Abstract
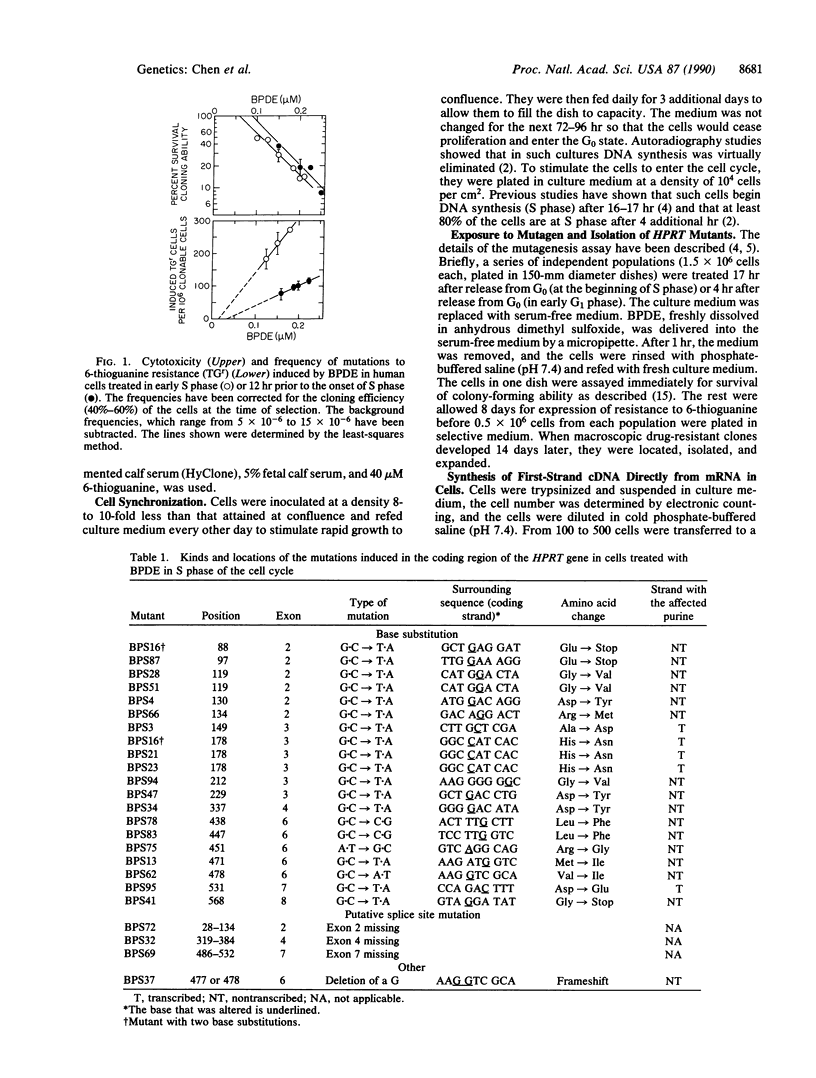
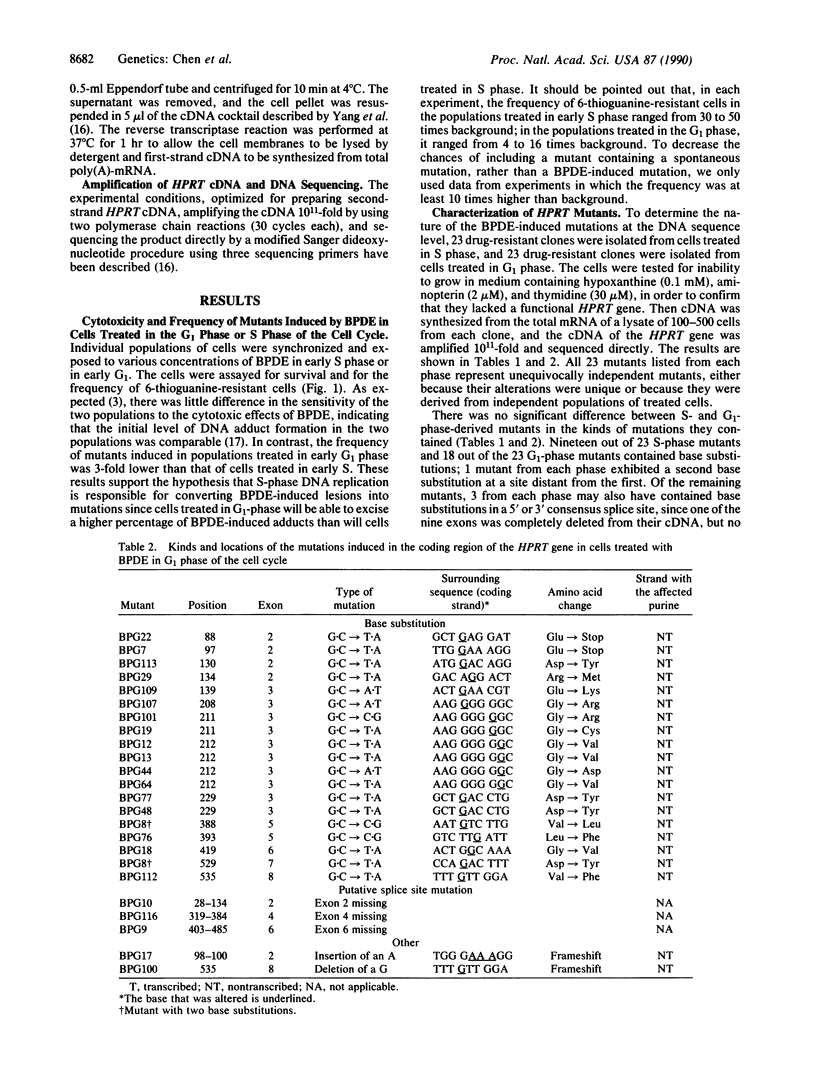
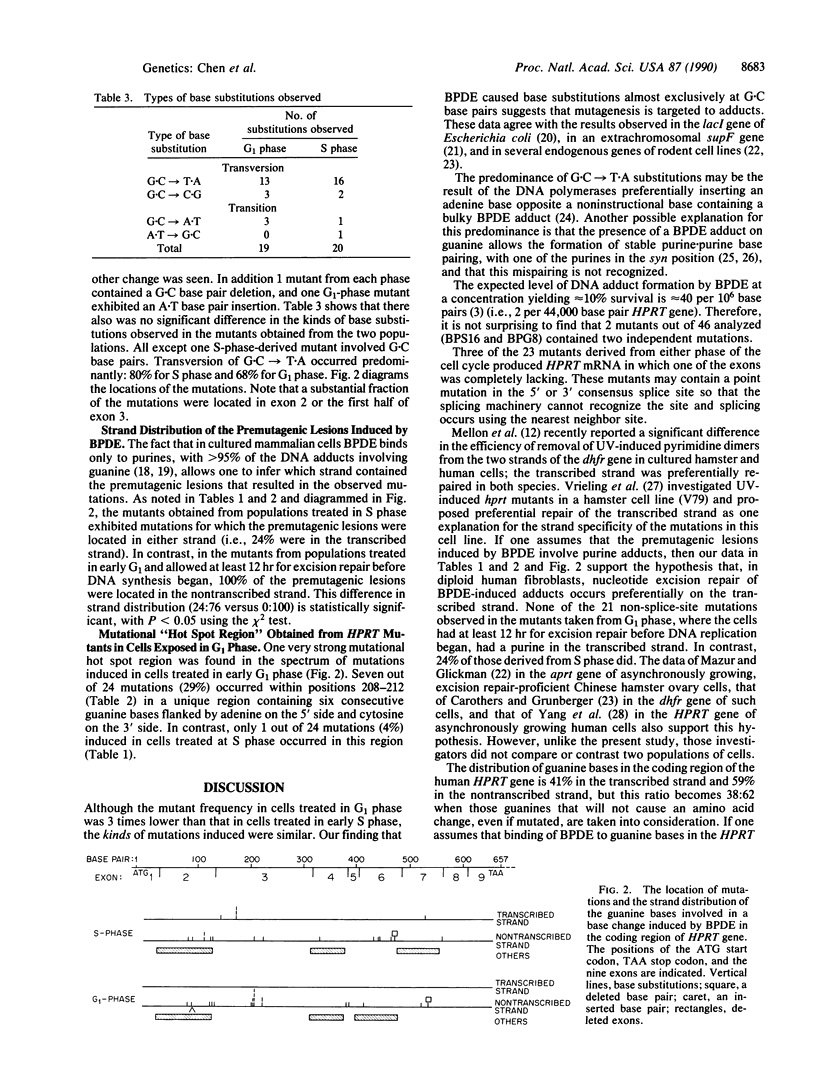
(+/-)-7 beta,8 alpha-Dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10- tetrahydrobenzo[a]pyrene (BPDE) is a direct-acting carcinogen that forms DNA adducts only with purines, predominantly (greater than 95%) with guanine. To investigate the effect of nucleotide excision repair on the kinds and locations (spectra) of mutations induced in diploid human fibroblasts by BPDE, we synchronized cells and exposed them to BPDE either at the beginning of S phase just when the target gene hypoxanthine (guanine) phosphoribosyltransferase (HPRT) is replicated or 12 hr prior to the beginning of S phase (early G1 phase). Clones resistant to 6-thioguanine were isolated, and the mRNA in lysates of 100-500 cells from each mutant clone was used to synthesize cDNA. HPRT cDNA was amplified 10(11)-fold by the polymerase chain reaction and then sequenced directly. The mutants derived from the two populations did not differ in the kinds of mutations; 19/20 of the base substitutions in cells taken from S phase and 19/19 of those from G1 phase involved G.C base pairs, predominantly G.C----T.A. However, they differed significantly in the distribution of the mutations in the coding region of the gene. In the cells from G1 phase, 29% of the mutations were clustered within a unique run of six guanine bases; in the S-phase cells, only 4% were located there. Assuming that the premutagenic BPDE-induced lesions involved purines, in the cells treated at the beginning of S phase, 24% of these lesions were located in the transcribed strand, whereas in the G1-treated cells, none were. This suggests that in the HPRT gene of diploid human cells excision repair of BPDE adducts occurs preferentially on the transcribed strand.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Selsing E. Letter: The structure of polydeoxyguanylic acid with polydeoxycytidylic acid. J Mol Biol. 1974 Sep 15;88(2):551–552. doi: 10.1016/0022-2836(74)90502-6. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Boles T. C., Hogan M. E. High-resolution mapping of carcinogen binding sites on DNA. Biochemistry. 1986 May 20;25(10):3039–3043. doi: 10.1021/bi00358a045. [DOI] [PubMed] [Google Scholar]
- Brown T., Leonard G. A., Booth E. D., Chambers J. Crystal structure and stability of a DNA duplex containing A(anti).G(syn) base-pairs. J Mol Biol. 1989 May 20;207(2):455–457. doi: 10.1016/0022-2836(89)90268-4. [DOI] [PubMed] [Google Scholar]
- Carothers A. M., Grunberger D. DNA base changes in benzo[a]pyrene diol epoxide-induced dihydrofolate reductase mutants of Chinese hamster ovary cells. Carcinogenesis. 1990 Jan;11(1):189–192. doi: 10.1093/carcin/11.1.189. [DOI] [PubMed] [Google Scholar]
- Cordeiro-Stone M., Boyer J. C., Smith B. A., Kaufmann W. K. Effect of benzo[a]pyrene-diol-epoxide-I on growth of nascent DNA in synchronized human fibroblasts. Carcinogenesis. 1986 Oct;7(10):1775–1781. doi: 10.1093/carcin/7.10.1775. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Warren A. J., Porter J., Atkins D., Miller J. H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham J. W., Greenberg D. S., Kaufman D. G., Smith G. J. Cycle-related toxicity and transformation in 10T1/2 cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4813–4817. doi: 10.1073/pnas.77.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konze-Thomas B., Hazard R. M., Maher V. M., McCormick J. J. Extent of excision repair before DNA synthesis determines the mutagenic but not the lethal effect of UV radiation. Mutat Res. 1982 Jun;94(2):421–434. doi: 10.1016/0027-5107(82)90305-0. [DOI] [PubMed] [Google Scholar]
- Kootstra A., Lew L. K., Nairn R. S., MacLeod M. C. Preferential modification of GC boxes by benzo[a]pyrene-7,8-diol-9,10-epoxide. Mol Carcinog. 1989;1(4):239–244. doi: 10.1002/mc.2940010406. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Dorney D. J., Mendrala A. L., Konze-Thomas B., McCormick J. J. DNA excision-repair processes in human cells can eliminate the cytotoxic and mutagenic consequences of ultraviolet irradiation. Mutat Res. 1979 Sep;62(2):311–323. doi: 10.1016/0027-5107(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Rowan L. A., Silinskas K. C., Kateley S. A., McCormick J. J. Frequency of UV-induced neoplastic transformation of diploid human fibroblasts is higher in xeroderma pigmentosum cells than in normal cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2613–2617. doi: 10.1073/pnas.79.8.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur M., Glickman B. W. Sequence specificity of mutations induced by benzo[a]pyrene-7,8-diol-9,10-epoxide at endogenous aprt gene in CHO cells. Somat Cell Mol Genet. 1988 Jul;14(4):393–400. doi: 10.1007/BF01534647. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Norman D., Abuaf P., Hingerty B. E., Live D., Grunberger D., Broyde S., Patel D. J. NMR and computational characterization of the N-(deoxyguanosin-8-yl)aminofluorene adduct [(AF)G] opposite adenosine in DNA: (AF)G[syn].A[anti] pair formation and its pH dependence. Biochemistry. 1989 Sep 5;28(18):7462–7476. doi: 10.1021/bi00444a046. [DOI] [PubMed] [Google Scholar]
- Osborne M. R., Beland F. A., Harvey R. G., Brookes P. The reaction of (+/-)-7alpha, 8beta-dihydroxy-9beta, 10beta-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene with DNA. Int J Cancer. 1976 Sep 15;18(3):362–368. doi: 10.1002/ijc.2910180315. [DOI] [PubMed] [Google Scholar]
- Ryan P. A., Maher V. M., McCormick J. J. Modification of MCDB 110 medium to support prolonged growth and consistent high cloning efficiency of diploid human fibroblasts. Exp Cell Res. 1987 Oct;172(2):318–328. doi: 10.1016/0014-4827(87)90390-9. [DOI] [PubMed] [Google Scholar]
- Straub K. M., Meehan T., Burlingame A. L., Calvin M. Identification of the major adducts formed by reaction of benzo(a)pyrene diol epoxide with DNA in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5285–5289. doi: 10.1073/pnas.74.12.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Vrieling H., Van Rooijen M. L., Groen N. A., Zdzienicka M. Z., Simons J. W., Lohman P. H., van Zeeland A. A. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Fujii S., van Boom J. H., Rich A. Molecular structure of the octamer d(G-G-C-C-G-G-C-C): modified A-DNA. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3968–3972. doi: 10.1073/pnas.79.13.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Parks W. C., Wigle J. C., Maher V. M., McCormick J. J. Fibroblasts from patients with inherited predisposition to retinoblastoma exhibit normal sensitivity to the mutagenic effects of ionizing radiation. Mutat Res. 1986 Oct;175(2):107–114. doi: 10.1016/0165-7992(86)90133-8. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Maher V. M., McCormick J. J. Excision repair of UV- or benzo[a]pyrene diol epoxide-induced lesions in xeroderma pigmentosum variant cells is 'error free'. Mutat Res. 1985 Nov;146(3):285–294. doi: 10.1016/0167-8817(85)90070-7. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Young A. B., Kelley W. N. Hypoxanthine-guanine phosphoribosyltransferase deficiency. The molecular basis of the clinical syndromes. N Engl J Med. 1983 Oct 13;309(15):900–910. doi: 10.1056/NEJM198310133091507. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Amplification and direct nucleotide sequencing of cDNA from the lysate of low numbers of diploid human cells. Gene. 1989 Nov 30;83(2):347–354. doi: 10.1016/0378-1119(89)90121-2. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Kinds of mutations formed when a shuttle vector containing adducts of (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9, 10-tetrahydrobenzo[a]pyrene replicates in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3787–3791. doi: 10.1073/pnas.84.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. L., Maher V. M., McCormick J. J. Error-free excision of the cytotoxic,mutagenic N2-deoxyguanosine DNA adduct formed in human fibroblasts by (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5933–5937. doi: 10.1073/pnas.77.10.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. L., Maher V. M., McCormick J. J. Relationship between excision repair and the cytotoxic and mutagenic effect of the 'anti' 7,8-diol-9,10-epoxide of benzo[a]pyrene in human cells. Mutat Res. 1982 Jun;94(2):435–447. doi: 10.1016/0027-5107(82)90306-2. [DOI] [PubMed] [Google Scholar]



