers in children and adolescents (positive regression slopes of 6.4, 65.5, and 103, respectively). |
| 5 | Davis et al., 2012 | Cross-sectional study | Children from the NHANES (n = 2323). | 48-Hour dietary questionnaire. Rice eater defined as 1/4 cup of cooked rice per day. | - | - | Spot urine | Children “rice eaters” had 1.6-fold higher t-As urinary compared to those non “rice eaters” (from 8.9 μg/L to 5.5 μg/L). An increase of 14.2% of urinary t-As was associated with each 1/4 cup of cooked rice consumption (14.1 g dry weight). |
| 6 | Kordas et al., 2016 | Cross sectional study | Young children of 5–8 years of age (n = 328) residing in Montevideo, Uruguay. | Two 24-hour dietary recalls including rice consumption. Children defined as consuming <5 g rice/day vs children ≥5 g rice/day | - | 0.45 μg/L | Spot urine | Sum of urinary arsenic concentrations (iAs, MMA, and DMA) increased from 11.0 μg/L to 13.5 μg/L with rice consumption ≥5 g rice/day. |
| 7 | Davis et al., 2014a | Cross-sectional study | Adults and children from NHANES (n = 20.497). | 48-Hour recall dietary questionnaire including rice consumption. | - | - | Spot urine | Association between rice consumption and urinary t-As among both children and adults. An increase of 10 g (dry weight) rice per day was associated with an increase of 9.6% urinary t-As, and an increase of 8.6% in urinary DMA. |
| 8 | Wu et al., 2015 | Cross-sectional study | U.S. adults (n = 6677) belonging to NHANES. | 48-Hour recall dietary questionnaire including white and brown rice consumption. | - | - | Spot urine | Intake of white or brown rice were both associated with ~ 1.6-fold higher urinary sum of arsenic concentrations excluding arsenobetaine |
| compared to those who did not consume rice (from 7.9 μg/L to 13.1 μg/L). Among rice eaters, urinary sum of arsenic metabolites excluding arsenobetaine did not differ between participants who primarily consume white rice or brown rice. | ||||||||
| 9 | Gilbert-Diamond et al., 2011 | Cross sectional study | Pregnant women (n = 229) residing in New Hampshire. | 72-Hour recall dietary questionnaire including rice consumption. | - | Range from ≤0.07 μg/L to100 μg/L. Highly right-skewed. | Spot urine | Urinary t-As was 5.3 μg/L for rice consumers and 3.4 μg/L for non-consumers. Rice consumers had 0.07 μg/L higher i-As, 0.18 μg/L higher MMA, and 1.25 μg/L higher DMA than non-rice consumers. Rice consumption increased urinary loge t-As with a β of 0.009 (95% CI: 0.005, 0.013). |
| 10 | Davis et al., 2014b | Cross-sectional study | Mother-infant pairs (n = 170) residing in New Hampshire, and mother-infant pairs (n = 130) belonging to a validation cohort from Rhode Island. | 72-Hour recall dietary questionnaire including rice consumption. | - | - | Toenails | Toenail t-As concentration from infants exposed to rice consumption during pregnancy was 0.09 μg/g compared to 0.06 μg/g for those non-exposed. An increase of 1/4 cooked rice cups/day during pregnancy was associated with a 16.9% increase in infants toenail t-As concentration. |
| 11 | Cascio et al., 2011 | Prospective cohort study | Adults living in United Kingdom (U.K.) (n = 49). | Food frequency questionnaire. Bangladeshi with high consumption of rice (n = 37) vs white Caucasians with lower consumption of rice (n = 12) | - | Refers to regulation (<10 μg/kg) | Spot urine | U.K. Bangladeshi community had 30-fold higher rice consumption than U.K. white Caucasians. Similar urinary t-As concentrations were found for the Bangladeshi group (28.4 μg/L) and the white Caucasians group (20.6 μg/L). Urinary DMA and i-As concentrations for the U.K. Bangladeshi community was 5- and 2.5-fold higher than for the U.K. white Caucasians. |
| 12 | Wei et al., 2014 | Cross-sectional study | U.S. adults (n = 3027) belonging to the NHANES. | Food frequency questionnaire including rice consumption. Adults classified as low and high rice consumers (<twice a week vs ≥twice a week). | - | - | Spot urine | Participants that consumed rice more than twice a week had 1.1- and 1.2-fold higher urinary t-As (from 2.21 to 2.42 μg/g creatinine) and DMA (from 1.32 to 1.58 μg/g creatinine) compared to those that consume rice less than twice a week, respectively. |
| 13 | Melkonian et al., 2013 | Prospective cohort study | Adults living in Araihazar, Bangladesh (n = 18.470, with a subset of 4517 with urinary As metabolites). | Food frequency questionnaire including rice consumption. | - | 50% participants with access to well water <50 μg As/L. | Spot urine | Rice consumption was positively associated with urinary t-As (Multivariate model β = 0.041) (95% CI: 0.032, 0.051). |
| 14 | Park and Lee, 2013 | Cross sectional study | Korean adults (n = 3404) residing in South Korea belonging to the Korean NHANES. | Semiquantitative food frequency questionnaire including rice consumption. | - | - | Spot urine | Rice consumption was associated with a higher urinary t-As concentration. The adjusted means urinary t-As for low, moderate, and high rice consumption were 99.3, 105.4, and 120.1 μg/g creatinine. |
| 15 | Rivera-Nuñez et al., 2012 | Cross-sectional study | Adults (n = 343) residing in Michigan belonging to the case-control study of bladder cancer in Michigan. | Food frequency questionnaire including rice consumption. | - | ≤50 μg/L | Spot urine | Rice consumption was positively associated with sum of arsenic concentrations (i-As, MMA, and DMA) with β and R2 of 7.51 and 0.008, respectively. |
| 16 | Karagas et al., 2016 | Cross sectional study | Infants (n = 759) belonging to the NHBCS. | Questionnaire and diaries including rice and rice-based products. | Ranging from 4.6 to 201 μg i-As/kg. | Mainly <10 μg/kg | Spot urine | Urinary t-As was nearly double (from 4.97 μg/L to 9.53 μg/L) for infants consuming rice-based products compared to those that did not consume rice. Infant rice cereals and rice-based snacks were positively associated with urinary log10 t-As according to the estimate, β = 0.22 (95% CI: 0.11, 0.34) and 0.10 (95% CI: 0.04, 0.15), respectively. |
| 17 | Cleland et al., 2009 | Descriptive study | Korean women adults (n = 108) living in Washington State. | Food frequency questionnaire including rice consumption rates, which is an important food item in the Korean community. | - | <2 μg/L | Spot urine and hair | Estimated arsenic exposure from rice was a statistically significant predictor of sum of the urinary arsenic species, but only explained a small percentage of the variability. |
| 18 | Cottingham et al., 2013 | Cross-sectional study | Adults residing in New Hampshire residents (n = 852) belonging to the case-control study of bladder and skin cancer in New Hampshire. | Annual food frequency questionnaire recording average daily consumption of 120 different diet items. | - | <1 μg/L vs ≥ 1 μg/L | Toenail | Toenail t-As increased with household water arsenic concentration. No clear relationship was found between toenail t-As and rice consumption, which may be related to the low rice consumption of the study population (adjusted model β for brown rice = 0.23, p-value = 0.29; β for white rice = 0.065, p-value = 0.67). |
| 19 | Slotnick et al., 2007 | Cross-sectional study | Adults control participants (n = 440) belonging to the case-control of As in drinking water and bladder cancer in 11 counties o Michigan. | Food frequency questionnaire including rice consumption. | - | Range from <LOD to 99.9 μg/L with a median of 0.38 μg/L. | Toenail | Rice consumption was negatively associated with As toenail concentration (β = −0.137, R2 = 0.017). |
| 20 | MacIntosh et al., 1997 | Cross sectional study nested within prospective cohort | Females and males adults (n = 969) belonging to the 1984–1986 Nurses’ Health Study and the Health Professionals Follow-Up Study. | Semiquantitative food frequency questionnaire. | - | - | Toenail | Regression coefficient for brown rice consumption per 1 unit increase in daily average servings 0.494 (p-value = 0.0457) |
2. Biomarkers of human exposure to arsenic
Biomarkers are used to estimate internal dose because they: (1) represent an aggregate measure that accounts for all routes of exposure and (2) are independent of self-report. Human biological specimens used to estimate internal dose of arsenic exposure include urine, blood, nails, and hair (Marchiset-Ferlay et al., 2012).
Upon ingestion, i-As is rapidly metabolized by the body to monomethylarsonic acid (MMA) and DMA. MMA and DMA are among the end metabolites of the i-As metabolism which may be excreted in the urine unchanged from direct dietary exposure (Vahter, 2002). Urinary arsenic concentration measured in urine spot samples, normalized by creatinine or specific gravity to account for differences in urinary dilution, is a commonly used biomarker of short-term exposure. However, among individuals with consistent patterns of arsenic exposure urinary arsenic concentration appears to be a reliable source of long-term exposure as well (Kile et al., 2009; Marchiset-Ferlay et al., 2012; Navas-Acien et al., 2009).
The measurement of specific arsenic species in urine is performed by ion exchange high performance liquid chromatography coupled with either inductively coupled plasma mass spectrometry (HPLC-ICP-MS) or hydride generation atomic fluorescence spectrometry (HPLC-HG-AFS). Identification of specific arsenic species in urine is useful to both assess dietary exposure and explore arsenic metabolism (Chowdhury et al., 2003; Concha et al., 1998; Fängström et al., 2009). In addition, arsenic speciation in urine allows the subtraction of species considered relatively non-toxic including arsenobetaine and arsenocholine, predominantly found in seafood products (Navas-Acien et al., 2011). To a lesser extent human blood has been used as a biomarker of short-term arsenic exposure (Hall et al., 2007, 2006).
Human nail (both fingernail and toenail) and hair samples have been extensively used as a biomarker of long-term i-As exposure since unmetabolized i-As tends to be attracted to negative sulfhydryl-groups found in keratin-rich tissues of the integumentary system (Mandal et al., 2003; Slotnick and Nriagu, 2006). The use of hair and nail specimens has the added advantage of being easy to collect (e.g., toenail clippings are of low cost to collect from study participants). However, measurement of t-As in hair and nails (that represents primarily i-As) is potentially less sensitive than arsenic measured in urine. Concentration of t-As in nails and hair tend to provide a better estimate of long-term exposure than arsenic measured in urine due to their growth rate. Nevertheless, considerable care must be taken during nail and hair sample collection and laboratory analysis to avoid external contamination (Mandal et al., 2003). For instance, nails must be thoroughly washed prior to chemical digestion in the laboratory to remove trace contamination of nail polish, dirt or other products.
3. Literature review
3.1. Experimental studies
Epidemiologic evidence points to rice being an independent contributor to dietary i-As exposure. Bioaccessibility of arsenic in rice has been evaluated using in vitro gastro intestinal digestion simulation procedures. These studies have found that between 53% and 102% of t-As in rice is bioavailable (He et al., 2012; Signes-Pastor et al., 2012). One experiment followed two participants who, while consuming ≤ 1 μg/L arsenic water, were exposed to a wheat-based diet for five days and then switched to a rice-based diet (418 g/day) for another five days (He and Zheng, 2010). The two study participants’ urinary t-As concentrations doubled (primarily urinary DMA) upon switching from the wheat-based to the rice-based diet — from a mean of 8.2 μg of t-As during the wheat-based diet to 16.3 μg of t-As during the rice-based diet. It was estimated that approximately 63% of the t-As ingested from rice was excreted in the urine. A 7.3-fold increase in urinary t-As concentration (from 6.8 μg/L to 49.9 μg/L) that consisted primarily of DMA was reported in a study including six experimental and three control participants after following a five-day rice diet (300 g/day) (Meharg et al., 2014). In this study the five-day rice diet was prepared with rice containing i-As and DMA at a ratio of 1:1 and cooked with deionized water. The authors estimated that 40% of t-As ingested by consuming rice was excreted in the urine. Variation was observed in an individual’s concentrations of t-As urine concentrations following a standardized rice portion, as well as urinary t-As excretion over time.
3.2. Child and adult (non-pregnant) populations
In the non-experimental setting, one of the most common used methods to estimate dietary intake is the 24-hour dietary recall. This method includes having a participant recall everything consumed in the previous day via either an interview or by a diet diary. The 24-hour dietary recall using an in-person questionnaire interview has been used in the National Health and Nutrition Examination Survey (NHANES). The NHANES is a survey of U.S. non-institutionalized population that also collects objective health measurements designed to make national estimates on diet and health. Several studies have used the NHANES data to examine the association between food intake and urinary arsenic concentrations. These studies reported associations between rice intake and higher t-As exposure in both children and adults (Davis et al., 2012; Davis et al., 2014a; deCastro et al., 2014; Wu et al., 2015). Using NHANES data, rice and rice cakes/crackers intake in g/day was independently associated with an increase of urinary MMA and DMA concentrations among both younger (6–11 years) and older (12–19 years) children (deCastro et al., 2014). In this study, an increase of urinary DMA and MMA (untransformed β =105.6 and 5.6 for DMA and MMA, respectively p-value < 0.15 for both) also was associated with rice beverage consumption in adults between 20 and 84 years old. Using a combination of the NHANES data and the U.S. Department of Agriculture’s Food Commodity Intake Database, it was found that children 6–17 years old consuming rice had almost 2-fold higher urinary t-As — median urinary arsenic concentration of 8.9 μg/L compared to 5.5 μg/L among those non-rice consumers (Davis et al., 2012). Adjusted for other factors, an increase of ¼ cup of cooked rice per day (14.1 g dry weight) was associated with a 14.2% increase in urinary t-As concentration (Davis et al., 2012). In a follow-up study, the same authors found that an increase of 10 g of dry rice per day was associated with an increase of 9.6% urinary t-As, and an increase of 8.6% urinary DMA (Davis et al., 2014a). In both studies the majority of study participants reported to consume water from public sources, which due to regulation is expected to have relatively low i-As concentrations (<10 μg/L). Another study used NHANES data to examine the association between brown and polished white rice intake separately and urinary arsenic (both t-As and i-As) concentrations in adults (Wu et al., 2015). Both white and brown rice consumption were associated with higher urinary t-As. However, urinary t-As excluding arsenobetaine did not differ between participants who primarily consume white versus brown rice. Mean urinary concentrations of the sum of arsenic metabolites excluding arsenobetaine were 11.5 μg/L among participants who ate <1 cup/day white rice, 13.1 μg/L among those who ate ≥1 cup/day white rice, and 7.9 μg/L among non-rice eaters. For brown rice eaters, the mean urinary i-As concentrations were 10.9 μg/L among those who ate <1 cup/day brown rice only and 13.1 μg/L for those who ate ≥1 cup/day brown rice. The lack of an observed difference between white and brown rice was unexpected. White rice is known to contain less i-As than brown rice as brown rice tends to accumulates arsenic in the outer layers of the pericarp and aleurone, portions of the grain that are removed during the polishing process (Meharg et al., 2008). In the one contrasting study of only 12 adults, whose diets comprised a relatively small proportion of rice, rice intake did not appear to affect either urinary excretion of i-As and its methylated species (Lovreglio et al., 2012).
The FFQ is another common method used to estimate diet in particular long-term dietary patterns (e.g., over the past year). Consequently, among those with variable diets, it may not accurately represent recent, short-term intake — e.g., be reflective of the period of urinary arsenic excretion. The FFQ has been used to examine the association between diet and urinary arsenic concentration among Bangladeshi populations living in the United Kingdom (U.K.) — a group known to consume high amounts of rice (Cascio et al., 2011). Indeed, the Bangladeshi population living in the U.K. had up to 30-fold higher rice consumption compared to Caucasians residing in the U.K. Furthermore, urinary DMA and i-As concentrations were 5- and 2.5-fold higher for the Bangladeshi population (median of 16.9 μg DMA/L and 0.63 μg i-As/L) than for Caucasians (median of 3.16 μg DMA/L and 0.25 μg i-As/L), respectively.
Using NHANES, which also collected FFQ data, another study classified U.S. adults according to their rice frequency consumption as low (<twice a week) and high consumers (>twice a week) (Wei et al., 2014). Those with high rice consumption had higher mean urinary t-As (2.4 μg/g) and DMA (1.6 μg/g) creatinine-adjusted concentrations compared to those with lower rice intake (mean urinary t-As and DMA creatinine-adjusted concentrations of 2.2 and 1.3 μg/g respectively). A similar trend was found in a study population of adults residing in Michigan, U.S. (Rivera-Nuñez et al., 2012). While individuals who reported consuming rice in the FFQ were found to have higher urinary t-As concentrations than those who did not report consuming rice, rice explained only a small percentage of the variability in t-As concentrations (Rivera-Nuñez et al., 2012). In a large prospective cohort study of adults residing in Araihazar, Bangladesh, spot urine samples were collected along with a 39-item FFQ that inquired about rice consumption (Melkonian et al., 2013). Nearly half of the study population (n = 18.470) was exposed to i-As contaminated drinking water >50 μg/L and amount of rice intake was positively associated with urinary t-As concentration (β = 0.04, 95% CI: 0.03, 0.05). A clear correlation between rice consumption estimated by a FFQ and urinary t-As concentrations also has been reported for both the Korean population living in the U.S. and residing in South Korea (Cleland et al., 2009; Park and Lee, 2013).
Toenail t-As concentration, that represents long-term exposure particularly to i-As, has also been related to rice intake. To our knowledge, one of the first research studies on dietary exposure to arsenic used data from the Nurses’ Health Study and the Health Professionals Follow-Up Study and reported that consumption of brown rice (estimated using a FFQ) was positively associated with toenail t-As concentration (β = 0.40, p-value = 0.11) (MacIntosh et al., 1997). A more recent study including participants from a case-control study of bladder and skin cancer in New Hampshire found that toenail t-As concentration increased with household water i-As concentration, but did not identify a relationship between rice consumption and toenail t-As concentration (for brown rice adjusted β = 0.23, p-value = 0.29 and white rice β = 0.065, p-value = 0.67) (Cottingham et al., 2013). The lack of a relationship in this study may have resulted from the overall low rice consumption of the study population (only 1–3 times per month). Similar results were found in a case-control study carried out in 11 counties of Michigan where rice intake was negatively associated (β = −0.137, p-value <0.05) with toenail t-As concentration (Slotnick et al., 2007). In this study the authors used an FFQ where study participants were asked on average over the past year how often they consumed foods including soup, rice, and pasta. The authors report the negative association between rice and toenail arsenic may have been due to dietary intake of other foods that confounded the observed relationship.
3.3. Pregnancy and early childhood
Exposure to arsenic during pregnancy and early childhood is of particular concern due to the vulnerability of the fetus, infants, and young children to environmental contaminants. There is some evidence that exposure i-As early in life may impact growth and health throughout the lifespan (Farzan et al., 2013; IARC, 2004).
It is known that i-As crosses the placental barrier and appears in fetal tissue. Thus maternal exposure during pregnancy is an important area of inquiry. Dietary i-As exposure has been evaluated extensively in the New Hampshire Birth Cohort Study (NHBCS) — an ongoing prospective birth cohort study being conducted in New Hampshire, U.S. where low-level i-As exposure is known to occur (Davis et al., 2014b; Gilbert-Diamond et al., 2011; Karagas et al., 2016). In these studies rice consumption was estimated using a 3-day food diary and spot urinary samples were obtained from women during pregnancy. Mothers who consumed rice had 0.07 μg/L higher urinary i-As, 0.18 μg/L higher urinary MMA, and 1.25 μg/L higher urinary DMA compared to non-rice eaters after adjustment for estimated arsenic intake through the drinking water (Gilbert-Diamond et al., 2011). Among mother-infant pairs it was found that maternal rice intake, also determined with a 3-day food diary, was associated with infants toenail t-As concentration (Davis et al., 2014a, b). Specifically, an increase of 1/4 cup of rice per day was associated with a 16.9% increase in infants toenail t-As concentration indicating that maternal consumption of rice relates fetal exposure, and that infant toenails represent this exposure.
Among one-year old infants participating in the NHBCS, those who were reported to consume rice-based products had mean urinary t-As concentration nearly twice that of those who did not consume rice-based products (5.8 μg/L versus 2.8 μg/L respectively) (Karagas et al., 2016). A similar trend was found among a sample of children between the ages of 5 to 8 years old residing in Montevideo, Uruguay, whose rice intake was estimated using two 24-hour food recall periods (Kordas et al., 2016).
4. Conclusion
Differences in study design, i.e., methods of characterizing both arsenic exposure and rice consumption, geographic and ethnic differences in the study populations, and ages of the study participants all provide challenges to directly compare estimates of the association between rice intake and arsenic exposure. Nevertheless, across diverse populations, studies have consistently found positive associations between rice intake and arsenic exposure. These associations have been reported for infants, adolescents and adults. Most notably, the association between rice intake and arsenic exposure using biomarkers such as urinary arsenic (that represent an estimate of internal dose) has been demonstrated in two experimental studies where participants followed a controlled rice diet. Maternal rice intake during pregnancy also has been associated with infant toenail arsenic concentration at birth, suggesting in utero exposure occurs by rice consumption during pregnancy. Collectively, evidence points to rice being an independent source of arsenic exposure in populations around the world underscoring the importance of strategies to prevent this exposure and understanding its impact on human health.
HIGHLIGHTS.
Rice intake has been associated with increased urinary arsenic concentrations across numerous studies.
The association between rice intake and human exposure has been demonstrated in populations around the world.
Maternal rice intake during pregnancy is associated with total arsenic concentrations in infant toenails.
Acknowledgments
This paper, and the four others that accompany it (Punshon et al.; Cubadda et al.; Taylor et al.; Nachman et al.), is a product of the Collaborative on Food with Arsenic and Associated Risk and Regulation (C-FARR), a two year effort led by the Dartmouth Superfund Research Program and Children’s Environmental Health and Disease Prevention Research Center. The goal of C-FARR is to synthesize the current information pertaining to arsenic from soil to plate, based on key questions and knowledge gaps identified by policy stakeholders and scientists from interdisciplinary backgrounds, to inform future regulatory and policy decisions affecting dietary arsenic exposure.
Funding
The Collaborative on Food with Arsenic and associated Risk and Regulation (C-FARR) is supported by the Dartmouth College Toxic Metals Superfund Research Program (NIH NIEHS P42ES007373) and the Children’s Environmental Health and Disease Prevention Research Center at Dartmouth (US NIH NIEHS P01ES022832 and EPA RD83459901).
References
- Amaral AFS, Porta M, Silverman DT, Milne RL, Kogevinas M, Rothman N, Cantor KP, Jackson BP, Pumarega JA, López T, Carrato A, Guarner L, Real FX, Malats N. Pancreatic cancer risk and levels of trace elements. Gut. 2012;61:1583–1588. doi: 10.1136/gutjnl-2011-301086. http://dx.doi.org/m1136/gutjnl-2011-301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastias JM, Bermudez M, Carrasco J, Espinoza O, Munoz M, Galotto MJ, Munoz O. Determination of dietary intake of total arsenic, inorganic arsenic and total mercury in the Chilean school meal program. Food Sci Technol Int. 2010;16:443–450. doi: 10.1177/1082013210367956. http://dx.doi.org/10.1177/1082013210367956. [DOI] [PubMed] [Google Scholar]
- Cascio C, Raab A, Jenkins RO, Feldmann J, Meharg AA, Haris PI. The impact of a rice based diet on urinary arsenic. J Environ Monit. 2011;13:257–265. doi: 10.1039/c0em00482k. http://dx.doi.org/10.1039/c0em00482k. [DOI] [PubMed] [Google Scholar]
- deCastro BR, Caldwell KL, Jones RL, Blount BC, Pan Y, Ward C, Mortensen ME. Dietary sources of methylated arsenic species in urine of the United States population, NHANES 2003–2010. PLoS One. 2014;9:e108098. doi: 10.1371/journal.pone.0108098. http://dx.doi.org/10.1371/journal.pone.0108098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury UK, Rahman MM, Sengupta MK, Lodh D, Chanda CR, Roy S, Quamruzzaman Q, Tokunaga H, Ando M, Chakraborti D. Pattern of excretion of arsenic compounds arsenite, arsenate, MMA(V), DMA(V) in urine of children compared to adults from an arsenic exposed area in Bangladesh. J Environ Sci Health A. 2003;38:87–113. doi: 10.1081/ese-120016883. http://dx.doi.org/10.1081/ese-120016883. [DOI] [PubMed] [Google Scholar]
- Cleland B, Tsuchiya A, Kalman DA, Dills R, Burbacher TM, White JW, Faustman EM, Mariën K. Arsenic exposure within the Korean community (United States) based on dietary behavior and arsenic levels in hair, urine, air, and water. Environ Health Perspect. 2009;117:632–638. doi: 10.1289/ehp.11827. http://dx.doi.org/10.1289/ehp.11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha G, Nermell B, Vahter M. Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina. Environ Health Perspect. 1998;106:355–359. doi: 10.1289/ehp.98106355. http://dx.doi.org/m1289/ehp.98106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham KL, Karimi R, Gruber JF, Zens MS, Sayarath V, Folt CL, Punshon T, Morris JS, Karagas MR. Diet and toenail arsenic concentrations in a New Hampshire population with arsenic-containing water. Nutr J. 2013;12:149. doi: 10.1186/1475-2891-12-149. http://dx.doi.org/10.1186/1475-2891-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in U.S. children. Environ Health Perspect. 2012;120:1418–1424. doi: 10.1289/ehp.1205014. http://dx.doi.org/10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Gilbert-Diamond D, Karagas MR, Li Z, Moore JH, Williams SM, Frost HR. A dietary-wide association study (DWAS) of environmental metal exposure in US children and adults. PLoS One. 2014a;9 doi: 10.1371/journal.pone.0104768. http://dx.doi.org/10.1371/journal.pone.0104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Li Z, Gilbert-Diamond D, Mackenzie TA, Cottingham KL, Jackson BP, Lee JS, Baker ER, Marsit CJ, Karagas MR. Infant toenails as a biomarker of in utero arsenic exposure. J Expo Sci Environ Epidemiol. 2014b;24:467–473. doi: 10.1038/jes.2014.38. http://dx.doi.org/10.1038/jes.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA. European Food Safety Authority. Scientific Opinion on Arsenic in Food. EFSA panel on contaminants in food chain (CONTAM) 2009 doi: http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/1351.pdf.
- Ettinger AS, Zota AR, Amarasiriwardene CJ, Hopkins MR, Schwartz J, Hu H, Wright RO. Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environ Health Perspect. 2009;117:1059–1064. doi: 10.1289/ehp0800533. http://dx.doi.org/10.1289/ehp0800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fängström B, Hamadani J, Nermell B, Grandér M, Palm B, Vahter M. Impaired arsenic metabolism in children during weaning. Toxicol Appl Pharmacol. 2009;239:208–214. doi: 10.1016/j.taap.2008.12.019. http://dx.doi.org/10.1016/j.taap.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Karagas MR, Chen Y. In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol. 2013;272:384–390. doi: 10.1016/j.taap.2013.06.030. http://dx.doi.org/10.1016/j.taap.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, Baker ER, Jackson BP, Folt CL, Karagas MR. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci U S A. 2011;108:20656–20660. doi: 10.1073/pnas.1109127108. http://dx.doi.org/10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Chen Y, Ahsan H, Slavkovich V, van Geen A, Parvez F, Graziano J. Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology. 2006;225:225–233. doi: 10.1016/j.tox.2006.06.010. http://dx.doi.org/10.1016/j.tox.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Hall M, Gamble M, Slavkovich V, Liu X, Levy D, Cheng Z, van Geen A, Yunus M, Rahman M, Pilsner JR, Graziano J. Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect. 2007;115:1503–1509. doi: 10.1289/ehp.9906. http://dx.doi.org/10.1289/ehp.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zheng Y. Assessment of in vivo bioaccessibility of arsenic in dietary rice by a mass balance approach. Sci Total Environ. 2010;408:1430–1436. doi: 10.1016/j.scitotenv.2009.12.043. http://dx.doi.org/10.1016/j.scitotenv.2009.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Pedigo CE, Lam B, Cheng ZQ, Zheng Y. Bioaccessibility of arsenic in various types of rice in an in vitro gastrointestinal fluid system. J Environ Sci Health B-Pestic Food Contam Agric Wastes. 2012;47:74–80. doi: 10.1080/03601234.2012.611431. http://dx.doi.org/10.1080/03601234.2012.611431. [DOI] [PubMed] [Google Scholar]
- IARC. International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Drinking-water Disinfectants and Contaminants, Including Arsenic. 2004 doi: monographs.iarc.fr/ENG/Monographs/vol84/mono84.pdf. [PMC free article] [PubMed]
- Jackson BP, Taylor VF, Punshon T, Cottingham KL. Arsenic concentration and speciation in infant formulas and first foods. Pure Appl Chem. 2012;84:215–223. doi: 10.1351/PAC-CON-11-09-17. http://dx.doi.org/10.1351/PAC-CON-11-09-17.Arsenic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Morris JS, Tosteson TD, Weiss JE, Spencer SK, Greenberg ER. Skin cancer risk in relation to toenail arsenic concentrations in a US population-based case-control study. Am J Epidemiol. 2001;153:559–565. doi: 10.1093/aje/153.6.559. http://dx.doi.org/10.1093/aje/153.6.559. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Morris JS, Demidenko E, Mott LA, Heaney J, Schned A. Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer Causes Control. 2004;15:465–472. doi: 10.1023/B:CACO.0000036452.55199.a3. http://dx.doi.org/10.1023/B:CACO.0000036452.55199.a3. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Punshon T, Sayarath V, Jackson BP, Folt CL, Cottingham KL. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;3766:1–8. doi: 10.1001/jamapediatrics.2016.0120. http://dx.doi.org/10.1001/jamapediatrics.2016.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Hoffman E, Hsueh YM, Afroz S, Quamruzzaman Q, Rahman M, Mahiuddin G, Ryan L, Christiani DC. Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environ Health Perspect. 2009;117:455–460. doi: 10.1289/ehp.11251. http://dx.doi.org/10.1289/ehp.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas K, Queirolo EI, Mañay N, Peregalli F, Hsiao PY, Lu Y, Vahter M. Low-level arsenic exposure: nutritional and dietary predictors in first-grade Uruguayan children. Environ Res. 2016;147:16–23. doi: 10.1016/j.envres.2016.01.022. http://dx.doi.org/10.1016/j.envres.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi G, Vahter M, Clemens F, Goessler W, Gurzau E, Hemminki K, Hough R, Koppova K, Kumar R, Rudnai P, Surdu S, Fletcher T. Inorganic arsenic and basal cell carcinoma in areas of Hungary, Romania, and Slovakia: a case-control study. Environ Health Perspect. 2012;120:721–726. doi: 10.1289/ehp.1103534. http://dx.doi.org/10.1289/ehp.1103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovreglio P, D’Errico MN, Gilberti ME, Drago I, Basso A, Apostoli P, Soleo L. The influence of diet on intra and inter-individual variability of urinary excretion of arsenic species in Italian healthy individuals. Chemosphere. 2012;86:898–905. doi: 10.1016/j.chemosphere.2011.10.050. http://dx.doi.org/10.1016/j.chemosphere.2011.10.050. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A. 2008;105:9931–9935. doi: 10.1073/pnas.0802361105. http://dx.doi.org/10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh DL, Williams PL, Hunter DJ, Sampson LA, Morris SC, Willett WC, Rimm EB. Evaluation of a food frequency questionnaire-food composition approach for estimating dietary intake of inorganic arsenic and methylmercury. Cancer Epidemiol Biomark Prev. 1997;6:1043–1050. [PubMed] [Google Scholar]
- Mandal BK, Ogra Y, Suzuki KT. Speciation of arsenic in human nail and hair from arsenic-affected area by HPLC-inductively coupled argon plasma mass spectrometry. Toxicol Appl Pharmacol. 2003;189:73–83. doi: 10.1016/s0041-008x(03)00088-7. http://dx.doi.org/10.1016/S0041-008X(03)00088-7. [DOI] [PubMed] [Google Scholar]
- Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat MP. What is the best biomarker to assess arsenic exposure via drinking water? Environ Int. 2012 doi: 10.1016/j.envint.2011.07.015. http://dx.doi.org/10.1016/j.envint.2011.07.015. [DOI] [PubMed]
- Meacher DM, Menzel DB, Dillencourt MD, Bic LF, Schoof Ra, Yost LJ, Eickhoff JC, Farr CH. Estimation of multimedia inorganic arsenic intake in the U.S. population. Hum Ecol Risk Assess Int J. 2002;8:1697–1721. http://dx.doi.org/10.1080/20028091057565. [Google Scholar]
- Meharg AA, Lombi E, Williams PN, Scheckel KG, Feldmann J, Raab A, Zhu YG, Islam R. Speciation and localization of arsenic in white and brown rice grains. Environ Sci Technol. 2008;42:1051–1057. doi: 10.1021/es702212p. http://dx.doi.org/10.1021/es702212p. [DOI] [PubMed] [Google Scholar]
- Meharg AA, Williams PN, Adomako EE, Lawgali YY, Deacon C, Villada A, Cambell RCJ, Sun G, Zhu YG, Feldmann J, Raab A, Zhao FJ, Islam R, Hossain S, Yanai J. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol. 2009;43:1612–1617. doi: 10.1021/es802612a. http://dx.doi.org/10.1021/es802612a. [DOI] [PubMed] [Google Scholar]
- Meharg AA, Williams PN, Deacon CM, Norton GJ, Hossain M, Louhing D, Marwa E, Lawgalwi Y, Taggart M, Cascio C, Haris P. Urinary excretion of arsenic following rice consumption. Environ Pollut. 2014;194:181–187. doi: 10.1016/j.envpol.2014.07.031. http://dx.doi.org/10.1016/j.envpol.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Melkonian S, Argos M, Hall MN, Chen Y, Parvez F, Pierce B, Cao H, Aschebrook-Kilfoy B, Ahmed A, Islam T, Slavcovich V, Gamble M, Haris PI, Graziano JH, Ahsan H. Urinary and dietary analysis of 18,470 Bangladeshis reveal a correlation of rice consumption with arsenic exposure and toxicity. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0080691. http://dx.doi.org/10.1371/journal.pone.0080691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani N, Chiba Y, Yamaji N, Ma JF. Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell. 2009;21:2133–2142. doi: 10.1105/tpc.109.067884. http://dx.doi.org/10.1105/tpc.109.067884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300:814–822. doi: 10.1001/jama.300.7.814. http://dx.doi.org/10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, Silbergeld EK, Guallar E. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the strong heart study. Environ Health Perspect. 2009;117:1428–1433. doi: 10.1289/ehp.0800509. http://dx.doi.org/10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res. 2011;111:110–118. doi: 10.1016/j.envres.2010.10.009. http://dx.doi.org/10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton GJ, Pinson SRM, Alexander J, McKay S, Hansen H, Duan GL, Rafiqul Islam M, Islam S, Stroud JL, Zhao FJ, McGrath SP, Zhu YG, Lahner B, Yakubova E, Guerinot M, Lou, Tarpley L, Eizenga GC, Salt DE, Meharg AA, Price AH. Variation in grain arsenic assessed in a diverse panel of rice (Oryza sativa) grown in multiple sites. New Phytol. 2012;193:650–664. doi: 10.1111/j.1469-8137.2011.03983.x. http://dx.doi.org/10.1111/j.1469-8137.2011.03983.x. [DOI] [PubMed] [Google Scholar]
- Orloff K, Mistry K, Metcalf S. Biomonitoring for environmental exposures to arsenic. J Toxicol Environ Health B Crit Rev. 2009;12:509–524. doi: 10.1080/10937400903358934. http://dx.doi.org/10.1080/10937400903358934. [DOI] [PubMed] [Google Scholar]
- Park S, Lee BK. Strong positive associations between seafood, vegetables, and alcohol with blood mercury and urinary arsenic levels in the Korean adult population. Arch Environ Contam Toxicol. 2013;64:160–170. doi: 10.1007/s00244-012-9808-x. http://dx.doi.org/10.1007/s00244-012-9808-x. [DOI] [PubMed] [Google Scholar]
- Rivera-Nuñez Z, Meliker JR, Meeker JD, Slotnick MJ, Nriagu JO. Urinary arsenic species, toenail arsenic, and arsenic intake estimates in a Michigan population with low levels of arsenic in drinking water. J Expo Sci Environ Epidemiol. 2012;22:182–190. doi: 10.1038/jes.2011.27. http://dx.doi.org/10.1038/jes.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Tjell JC, Sloth JJ, Holm PE. Review of arsenic contamination, exposure through water and food and low cost mitigation options for rural areas. Appl Geochem. 2014;41:11–33. http://dx.doi.org/10.1016/j.apgeochem.2013.11.012. [Google Scholar]
- Signes-Pastor AJ, Al-Rmalli SW, Jenkins RO, Carbonell-Barrachina AA, Haris PI. Arsenic bioaccessibility in cooked rice as affected by arsenic in cooking water. J Food Sci. 2012;77:T201–T206. doi: 10.1111/j.1750-3841.2012.02948.x. http://dx.doi.org/10.1111/j.1750-3841.2012.02948.x. [DOI] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Carey M, Carbonell-Barrachina AA, Moreno-Jiménez E, Green AJ, Meharg AA. Geographical variation in inorganic arsenic in paddy field samples and commercial rice from the Iberian Peninsula. Food Chem. 2016a;202:356–363. doi: 10.1016/j.foodchem.2016.01.117. http://dx.doi.org/10.1016/j.foodchem.2016.01.117. [DOI] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Carey M, Meharg AA. Inorganic arsenic in rice-based products for infants and young children. Food Chem. 2016b;191:128–134. doi: 10.1016/j.foodchem.2014.11.078. http://dx.doi.org/10.1016/j.foodchem.2014.11.078. [DOI] [PubMed] [Google Scholar]
- Slotnick MJ, Nriagu JO. Validity of human nails as a biomarker of arsenic and selenium exposure: a review. Environ Res. 2006 doi: 10.1016/j.envres.2005.12.001. http://dx.doi.org/10.1016/j.envres.2005.12.001. [DOI] [PubMed]
- Slotnick MJ, Meliker JR, AvRuskin GA, Ghosh D, Nriagu JO. Toenails as a biomarker of inorganic arsenic intake from drinking water and foods. J Toxicol Environ Health A. 2007;70:148–158. doi: 10.1080/15287390600755232. http://dx.doi.org/10.1080/15287390600755232. [DOI] [PubMed] [Google Scholar]
- Sohel N, Persson LA, Rahman M, Streatfield PK, Yunus M, Ekström EC, Vahter M. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology. 2009;20:824–830. doi: 10.1097/EDE.0b013e3181bb56ec. http://dx.doi.org/10.1097/EDE.0b013e3181bb56ec. [DOI] [PubMed] [Google Scholar]
- States JC, Barchowsky A, Cartwright IL, Reichard JF, Futscher BW, Lantz RC. Arsenic toxicology: translating between experimental models and human pathology. Environ Health Perspect. 2011 doi: 10.1289/ehp.1103441. http://dx.doi.org/10.1289/ehp.1103441. [DOI] [PMC free article] [PubMed]
- Tao SS, Bolger PM. Dietary arsenic intakes in the United States: FDA total diet study, September 1991-December 1996. Food Addit Contam. 1999;16:465–472. doi: 10.1080/026520399283759. http://dx.doi.org/10.1080/026520399283759. [DOI] [PubMed] [Google Scholar]
- Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, Karagas MR, Francesconi KA. Human exposure to organic arsenic species from seafood. Sci Total Environ. 2016 doi: 10.1016/j.scitotenv.2016.12.113. http://dx.doi.org/10.1016/j.scitotenv.2016.12.113. [DOI] [PMC free article] [PubMed]
- UN FAO/WHO. United Nations Food and Agricultural Organitzation/World Health Organitzation. Safety evaluation of certain contaminants in food. Prepared by the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (jecfa) FAO Food and Nutrition Paper. 2011 doi: apps.who.int/iris/bitstream/10665/44520/1/9789241660631_eng.pdf. [PubMed]
- Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002:181–182. 211–217. doi: 10.1016/s0300-483x(02)00285-8. http://dx.doi.org/10.1016/S0300-483X(02)00285-8. [DOI] [PubMed]
- Wei Y, Zhu J, Nguyen A. Rice consumption and urinary concentrations of arsenic in US adults. Int J Environ Health Res. 2014;24:459–470. doi: 10.1080/09603123.2013.857393. http://dx.doi.org/10.1080/09603123.2013.857393. [DOI] [PubMed] [Google Scholar]
- Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol. 2005;39:5531–5540. doi: 10.1021/es0502324. http://dx.doi.org/10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- Williams PN, Raab A, Feldmann J, Meharg AA. Market basket survey shows elevated levels of As in South Central U.S. processed rice compared to California: consequences for human dietary exposure. Environ Sci Technol. 2007a;41:2178–2183. doi: 10.1021/es061489k. http://dx.doi.org/10.1021/es061489k. [DOI] [PubMed] [Google Scholar]
- Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol. 2007b;41:6854–6859. doi: 10.1021/es070627i. http://dx.doi.org/10.1021/es070627i. [DOI] [PubMed] [Google Scholar]
- Wu H, Grandjean P, Hu FB, Sun Q. Consumption of white rice and brown rice and urinary inorganic arsenic concentration. Epidemiology. 2015;26:e65–e67. doi: 10.1097/EDE.0000000000000369. http://dx.doi.org/10.1097/EDE.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003-2004 NHANES data. Environ Health Perspect. 2010;118:345–350. doi: 10.1289/ehp.0901205. http://dx.doi.org/10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost LJ, Tao SH, Egan SK, Barraj LM, Smith KM, Tsuji JS, Lowney YW, Schoof RA, Rachman NJ. Estimation of dietary intake of inorganic arsenic in U.S. children. Hum Ecol Risk Assess Int J. 2004;10:473–483. http://dx.doi.org/10.1080/10807030490452151. [Google Scholar]


