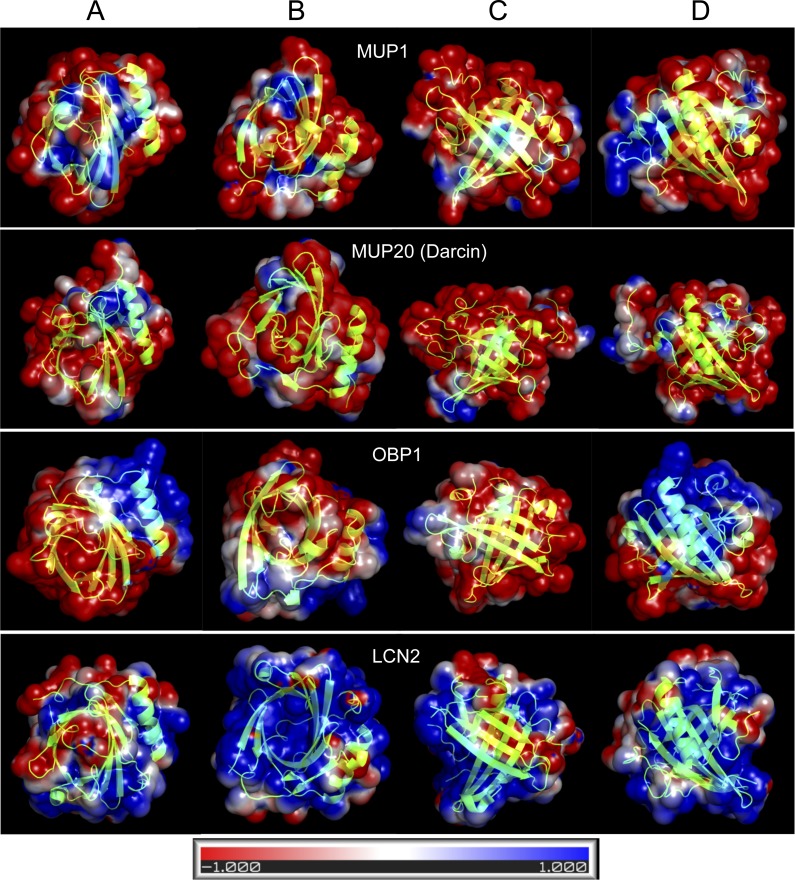
Figure 4. Graphical representation of the tertiary structure of MUP1, MUP20, OBP1, and LCN2 with electrostatics modelling, and scaled from -1kTe (red, negative) to +1kTe (blue, positive).
Each protein is demonstrated in the four views: lower part of the barrel (A), opening of the barrel (B), and the two side views (C, D). Although, their structures are highly similar due to their beta-barrel structures, the distribution of positive and negative charges are non-random with OBP1 and LCN2 being amphipathic. Note the positively charged amino acid residues of LCN2 at the opening of the barrel, which bind negatively charged siderophores, whilst OBP1 has most positively charged residua on its surface and alpha helix.