Abstract
The multicenter retrospective study conducted in 38 centers from 20 countries including 172 adult patients with CNS MM aimed to describe the clinical and pathological characteristics and outcomes of patients with multiple myeloma (MM) involving the central nervous system (CNS). Univariate and multivariate analyses were performed to identify prognostic factors for survival. The median time from MM diagnosis to CNS MM diagnosis was 3 years. Thirty-eight patients (22%) were diagnosed with CNS involvement at the time of initial MM diagnosis and 134 (78%) at relapse/progression. Upon diagnosis of CNS MM, 97% patients received initial therapy for CNS disease, of which 76% received systemic therapy, 36% radiotherapy and 32% intrathecal therapy. After a median follow-up of 3.5 years, the median overall survival (OS) from the onset of CNS involvement for the entire group was 7 months. Untreated and treated patients had median OS of 2 and 8 months, respectively (p<0.001). At least one previous line of therapy for MM before the diagnosis of CNS disease and >1 cytogenetic abnormality detected by FISH were independently associated with worse OS. The median OS for patients with 0, 1 and 2 of these risk factors were 25 months, 5.5 months and 2 months, respectively (p<0.001). Neurological manifestations, not considered chemotherapy-related, observed at any time after initial diagnosis of MM should raise a suspicion of CNS involvement. Although prognosis is generally poor, the survival of previously untreated patients and patients with favorable cytogenetic profile might be prolonged due to systemic treatment and/or radiotherapy.
Keywords: multiple myeloma, therapy, central nervous system
INTRODUCTION
During recent years an increasing attention has been paid to extramedullary involvement by multiple myeloma (MM). At the time of diagnosis, extramedullary MM is found in approximately 7% of patients, while another 6% may develop extramedullary lesions later in their disease course [1]. However, the central nervous system (CNS) is a very rare location of extramedullary involvement and is diagnosed in less than 1% of MM patients [2]. Consequently, the available data on CNS MM are extremely sparse and originate mostly from single case reports and retrospective studies on a limited number of patients. Therefore, data regarding characteristics, diagnosis, treatment algorithms and outcomes of patients with CNS MM are currently lacking.
We describe the clinical and pathological characteristics of 172 patients with CNS MM in international retrospective analysis. We also present diagnostic methodologies and therapeutic approaches and their impact on survival.
METHODS
Patient Selection
This was a multi-institutional, retrospective study conducted in 38 centers from 20 countries in Europe (Belgium, Czech Republic, Denmark, France, Germany, Greece, Italy, Holland, Norway, Poland, Spain, Sweden), Asia (Hong Kong, Japan, Turkey), South America (Argentina, Brazil), Australia, the United States and Canada. Patients were identified through a database search at each of the participating institutions. Adult (≥18 years) patients with a pathological and/or radiological diagnosis of CNS MM between January 1995 and December 2014 were included. CNS involvement was defined as histologically or radiologically proven plasmacytoma arising from the CNS in a location non-contiguous with a bone.
Data Collection
Clinical data included age at the time of MM diagnosis and at the time of CNS involvement, ISS stage, cytogenetic abnormalities, time from MM diagnosis to CNS MM diagnosis, gender, number and type of therapies previous to CNS involvement, symptoms at the time of CNS MM diagnosis, Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) scan findings, number and types of therapies received for CNS MM, overall survival (OS) and cause of death. Laboratory data included: immunoglobulin isotype, beta-2-microglobulin (B2M), albumin, lactate dehydrogenase (LDH) levels at CNS MM diagnosis. Pathological data included findings in cerebrospinal fluid (CSF) cytology and flow cytometry.
Statistical Analysis
Continuous and categorical variables are presented using descriptive statistics. Time from MM diagnosis to CNS MM diagnosis and OS were estimated using the Kaplan-Meier method. The log-rank test was used to compare OS estimates according to prognostic factors. Univariate and multivariate hazard ratios (HRs) were calculated using the Cox proportional-hazard regression method. The outcome measure was the HR with 95% CI. For the logistic and survival regression models, univariate analysis (UVA) was performed for each variable, and only the variables with p-values <0.1 were included in the multivariate analysis (MVA). P-values <0.05 were considered statistically significant in the MVA. All calculations and graphs were obtained using STATA 13.1 (StataCorp LP, College Station, TX, USA).
RESULTS
Clinical Characteristics
A total of 172 patients met the predetermined criteria for inclusion in this study. The median age at diagnosis of MM was 53 years (range 31–82 years), and the median age of CNS MM diagnosis was 56 years (range 33–82 years). The median time from MM diagnosis to the development of lesions in the CNS was 25 months (range 0–216 months; Figure 1A). Thirty-eight patients (22%) were diagnosed with CNS involvement at the initial diagnosis of MM (primary CNS MM). The median number of prior therapies before CNS MM involvement was 2 (range 0–8); 69% of previously treated patients had received alkylators, 59% IMIDs, 58% proteasome inhibitors, 54% autologous SCT and 5% allogeneic SCT. The most common symptoms at presentation were visual changes (36%), radiculopathy (27%), headache (25%), confusion (21%), dizziness (7%) and seizures (6%). Selected clinical characteristics are shown in Table 1.
Figure 1.
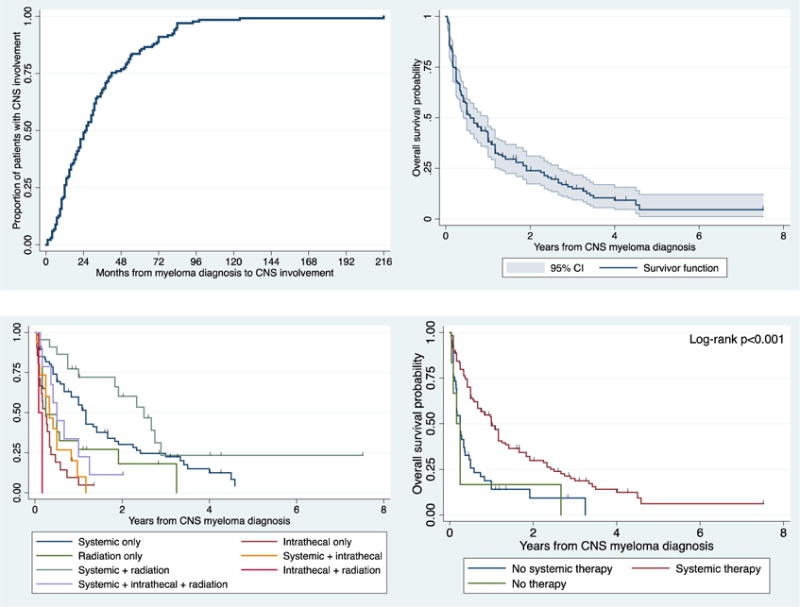
Time from MM diagnosis to diagnosis of CNS involvement by MM (A), and OS estimates in all patients with CNS MM (B), in patients treated with different types of therapy (C), and in patients received or did not receive systemic therapy (D).
Table 1.
Selected clinical characteristics of patients with CNS myeloma
| Characteristic | N/median (%/range) |
|---|---|
| Age, in years (n=171) | |
| At myeloma diagnosis | 53 (31–82) |
| At CNS myeloma diagnosis | 56 (33–82) |
| Sex (n=171) | |
| Male | 94 (55%) |
| Female | 77 (45%) |
| Previous lines of myeloma therapy (n=172) | |
| 0 previous lines | 43 (25%) |
| 1–2 previous lines | 63 (37%) |
| >2 previous lines | 66 (38%) |
| Heavy chain isotype (n=166) | |
| IgG | 83 (50%) |
| IgA | 45 (27%) |
| IgD | 4 (2%) |
| Biclonal | 2 (1%) |
| No heavy chain | 32 (19%) |
| Light chain isotype (n=172) | |
| Kappa | 89 (52%) |
| Lambda | 73 (42%) |
| No light chain | 1 (0.6%) |
| Biclonal | 9 (5%) |
| LDH levels (n=88) | |
| Normal | 47 (53%) |
| Elevated | 41 (47%) |
| ISS stage (n=148) | |
| Stage I | 47 (32%) |
| Stage II | 61 (41%) |
| Stage III | 40 (27%) |
| Symptoms at presentation (n=146) | |
| Visual changes | 52 (36%) |
| Radiculopathy | 40 (27%) |
| Headache | 37 (25%) |
| Change in mental status | 31 (21%) |
| Peripheral neuropathy | 13 (9%) |
| Dizziness | 10 (7%) |
| Seizures | 9 (6%) |
| Auditory changes | 1 (1%) |
| FISH abnormalities | |
| Del13q | 48/122 (39%) |
| Del17p | 28/122 (23%) |
| t(4;14) | 15/122 (12%) |
| t(11;14) | 9/122 (7%) |
| Number of FISH abnormalities | |
| No abnormalities | 45/122 (37%) |
| 1 abnormality | 36/122 (30%) |
| 2 abnormalities | 23/122 (19%) |
| >2 abnormalities | 18/122 (15%) |
| CSF flow cytometry profile | |
| CD45 | 18/34 (53%) |
| CD19 | 3/34 (9%) |
| CD20 | 3/27 (11%) |
| CD4 | 0/5 (0%) |
| CD8 | 0/6 (0%) |
| CD56 | 35/50 (70%) |
| CD38 | 62/65 (95%) |
| CD138 | 31/33 (94%) |
CNS: central nervous system; Ig: immunoglobulin; ISS: International Staging System; LDH: lactate dehydrogenase
Imaging Studies
MRI of the brain and/or spine was performed in 156 patients (91%) showing evidence of disease in 145 of them (93%). CT scans of the brain and/or spine were performed in 53 patients (31%), and showed evidence of disease in 43 of them (81%). No MRIs or CT scans were performed in 5 patients (3%). Out of 167 patients who underwent MRI and/or CT, leptomeningeal involvement was identified in 95 patients (57%) and a cerebral mass lesion in 89 patients (53%); leptomeningeal involvement only was identified in 63 patients (38%), mass only in 57 (34%), mass and leptomeningeal involvement in 32 (19%), and no mass or leptomeningeal involvement in 15 (9%).
Pathological Features
FISH analyses prior to the diagnosis of CNS MM were available for 122 patients (71%). The FISH profile in these patients is shown in Table 1. CSF cytology was performed in 96 patients (56%), and showed evidence of plasma cells in 86 (90%). CSF flow cytometry was performed in 80 patients (47%), and showed a monoclonal plasma cell population in 73 (91%). The flow cytometry profile is shown in Table 1.
Treatment and Causes of Death
Of the 172 patients in our study, 166 (97%) received initial therapy for CNS MM consisting of systemic therapy in 117 (76%) patients, radiotherapy in 56 (36%) patients, intrathecal therapy in 49 (32%) patients and steroids only in 5 (3%) patients; 1 (1%) patient underwent mass resection and 32 (21%) patients were given autologous or allogeneic SCT after induction phase. Systemic chemotherapy included: IMIDs in 50 (43%) patients, proteasome inhibitors in 39 (33%) patients and other chemotherapy regimens in 28 (24%) patients. Details on the type of initial CNS MM therapy are shown in Table 2. Seventy-three patients (44%) went on to receive second line therapy, 28 (17%) received third line therapy, and 1 (1%) patient received fourth line therapy. At the time of this report, 139 patients (81%) have died. The causes of death are shown in Table 2.
Table 2.
Frontline therapies and causes of death in CNS myeloma patients
| Initial therapy (n=166) | N (%) |
|---|---|
| Systemic therapy only | 69 (40%) |
| Systemic + radiotherapy | 22 (13%) |
| Intrathecal therapy only | 21 (12%) |
| Radiotherapy only | 20 (12%) |
| Systemic + intrathecal therapy | 16 (9%) |
| Systemic + intrathecal + radiotherapy | 10 (6%) |
| Steroids only | 5 (3%) |
| Intrathecal + radiotherapy | 2 (1%) |
| Resection + radiotherapy | 1 (1%) |
| Systemic therapy (n=117) | |
| Chemotherapy + proteasome inhibitors | 36 (23%) |
| Chemotherapy | 27 (18%) |
| Chemotherapy + IMIDs | 19 (12%) |
| IMIDs | 14 (9%) |
| Proteasome inhibitors + IMIDs | 12 (8%) |
| Chemotherapy + proteasome inhibitors + IMIDs | 5 (3%) |
| Proteasome inhibitors | 3 (2%) |
| Other | 1 (1%) |
| Intrathecal therapy (n=49) | |
| Methotrexate + cytarabine | 21 (43%) |
| Methotrexate | 7 (14%) |
| Cytarabine | 3 (6%) |
| Thiotepa | 2 (4%) |
| Unknown | 16 (33%) |
| Causes of death (n=139) | |
| Disease progression | 120 (86%) |
| Infection | 13 (9%) |
| Bleeding | 2 (1%) |
| Stroke | 1 (1%) |
| Acute myeloid leukemia | 1 (1%) |
| Congestive heart failure | 1 (1%) |
| Multiorgan failure | 1 (1%) |
IMIDs: immunomodulators
Survival Analyses and Prognostic Factors
After a median follow-up of 3.5 years, the median OS for the entire group was 6.7 months (Figure 1B). The patients who received no treatment for CNS MM had a median OS of 2 months, and the treated patients had a median OS of 7 months (HR 1.1, 95% CI 0.99–1.22; p=0.07). The 1-, 2- and 3-year OS rates were 38% (95% CI 31–46%), 24% (17–31%) and 15% (9–22%), respectively. We then evaluated the effect of initial salvage treatment of CNS MM on OS. Patients who received systemic therapy only and systemic therapy plus radiotherapy appeared to have better OS but the OS in patients in all the other treatment groups were not significantly different than the OS of patients who were not treated (Figure 1C). We then divided patients into 2 groups: a group of patients who received systemic therapy (with or without intrathecal and/or radiotherapy; n=117), and patients who received no systemic therapy (resection and radiotherapy, intrathecal therapy and radiotherapy, steroids only, radiotherapy only, intrathecal therapy only; n=49). The median OS for patients who received systemic therapy vs those who received no systemic therapy was 12 months and 3 months, respectively (HR 0.44, 95% CI 0.29–0.65; p<0.001; Figure 1D).
In the univariate analysis, 1 or more lines of therapy for MM prior to CNS MM diagnosis vs. no previous therapy (Figure 2A), ISS staging (Figure 2B), and >1 FISH abnormality vs. 0–1 abnormalities (Figure 2C) were associated with a worse OS. The presence of a mass on MRI/CT was associated with a better OS (Figure 2D) when compared with the presence of leptomeningeal enhancement. Age, sex, immunoglobulin isotype were not associated with worse or better OS. Although significant in the univariate model, elevated LDH level at CNS MM diagnosis was not included in the multivariate model because there were less than 100 observations. In the multivariate analysis, 1+ previous lines of therapy for MM vs. no previous therapy and >1 FISH abnormality vs. 0–1 abnormalities were independently associated with worse OS. The univariate and multivariate models are shown in Table 3.
Figure 2.

OS estimates in patients with CNS myeloma, according to previous lines of therapy (A), ISS stage (B), number of FISH abnormalities (C), and presence of mass on MRI/CT (D).
Table 3.
Univariate and multivariate analysis for overall survival in patients with CNS myeloma
| Factor | Median OS (months) |
Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | |||||
| At myeloma diagnosis | – | 1.00 (0.98–1.01) | 0.90 | ||
| At CNS myeloma diagnosis | – | 1.00 (0.99–1.02) | 0.65 | ||
| Sex | |||||
| Female | 6.7 months | 1.00 | |||
| Male | 6 months | 1.19 (0.84–1.67) | 0.33 | ||
| Previous lines of therapy | |||||
| No previous | 12 months | 1.00 | 1.00 | ||
| 1+ previous line | 4.4 months | 2.91 (1.89–4.47) | <0.001 | 2.22 (1.27–3.86) | 0.005 |
| ISS staging | |||||
| ISS stage I | 12 months | 1.00 | 1.00 | ||
| ISS stage II–III | 5 months | 1.88 (1.24–2.85) | 0.003 | 1.63 (0.85–2.17) | 0.19 |
| LDH at CNS myeloma diagnosis* | |||||
| Normal | 10 months | 1.00 | |||
| Elevated | 3.2 months | 2.55 (1.56–4.19) | <0.001 | ||
| Heavy chain isotype | |||||
| IgG | 7 months | 1.00 | |||
| IgA | 7 months | 0.98 (0.65–1.46) | 0.91 | ||
| IgD | 2 months | 1.96 (0.71–5.41) | 0.19 | ||
| Non-secretory | 8 months | 0.93 (0.58–1.48) | 0.76 | ||
| Biclonal | 3 months | 1.19 (0.29–4.86) | 0.81 | ||
| Light chain isotype | |||||
| Kappa | 6 months | 1.00 | |||
| Lambda | 6 months | 1.08 (0.76–1.52) | 0.67 | ||
| No light chain | 13 months | 0.70 (0.31–1.63) | 0.41 | ||
| Biclonal | 3 months | 3.62 (0.49–26.5) | 0.21 | ||
| FISH abnormalities | |||||
| 0–1 abnormalities | 8 months | 1.00 | 1.00 | ||
| >1 abnormality | 2.8 months | 2.09 (1.38–3.16) | <0.001 | 2.26 (1.41–3.61) | 0.001 |
| Leptomeningeal enhancement | |||||
| Absent | 8 months | 1.00 | |||
| Present | 6 months | 1.20 (0.85–1.70) | 0.30 | ||
| Mass | |||||
| Absent | 4 months | 1.00 | 1.00 | ||
| Present | 13 months | 0.44 (0.31–0.62) | <0.001 | 0.88 (0.57–1.36) | 0.57 |
OS: overall survival; HR: hazard ratio; CI: confidence interval; CNS: central nervous system; ISS: International Staging System; LDH: lactate dehydrogenase; Ig: immunoglobulin; FISH: fluorescence in situ hybridization
Using number of previous lines of therapy prior to CNS MM diagnosis and number of adverse FISH abnormalities, we then generated a score in which patients were divided in 3 groups: a group with no previous therapies and 0–1 FISH abnormalities (0 risk factors; n=16, 13%), a group with either >1 previous therapy or >1 FISH abnormality (1 risk factor; n=72, 59%), and a group with >1 previous therapy and >1 FISH abnormality at diagnosis (2 risk factors; n=34, 28%). The median OS for patients with 0, 1 and 2 risk factors were 25 months, 5.5 months (HR 2.25, 95% CI 1.20–4.20; p=0.01) and 2 months (HR 4.65, 95% CI 2.33–9.26; p<0.001), respectively (Log-rank for trend p<0.001; Figure 3A). In a subgroup sensitivity analysis, the increased score remained associated with shortened OS after removal of patients who were not treated (Figure 3B), in patients who received systemic therapy (Figure 3C), and in patients who did not receive systemic therapy (Figure 3D).
Figure 3.

OS estimates in patients with CNS myeloma according to prognostic score for the entire group (A), in patients who were treated (B), in patients who received systemic therapy (C), and in patients who did not receive systemic therapy (D).
DISCUSSION
In the present study of 172 CNS MM cases, the risk of CNS involvement was not associated with sex, as CNS MM was found with similar incidence in men and women. The median age of patients was 53 years, whereas the average age at myeloma onset is about 65–70 years old, which suggests that younger myeloma patients are more prone to develop lesions in CNS. This observation is consistent with other reports on CNS involvement in MM [3,4]. The time elapsed between initial MM diagnosis and detection of CNS involvement was relatively short (median of about 2 years). Remarkably, we showed that 22% of patients had CNS involvement at the time of MM diagnosis (primary CNS MM). Although in previously reported cases CNS involvement was also detected synchronically to primary MM, it is more often associated with more advanced stages of the disease (secondary CNS MM) [3,5–7]. However, the distribution of ISS stages in our cohort was relatively even without favoring more advanced stages, which suggests that development of CNS MM is not associated with advanced myeloma, but rather with other characteristics of the disease. Neurological symptoms documented in our patients were heterogeneous, and included supratentorial, meningeal and spinal manifestations. The presence of these symptoms in MM patients not thought to be chemotherapy-related should prompt the investigation for CNS involvement. Hypercalcemia, uremia, paraproteinemia and/or bone damage, however, can confound the symptoms [8].
We found IgA, IgD and biclonal MM subtypes in 27%, 2% and 1% of patients with CNS involvement, respectively. This distribution is consistent with previous reports [6,9,10]. Also, we found that deletions of 13q and 17p are the most frequent cytogenetic anomalies observed in patients with CNS MM. This is consistent with the observations from previous smaller studies [11,12]. The incidence of cytogenetic changes was not compared with any control group and we cannot conclude that any of changes makes the development of CNS MM more probable. However, the presence of adverse genetic abnormalities was associated with prognosis and it was incorporated into the proposed prognostic scoring system. Elevated LDH was one of the most common laboratory abnormalities present in our group. Although according to some authors, elevated activity of this enzyme may be linked to the risk of CNS MM [10], this association was not confirmed by other researchers [6]. Also, the lack of CD56 expression, an adhesion molecule of plasma cells, was postulated to play a role in CNS MM pathogenesis [12]. However, this hypothesis was not confirmed empirically [13]; also the vast majority (70%) of our patients who have been tested for this antigen showed its normal expression on plasma cells isolated from CSF.
Detection of CNS MM on the basis of imaging studies can be challenging. The lesions found within CNS may be heterogeneous, ranging from leptomeningeal infiltration to well-demarcated masses. The presence of mass or infiltration on imaging studies alone is insufficient for establishing the diagnosis of CNS MM due to high incidence of false positive and false negative results [11,14,15]. Contrast-enhanced MRI is more sensitive than CT and constitutes the method of choice in the detection of CNS MM [10,16,17], however it was also associated with a false negative rate of 10% [6]. Therefore it is preferable to perform imaging, pathological and CSF examination concurrently. Presence of clonal plasma cells in CSF, or pathological evidence of soft tissue infiltration, should point towards the diagnosis of CNS MM. However, in daily clinical practice, it is not always possible to obtain specimen for histopathological assessment due to poor performance status, end-stage disease or patient’s refusal. Moreover, the presence of plasma cells in CSF does not constitute a diagnosis of CNS MM, unless monoclonal [18]. On the other hand, plasma cells can be absent in CSF from patients with parenchymal infiltration or isolated changes in the dura mater [19]. Therefore, the absence of plasma cells in CSF does not rule out the diagnosis of CNS MM. CSF cytology should be accompanied by flow cytometry, as polyclonal plasma cells can be also found in other conditions [6]. Our study was retrospective and involved patients treated in different centers which did not follow the same diagnostic and treatment protocol and that’s way only limited proportion of patients were evaluated with all mentioned diagnostic procedures, i.e. MRI or CT, pathological and CSF examination. On the other hand our data represents a real-life routine practice and shows that the diagnosis of CNS MM can be established with limited number of diagnostic methods.
Data on treatment of CNS MM are sparse, and there is no standard of care in these cases. Our study showed that systemic treatment, alone or combined with radiotherapy, resulted in a significant improvement of survival in patients when compared to no systemic therapy. Due to marked heterogeneity of our group, we did not analyze the efficacy of specific treatments, but compared the outcomes of patients subjected to chemotherapy and/or radiotherapy with the results of individuals who were left untreated or offered other treatment modalities (for example only steroids, intrathecal therapy or radiotherapy). Some anti-MM agents can cross the blood-brain barrier. Thalidomide, for example, can be detected in cerebrospinal fluid after oral administration at 100 mg/day [18,20]. However, the effects of thalidomide can be delayed constituting a limitation in patients with rapidly progressing CNS MM [21]. Animal studies showed that lenalidomide [4] and pomalidomide [16], can penetrate to CNS. One study demonstrated that administration of pomalidomide to a patient with CNS involvement resulted in disappearance of plasma cells from CSF [22]. The penetration of bortezomib through the blood-brain barrier was limited in animal models [23]. Bendamustine can potentially be used in the management of CNS MM, as administration of this agent resulted in clinical improvement of patients with CNS lymphoma, although experiences with this agent are limited to 2 published case reports and no strong recommendation on bendamustine can be made [24,25].
Intrathecal agents have been used in CNS MM with conflicting results [9,12,26–29]. The usefulness of intrathecal agents is often put into question as they are usually used in combination with systemic therapies [27], and to this date, did not prove to be efficient as monotherapy [28]. Although whole brain radiation is a therapeutic option in CNS MM, its practical application is limited due to toxicity. Localized metastases to CNS can also be treated with low-dose radiotherapy [10]. The role of autologous hematopoietic stem cell transplantation (HSCT) in the management of CNS MM is unclear. Some authors point to potential beneficial effects of high-dose melphalan conditioning (200 mg/m2) prior to autologous HSCT [16,30]. Although there are few published reports documenting favorable effects of allogeneic HSCT in patients with CNS involvement, the graft-versus-myeloma effect is generally limited.
In our study, the patients treated only with intrathecal therapy or intrathecal therapy combined with radiotherapy had poor survival. It should be noted that only when combined with systemic therapy, intrathecal administration of cytotoxic agents enabled to prolong survival. Although the patients treated with systemic therapy combined with intrathecal and radiation therapy had poor outcomes when compared with systemic therapy only, but the number of patients who received such treatment was small and the data are probably biased. Altogether, our observations regarding treatment suggest that systemic therapy constitutes the basis of effective treatment of CNS involvement in myeloma patients. However, it should be emphasized that the data are retrospective and patients were selected to different treatment strategies, which would include the selection bias that systemic therapy was chosen for more fit patients.
The survival of patients with CNS MM is poor and in our study we observed a 75% mortality within 2 years of diagnosis. This is consistent with previous findings [10,16]. However, long-term survivors have been reported [31,32]. Little is known on the prognostic factors in CNS MM. Dr Paludo et al suggested that mSMART classification ingredients might play a role as prognostic factors in CNS MM patients [Paludo et al 3119 Myelomatous Involvement Of The Central Nervous System: Mayo Clinic Experience, ASH 2013]. We identified two significant predictors of unfavorable prognosis: at least one previous line of anti-MM therapy, and more than one cytogenetic abnormality in MM cells. The scoring system based on these two factors enabled us to stratify our patients in three groups. The proposed scoring system seemed to maintain its significance in patients treated with more effective as well as less effective therapies, and should be validated independently.
Due to its retrospective character, our study is not free from potential limitations, such as incomplete documentation or lack of uniform diagnostic and therapeutic protocols. Since all the patients included in the analysis were treated at tertiary centers, our sample might be subject of selection bias and was not necessarily representative of the whole population of CNS MM patients. Despite these limitations, our study is the largest analysis of CNS MM patients. Furthermore, due to the very low incidence of CNS MM, a prospective study of individuals with this condition, although highly desirable and needed, is unlikely to be conducted.
In conclusion, the neurological manifestations not associated with chemotherapy-related toxicities observed in patients with MM should raise a suspicion of CNS involvement. The diagnosis of CNS MM should be based on imaging studies, CSF cytology and flow cytometry, supplemented with histopathological examination in doubtful cases. Although prognosis is generally poor, especially in patients with a long history of chemotherapy and unfavorable cytogenetic profile, survival of individuals free from these negative prognostic factors can be prolonged due to administration of systemic treatment. The administration of intrathecal therapy alone or in combination with radiotherapy might not be sufficient to improve prognosis and prolong survival. Prospective multi-institutional studies are warranted to improve the outcome of patients with CNS MM.
References
- 1.Varettoni M, Corso A, Pica G, et al. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325–330. doi: 10.1093/annonc/mdp329. [DOI] [PubMed] [Google Scholar]
- 2.Fassas AB, Muwalla F, Berryman T, et al. Myeloma of the central nervous system: association with high-risk chromosomal abnormalities, plasmablastic morphology and extramedullary manifestations. Br J Haematol. 2002;117:103–108. doi: 10.1046/j.1365-2141.2002.03401.x. [DOI] [PubMed] [Google Scholar]
- 3.Abdallah AO, Atrash S, Shahid Z, et al. Patterns of central nervous system involvement in relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2014;14:211–214. doi: 10.1016/j.clml.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Kalff A, Low M, et al. Central nervous system multiple myeloma–potential roles for intrathecal therapy and measurement of cerebrospinal fluid light chains. Br J Haematol. 2013;162:371–375. doi: 10.1111/bjh.12404. [DOI] [PubMed] [Google Scholar]
- 5.Dennis M, Chu P. A case of meningeal myeloma presenting as obstructive hydrocephalus–a therapeutic challenge. Leuk Lymphoma. 2000;40:219–220. doi: 10.3109/10428190009054901. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwenhuizen L, Biesma DH. Central nervous system myelomatosis: review of the literature. Eur J Haematol. 2008;80:1–9. doi: 10.1111/j.1600-0609.2007.00956.x. [DOI] [PubMed] [Google Scholar]
- 7.Schluterman KO, Fassas AB, Van Hemert RL, et al. Multiple myeloma invasion of the central nervous system. Arch Neurol. 2004;61:1423–1429. doi: 10.1001/archneur.61.9.1423. [DOI] [PubMed] [Google Scholar]
- 8.Usnarska-Zubkiewicz L, Bilinska M, Koszewicz M, et al. Neuropathies in multiple myeloma and others monoclonal gammopathy. Acta Haematol Pol. 2008;39:53–62. [Google Scholar]
- 9.Petersen SL, Wagner A, Gimsing P. Cerebral and meningeal multiple myeloma after autologous stem cell transplantation. A case report and review of the literature. Am J Hematol. 1999;62:228–233. doi: 10.1002/(sici)1096-8652(199912)62:4<228::aid-ajh5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Yellu MR, Engel JM, Ghose A, et al. Overview of recent trends in diagnosis and management of leptomeningeal multiple myeloma. Hematol Oncol. 2014 Dec 19; doi: 10.1002/hon.2185. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Fassas AB, Ward S, Muwalla F, et al. Myeloma of the central nervous system: strong association with unfavorable chromosomal abnormalities and other high-risk disease features. Leuk Lymphoma. 2004;45:291–300. doi: 10.1080/10428190310001597964. [DOI] [PubMed] [Google Scholar]
- 12.Chang H, Sloan S, Li D, et al. Multiple myeloma involving central nervous system: high frequency of chromosome 17p13.1 (p53) deletions. Br J Haematol. 2004;127:280–284. doi: 10.1111/j.1365-2141.2004.05199.x. [DOI] [PubMed] [Google Scholar]
- 13.Marini A, Carulli G, Lari T, et al. Myelomatous meningitis evaluated by multiparameter flow cytometry: report of a case and review of the literature. J Clin Exp Hematop. 2014;54:129–136. doi: 10.3960/jslrt.54.129. [DOI] [PubMed] [Google Scholar]
- 14.Tsang CS, Ho LC, Tan TC. Intracranial multiple myeloma involving the dura. J Clin Neurosci. 2006;13:122–123. doi: 10.1016/j.jocn.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Shpilberg KA, Esses SJ, Fowkes ME, et al. Imaging of extraosseous intracranial and intraspinal multiple myeloma, including central nervous system involvement. Clin Imaging. 2015;39:213–219. doi: 10.1016/j.clinimag.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Cerase A, Tarantino A, Gozzetti A, et al. Intracranial involvement in plasmacytomas and multiple myeloma: a pictorial essay. Neuroradiology. 2008;50:665–674. doi: 10.1007/s00234-008-0390-x. [DOI] [PubMed] [Google Scholar]
- 17.Jurczyszyn A, Malkowski B, Czepiel J, et al. The importance of imaging techniques in the modern treatment of multiple myeloma. Przegl Lek. 2014;71:221–230. [PubMed] [Google Scholar]
- 18.Chen CI, Masih-Khan E, Jiang H, et al. Central nervous system involvement with multiple myeloma: long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br J Haematol. 2013;162:483–488. doi: 10.1111/bjh.12414. [DOI] [PubMed] [Google Scholar]
- 19.Mendez CE, Hwang BJ, Destian S, et al. Intracranial multifocal dural involvement in multiple myeloma: case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2010;10:220–223. doi: 10.3816/CLML.2010.n.035. [DOI] [PubMed] [Google Scholar]
- 20.Yutaka H, Mariko Y, Shinichiro O, et al. Thalidomide for the treatment of leptomeningeal multiple myeloma. Eur J Haematol. 2006;76:358–359. doi: 10.1111/j.1600-0609.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 21.Vicari P, Ribas C, Sampaio M, et al. Can thalidomide be effective to treat plasma cell leptomeningeal infiltration? Eur J Haematol. 2003;70:198–199. doi: 10.1034/j.1600-0609.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 22.Mussetti A, Dalto S, Montefusco V. Effective treatment of pomalidomide in central nervous system myelomatosis. Leuk Lymphoma. 2013;54:864–866. doi: 10.3109/10428194.2012.718343. [DOI] [PubMed] [Google Scholar]
- 23.Gozzetti A, Cerase A. Novel agents in CNS myeloma treatment. Centr Nerv Syst Agents Med Chem. 2014;14:23–27. doi: 10.2174/1871524914999140818111514. [DOI] [PubMed] [Google Scholar]
- 24.Renfrow JJ, Detroye A, Chan M, et al. Initial experience with bendamustine in patients with recurrent primary central nervous system lymphoma: a case report. J Neurooncol. 2012;107:659–663. doi: 10.1007/s11060-011-0788-x. [DOI] [PubMed] [Google Scholar]
- 25.Nahi H, Svedmyr E, Lerner R. Bendamustine in combination with high-dose radiotherapy and thalidomide is effective in treatment of multiple myeloma with central nervous system involvement. Eur J Hematol. 2014;92:454–455. doi: 10.1111/ejh.12247. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain MC, Glantz M. Myelomatous meningitis. Cancer. 2008;112:1562–1567. doi: 10.1002/cncr.23330. [DOI] [PubMed] [Google Scholar]
- 27.Grisold A, Weber C, Hainfellner J, et al. MRI negative meningeal myeloma with abducens nerve palsies responding to intrathecal chemotherapy. J Neurol Sci. 2014;347:359–360. doi: 10.1016/j.jns.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Marjanovic S, Mijuskovic Z, Stamatovic D, et al. Multiple myeloma invasion of the central nervous system. Vojnosanit Pregl. 2012;69:209–213. [PubMed] [Google Scholar]
- 29.Velasco R, Petit J, Llatjos R, et al. Can leptomeningeal myelomatosis be predicted in patients with IgD multiple myeloma? J Clin Neurosci. 2010;17:1071–1072. doi: 10.1016/j.jocn.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Gozzetti A, Cerase A, Lotti F, et al. Extramedullary intracranial localization of multiple myeloma and treatment with novel agents: a retrospective survey of 50 patients. Cancer. 2012;118:1574–1584. doi: 10.1002/cncr.26447. [DOI] [PubMed] [Google Scholar]
- 31.Haegelen C, Riffaud L, Bernard M, et al. Dural plasmacytoma revealing multiple myeloma. Case report J Neurosurg. 2006;104:608–610. doi: 10.3171/jns.2006.104.4.608. [DOI] [PubMed] [Google Scholar]
- 32.Pontikoglou C, Fragos C, Kolyvaki E, et al. Multiple myeloma involving the central nervous system: a report of two cases with unusual manifestations. Leuk Lymphoma. 2005;46:737–741. doi: 10.1080/10428190500032661. [DOI] [PubMed] [Google Scholar]


