Abstract
Purpose
In some cancers, the oncogenic consequences of inactivating the retinoblastoma protein (Rb) appear to be mediated by unrestrained activity of the inhibitor of DNA binding protein Id2. The role of Id2 has not yet been investigated in the prototype cancer Rb-defective cancer, retinoblastoma itself. This study investigated whether loss of Id2 modified the effects of Rb inactivation in a mouse model of retinoblastoma.
Methods
Id2 was analyzed in cultured cells using qPCR, Western blot, and colony formation assays. LHβ-Tag transgenic mice were crossed with Id2 heterozygotes to obtain mice with all three Id2 genotypes. Intraocular tumors were assessed for size, degree of differentiation, mitotic index, and tumor vascular density at 15 weeks of age.
Results
Retinoblastoma cell lines expressed low levels of Id2 mRNA and protein. Depletion of Id2 in Rb-inactivated cells increased clonogenic activity. Id2-deficient tumors in vivo were significantly larger, less differentiated, and more vascularized than Id2-wild-type tumors (P = 0.02, P = 0.01, P = 0.0001, respectively). There was a dosage effect for loss of each Id2 allele with respect to differentiation and vascular density.
Conclusions
Id2 suppresses rather than promotes tumor progression in this mouse model of retinoblastoma. Id2 can act as either an oncogene or a tumor suppressor depending on context.
Keywords: Id2, Pocket proteins, Rb protein, Retinoblastoma, Tumor suppressor
Introduction
Inhibitor of DNA binding (Id) proteins, four of which have been identified in mammals (Id1 to Id4), are dominant-negative inhibitors of basic helix-loop-helix (bHLH) transcription factors and have roles in cell proliferation, differentiation, angiogenesis, and tumorigenesis.1–4 In some cancers, Id2 appears to act as an oncogene.5 In neuroblastoma, Id2 is an effector of the N-myc oncogene.6 The Rb family of pocket proteins (Rb1, p107, and p130) can bind and inhibit Id2, and it has been suggested that the oncogenic consequences of Rb loss are due, at least in part, to the unrestrained activity of Id2.7–10
On the other hand, Id2 is dispensable for tumorigenesis in other myc-induced tumors, such as lymphoma and epidermal neoplasia.11,12 Recent studies suggest that Id2 can actually suppress the development of tumors in the intestine and mammary gland.13,14 Thus, the role of Id2 appears to be tissue specific and context dependent.15
Since Id2 has been implicated as a mediator of oncogenesis in Rb-deficient tumors, we wished to determine the role of Id2 in retinoblastoma itself. Id2 mRNA and protein were expressed at very low levels in retinoblastoma cells, and loss of Id2 in a transgenic model of retinoblastoma led to increased tumor size, loss of differentiation, and increased tumor vascular density. These results indicate that Id2 acts as a tumor suppressor, rather than an oncogene, in this model of retinoblastoma. These findings provide new insights into retinoblastoma tumor biology, and they reinforce further the concept that Id2 can act as either an oncogene or a tumor suppressor depending on context.
Materials and Methods
Cell Culture Studies
Y79 (ATCC HTB-18), WERI-RB-1 (ATCC HTB-169), and CHLA223 (gift of A. Linn Murphree, Childrens Hospital Los Angeles, Los Angeles, California, USA) retinoblastoma cells were measured for Id2 expression by realtime qPCR and Western blot analyses. Mel202 uveal melanoma cells (gift of B. Ksander, Harvard University, Boston, Massachusetts, USA) and U2OS osteosarcoma cells (ATCC HTB-96) were used as positive controls. Real-time PCR was performed using the IQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, California, USA) and qPCR was performed by monitoring the increase of SYBR Green fluorescence in realtime using i-Cycler (Bio-Rad Laboratories, Hercules, California, USA). Custom primers were synthetized by Integrated DNA Technologies (IDT, Coralville, Iowa, USA): UBC_F 5′-ATTTGGGTCGCGGTTCTTG-3′, UBC_R 5′-TGCCTTGACATTCTCGATGGT-3′, ID2_F5′-ATATCAGCATCCTGTCCTTGCAG-3′, ID2_R 5′-GAAATCATGAACACCGCTTATTCAG-3′. At least one primer of each pair crossed exon-exon boundaries to prevent amplification of genomic DNA, and primer quality (lack of primer-dimer amplification) was confirmed by melting curve analysis. Relative quantitation of the gene expression was performed using the standard curve method (user bulletin 2 of the ABI Prism 7700 Sequence Detection System, Applied Biosystems, Foster City, California, USA). In each experiment the relative amount of target gene mRNA was calculated from the standard curve and normalized to the relative amount of reference gene RNA (UBC RNA), which was obtained from a similar standard curve. Western blot was performed using an antibody that recognized Id2 (1:200; C-20; Santa Cruz Biotechnology, Santa Cruz, California, USA). Colony formation assays were performed with Rb-inactivated U2OS cells16 using Id2 sens and antisense7 (gift of A. Iavarone, Columbia University, New York, New York, USA), full-length Rb17 and RbΔ1018 (gift of J. Bartek, Danish Cancer Society, Copenhagen, Denmark) expression vectors, as described previously.19,20 Transfections (24h) were done using Effectene (Qiagen, Valencia, California, USA).
Generation of LHβ-Tag/Id2 Mice, Genotyping and Histopathology
Animal experiments were approved by the Washington University Animal Studies Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. LHβ-Tag mice (gift of D.M. Albert, University of Wisconsin, Madison, Wisconsin, USA) were generated as previously described21 and maintained on a CB6F1 (C57BL/6 × BALB/c) background. These mice have retina-specific expression of large T antigen, which blocks Rb, as well as the other two pocket proteins, p107 and p130.21 By 6–8 weeks of age, these animals develop multifocal, bilateral retinal tumors with histopathologic features closely resembling human retinoblastoma.21 LHβ-Tag mice were crossed with Id2 heterozygotes on the 129/Sv background22 (gift of Y. Yokota, Fukui Medical University, Fukui, Japan), which were subsequently interbred to generate LHβ-Tag mice with all three Id2 genotypes (129/Sv background ranged from 50–75% for LHβ-Tag/Id2 mice and corresponding control mice). All mice were genotyped for SV40 Tag and Id2 by PCR analysis of tail biopsies as previously described.21,22 Mice were euthanized at 15 weeks of age. It was not possible to evaluate mice beyond this age because they began to develop advanced eye tumors that required euthanasia to avoid discomfort. Eyes were fixed in formalin and embedded in paraffin (ten LHβ-Tag/Id2+/+, ten LHβ-Tag/Id2+/-, and four LHβ-Tag/Id2-/-, with each eye measured in triplicate). Four micron sections were stained with hematoxylin and eosin and examined in a masked fashion.
Analysis of Ocular Tumors
Tumor diameter was measured as the ratio of the largest linear basal tumor diameter to the largest linear retinal diameter, measured on histopathologic sections through the region of maximum tumor diameter. This technique allowed us to normalize for minor differences in eye size and sectioning. For these measurements, photomicrographic images were taken of coronal sections through the largest diameter of the tumor and uploaded into ImageJ software for analysis (http://rsb.info.nih.gov/ij/). The degree of tumor differentiation was measured as the number of photoreceptor rosettes per 40× field.23 The mitotic index was measured as the number of mitotic figures per 40× field. Vascular density was measured as the number of intratumoral vessels per 40× field. At least ten 40× fields were examined. Measurements were normalized for tumor area.
Statistical Analysis
Results were analyzed for statistical significance by two-tailed t-test using MedCalc software version 9.5.1.0 (http://medcalcsoftware.com/medcalc.php); P < 0.05 was considered statistically significant. Since the variability from eye to eye is similar to that from animal to animal, it is customary in this inbred line to consider each eye as an independent “experiment.” Thus, the error bars and p values were calculated from the individual eyes in each group.
Results
Id2 mRNA and protein were expressed at very low levels in human retinoblastoma cells (Figure 1A and 1B). Depletion of Id2 in Rb-inactivated U2OS cells using an antisense expression vector led to a significant increase in anchorage-independent growth and colony formation compared to the cells transfected with Id2 sense or Rb expression constructs (Figure 1C). This clonogenic activity was blocked by co-expression of a constitutively active Rb allele (RbΔ10) (Figure 1C).
Figure 1.
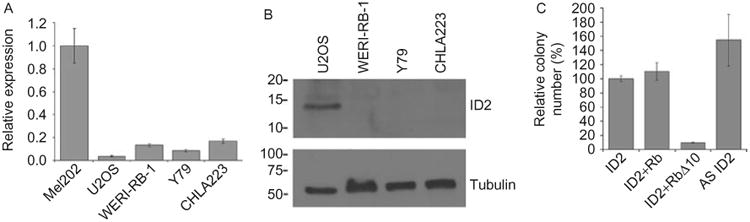
Id2 mRNA and protein expression in retinoblastoma cell lines. (A) Real-time qPCR; (B) Western blot; (C) Colony formation assays in Rb-inactivated U2OS cells using the indicated expression constructs. Mel202 and U2OS cell lines were used as positive controls. Error bars represent standard error.
LHβ-Tag transgenic retinoblastoma mice were crossed with Id2 heterozygotes to create mice with all three Id2 genotypes.21,22 Mice of all three Id2 genotypes developed intraocular retinoblastomas by 15 weeks of age (Figure 2). The mean diameter of the LHβ-Id2-/- tumors was significantly greater than that of the LHβ-Id2+/+ tumors (P = 0.02) and LHβ-Id2+/- tumors (P = 0.02) (Figure 3A). There was no significant difference in diameter between the LHβ-Id2+/+ and LHβ-Id2+/- tumors (P = 0.9). The degree of tumor differentiation of LHβ-Id2+/+ tumors was significantly greater than the LHβ-Id2-/- tumors (P = 0.01) and LHβ-Id2+/- tumors (P = 0.02) (Figure 3B). The vascular density of the LHβ-Id2-/- tumors was significantly greater than the LHβ-Id2+/+ tumors (P = 0.0001) and LHβ-Id2+/- tumors (P = 0.0001) (Figure 3C). The mitotic index was not significantly different between Id2 deficient and Id2 wild-type tumors (data not shown). Metastasis was not detected at necropsy in any of the animals at the age of 15 weeks, and it was not possible to keep the animals alive longer due to concerns about discomfort associated with the development of large eye tumors.
Figure 2.
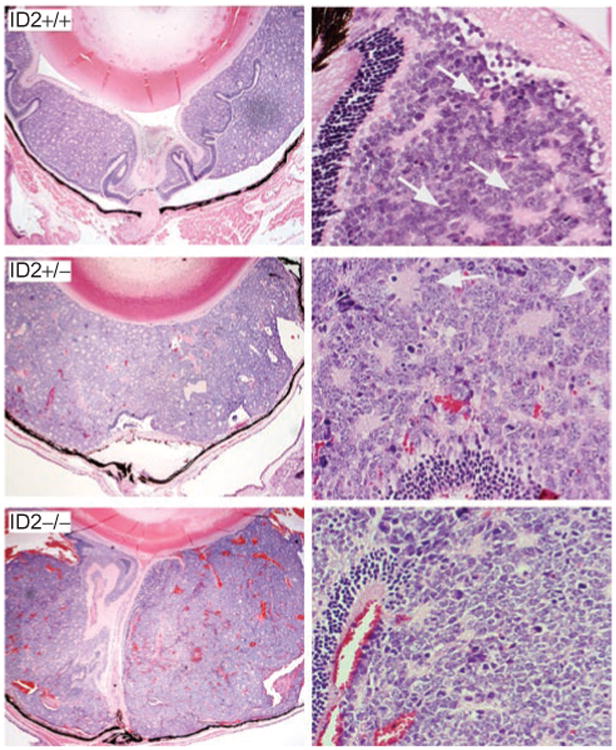
Histopathology of LHβ-Tag retinoblastomas with three Id2 genotypes, as indicated. Images were obtained at 10× magnification (left panels) and 40× magnification (right panels). Arrows in right panels indicate photoreceptor rosettes.
Figure 3.
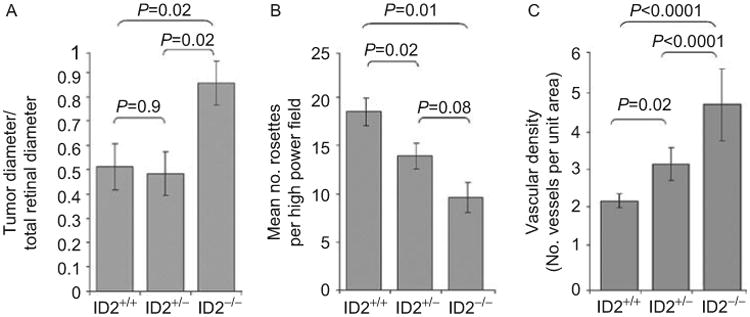
Histomorphometric analysis of LHβ-Tag retinoblastomas with three Id2 genotypes, as indicated: (A) Tumor size; (B) Photoreceptor differentiation; (C) Vascular density. Error bars represent standard error. The error bars and p values were calculated from individual eyes in each group (ten LHβ-Tag/Id2+/+, ten LHβ-Tag/Id2+/-, and four LHβ-Tag/Id2-/-).
Discussion
Since Rb can negatively regulate Id2, and loss of Rb can promote tumorigenesis by unleashing oncogenic activities of Id2,7–10 we predicted initially that deletion of Id2 in LHβ-Tag mice would delay or prevent the development of retinoblastoma. Instead, all Id2-deficient LHβ-Tag mice developed retinoblastomas, and these tumors were significantly larger, less differentiated, and more vascularized than Id2 wild-type tumors. It is unlikely that the observed phenotypic differences were due to differences in genetic backgrounds since the same background was present in LHβ-Id2+/+, LHβ-Id2+/-, and LHβ-Id2-/- mice.
Id2 is highly expressed in the developing and adult retina,20 and it is down-regulated in human retinoblastomas.24 These observations, coupled with our findings reported herein, suggest that Id2 may function as a tumor suppressor in the retina and that loss of Id2 may be an important step in retinoblastoma progression. The fact that Id2 deficiency alone does not lead to retinal tumors indicates that Id2 loss is not an initiating event but, rather, an event that occurs during tumor progression. This possibility is consistent with the “M3” concept described by Gallie and co-workers.25 Once tumor formation has occurred, as a result of Rb inactivation, the subsequent loss of Id2 appears to convey a selective growth advantage by increasing tumor vascularity and decreasing the limits on growth associated with differentiation. A similar role for Id2 has recently been shown in hepatocellular carcinoma.26 Retinoblastoma joins a growing list of cancers in which Id2 appears to function as a tumor suppressor, including breast,13 intestinal,14 prostate,27 and hepatocellular carcinoma.26 Further, we recently showed that Id2 suppresses tumor progression in uveal (ocular) melanoma, where inactivation of Id2 leads to more aggressive, dedifferentiated, stem-like cancer cells.20
The observed association between Id2 loss and increased tumor vascularization was of particular interest. Intratumoral vascularity induced by vascular endothelial growth factor (VEGF)-mediated angiogenesis is prevalent in human retinoblastoma.28 Indeed, increased intratumoral vascularity is associated with increased tumor aggressiveness.29 Rapidly growing retinoblastomas develop regions of hypoxia that induce expression of VEGF, hypoxia inducible factor 1-alpha (HIF1A), and other proteins that can promote angiogenesis and tumor progression.30 Id2 inhibits VEGF and HIF1A, and loss of Id2 is associated with increased tumor aggressiveness by allowing increased VEGF expression.26 Thus, Id2 may function as a tumor suppressor in retinoblastoma, at least in part, by inhibiting angiogenesis. These findings provide new insights into the pathogenesis of retinoblastoma and may lead to new therapeutic targets in the treatment of retinoblastoma.
Acknowledgments
The authors wish to thank the Immunomorphology Core Lab for preparation of histopathologic sections. This research was supported by Grant R01 EY1316905 from the National Eye Institute, Knights Templar Eye Foundation (Postdoctoral Training Award; DM), Research to Prevent Blindness, Horncrest Foundation (JWH), Fonds de la recherche en santé du Québec (Postdoctoral Training Award; SL), and departmental grants from Research to Prevent Blindness and National Eye Institute Vision Core Grant P30 EY 02687.
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Yokota Y. Id and development. Oncogene. 2001;20:8290–8298. doi: 10.1038/sj.onc.1205090. [DOI] [PubMed] [Google Scholar]
- 2.Sikder HA, Devlin MK, Dunlap S, et al. Id proteins in cell growth and tumorigenesis. Cancer Cell. 2003;3:525–530. doi: 10.1016/s1535-6108(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 3.Gabellini C, Del Bufalo D, Zupi G. Involvement of RB gene family in tumor angiogenesis. Oncogene. 2006;25:5326–5332. doi: 10.1038/sj.onc.1209631. [DOI] [PubMed] [Google Scholar]
- 4.Lasorella A, Uo T, Iavarone A. Id proteins at the cross-road of development and cancer. Oncogene. 2001;20:8326–8333. doi: 10.1038/sj.onc.1205093. [DOI] [PubMed] [Google Scholar]
- 5.Kleeff J, Ishiwata T, Friess H, et al. The helix-loop-helix protein Id2 is overexpressed in human pancreatic cancer. Cancer Res. 1998;58:3769–3772. [PubMed] [Google Scholar]
- 6.Lasorella A, Boldrini R, Dominici C, et al. Id2 is critical for cellular proliferation and is the oncogenic effector of N-myc in human neuroblastoma. Cancer Res. 2002;62:301–306. [PubMed] [Google Scholar]
- 7.Iavarone A, Garg P, Lasorella A, et al. The helix-loop-helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev. 1994;8:1270–1284. doi: 10.1101/gad.8.11.1270. [DOI] [PubMed] [Google Scholar]
- 8.Lasorella A, Iavarone A, Israel MA. Id2 specifically alters regulation of the cell cycle by tumor suppressor proteins. Molec Cell Biol. 1996;16:2570–2578. doi: 10.1128/mcb.16.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasorella A, Noseda M, Beyna M, et al. Id2 is a retinoblastoma protein target and mediates signaling by Myc onco-proteins. Nature. 2000;407:592–598. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]
- 10.Lasorella A, Rothschild G, Yokota Y, et al. Id2 mediates tumor initiation, proliferation, and angiogenesis in Rb mutant mice. Mol Cell Biol. 2005;25:3563–3574. doi: 10.1128/MCB.25.9.3563-3574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilsson JA, Nilsson LM, Keller U, et al. Id2 is dispensable for myc-induced lymphomagenesis. Cancer Res. 2004;64:7296–7301. doi: 10.1158/0008-5472.CAN-04-2133. [DOI] [PubMed] [Google Scholar]
- 12.Murphy DJ, Swigart LB, Israel MA, et al. Id2 is dispensable for Myc-induced epidermal neoplasia. Mol Cell Biol. 2004;24:2083–2090. doi: 10.1128/MCB.24.5.2083-2090.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itahana Y, Singh J, Sumida T, et al. Role of Id2 in the maintenance of a differentiated and noninvasive phenotype in breast cancer cells. Cancer Res. 2003;63:7098–7105. [PubMed] [Google Scholar]
- 14.Russell RG, Lasorella A, Dettin LE, et al. Id2 drives differentiation and suppresses tumor formation in the intestinal epithelium. Cancer Res. 2004;64:7220–7225. doi: 10.1158/0008-5472.CAN-04-2095. [DOI] [PubMed] [Google Scholar]
- 15.Kowanetz M, Valcourt U, Bergstrom R, et al. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol Cell Biol. 2004;24:4241–4254. doi: 10.1128/MCB.24.10.4241-4254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashizawa S, Nishizawa H, Yamada M, et al. Collective inhibition of pRB family proteins by phosphorylation in cells with p16INK4a loss or cyclin E overexpression. J Biol Chem. 2001;4:4. doi: 10.1074/jbc.M007992200. [DOI] [PubMed] [Google Scholar]
- 17.Harbour JW, Luo RX, Dei Sante A, et al. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 18.Lukas J, Sorensen CS, Lukas C, et al. p16INK4a, but not constitutively active pRb, can impose a sustained G1 arrest: Molecular mechanisms and implications for oncogenesis. Oncogene. 1999;18:3930–3935. doi: 10.1038/sj.onc.1202777. [DOI] [PubMed] [Google Scholar]
- 19.Ma D, Zhou P, Harbour JW. Distinct mechanisms for regulating the tumor suppressor and antiapoptotic functions of Rb. J Biol Chem. 2003;278:19358–19366. doi: 10.1074/jbc.M301761200. [DOI] [PubMed] [Google Scholar]
- 20.Onken MD, Ehlers JP, Worley LA, et al. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res. 2006;66:4602–4609. doi: 10.1158/0008-5472.CAN-05-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Windle JJ, Albert DM, O'Brien JM, et al. Retinoblastoma in transgenic mice. Nature. 1990;343:665–669. doi: 10.1038/343665a0. [DOI] [PubMed] [Google Scholar]
- 22.Yokota Y, Mansouri A, Mori S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 23.Eagle RC., Jr High-risk features and tumor differentiation in retinoblastoma: A retrospective histopathologic study. Arch Pathol Lab Med. 2009;133:1203–1209. doi: 10.5858/133.8.1203. [DOI] [PubMed] [Google Scholar]
- 24.Chakraborty S, Khare S, Dorairaj SK, et al. Identifcation of genes associated with tumorigenesis of retinoblastoma by microarray analysis. Genomics. 2007;90:344–353. doi: 10.1016/j.ygeno.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Corson TW, Gallie BL. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer. 2007;46:617–634. doi: 10.1002/gcc.20457. [DOI] [PubMed] [Google Scholar]
- 26.Tsunedomi R, Iizuka N, Tamesa T, et al. Decreased ID2 promotes metastatic potentials of hepatocellular carcinoma by altering secretion of vascular endothelial growth factor. Clin Cancer Res. 2008;14:1025–1031. doi: 10.1158/1078-0432.CCR-07-1116. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhary J, Schmidt M, Sadler-Riggleman I. Negative acting HLH proteins Id 1, Id 2, Id 3, and Id 4 are expressed in prostate epithelial cells. Prostate. 2005;64:253–264. doi: 10.1002/pros.20238. [DOI] [PubMed] [Google Scholar]
- 28.Jockovich ME, Murray TG, Escalona-Benz E, et al. Anecortave acetate as single and adjuvant therapy in the treatment of retinal tumors of LH(BETA)T(AG) mice. Invest Ophthalmol Vis Sci. 2006;47:1264–1268. doi: 10.1167/iovs.05-1194. [DOI] [PubMed] [Google Scholar]
- 29.Finger PT, Harbour JW, Karcioglu ZA. Risk factors for metastasis in retinoblastoma. Surv Ophthalmol. 2002;47:1–16. doi: 10.1016/s0039-6257(01)00279-x. [DOI] [PubMed] [Google Scholar]
- 30.Kvanta A, Steen B, Seregard S. Expression of vascular endothelial growth factor (VEGF) in retinoblastoma but not in posterior uveal melanoma. Exp Eye Res. 1996;63:511–518. doi: 10.1006/exer.1996.0141. [DOI] [PubMed] [Google Scholar]


