Abstract
Wearable sensors give the users convenience in daily health monitoring, though several challenges in such sensor systems should be overcome. This paper discusses the challenges in wearable health monitoring sensors and solutions for multi-modal and multi-functional wrist-worn devices based on novel circuit design techniques to reject DC offset. Furthermore, this paper also presents a novel sophisticated algorithm to reject motion artifacts. The system has the capability to simultaneously acquire several bio-signals (i.e. electrocardiogram, PPG, and body-electrode impedance). The system can also help patients who want to monitor their psychological signals to mitigate health risks.
I. Introduction
Wearable health monitoring technology attracts serious attention from both the research community and the industry as it will transform the healthcare system in more effective and convenient ways. Recent technological advances in wearable sensors, low-power integrated circuits, and wireless communications have enabled to monitor significant physiological signals such as heart rate, electrocardiogram (ECG), oxygen saturation, respiration rate, body temperature, blood pressure, galvanic skin response, electroencephalogram (EEG), and blood glucose [1, 2, 3]. In parallel with these advances, wireless personal or body networks (WPANs) have been introduced to integrate seamlessly these sensors into for wireless health monitoring [2].
Meanwhile, the demand on measuring reliable physiological signals during severe circumstances such as fitness training as well as daily living is increasing. This requires higher front-end dynamic ranges and integration of data processing and artifact removal algorithms in the wearable sensor system. Thus, each sensor produces at least 16 bit digital stream data with up to a sampling rate of 250~1,000; the mobile host should be capable of processing data from multiple sensors.
This approach still requires overcoming several major challenges. For example, it needs breakthrough in sensor hardware such as on-sensor signal processing to reduce data transfer rates and computing burdens in the mobile host. With state-of-the-art technologies, such sensors still remain larger in size and consume higher battery power than ideal. Given situation, one should trade-off between user comfort, long-term wearability and reliable data communications. Yet, most people may not want to wear uncomfortable sensors to monitor their health unless they have serious health problems.
Thus, we believe the best solution for wearable health monitoring system is to integrate as much functionalities and sensing modalities into one form-factored device to maximize user comport. One of the best locations for the wearable sensors is the wrist as people have been wearing wrist watches for more than hundred years while we can monitor many significant physiological signals with the wrist. In terms of technical challenges, however, the wrist may be not the best place to locate such sensors because of motion artifacts and size limitation. For instance, one can measure oxygen saturation from ear, forehead, finger, or wrist, but the motion at the ear or forehead is relatively smaller comparing to the motion measured at the wrist or finger. Technical solutions to overcome these issues should be properly discussed to develop wrist-worn health monitoring devices.
Thus, this paper addresses the technical challenges to implement multi-functionality and multi-modality into a wrist-worn health monitoring system in Section II. Next, possible solutions associated with the challenges are discussed in Section III and IV. Section V presents the conclusion and future direction.
II. Challenges in Bio-sensing from Wrist
A. Multi-Modality/Multi-functionality
Due to power consumption and size limitation, most wrist-worn devices support either photoplethysmography (PPG) or ECG sensor with an accelerometer [3, 4]. People can monitor their sleep states and/or calorie burning by measuring heart rate and activities [3]. Adding potentiostat into the system, however, one can actually do much more; it will enable to estimate user’s body contents (muscle and fat) and stress level by measuring body impedance and galvanic skin response, respectively.
Integration of multi-modal sensing is not trivial since the challenges encompass the limitations of power consumption, the electronics size, and data transfer bandwidth. By even simple integration of several analog front-ends, one can expect significant reduction of power consumption and area of the system. However, to further improve these, it is required that novel system architecture and circuit design to make the circuit multi-functional and to share many circuit components (such as analog-to-digital converter) with other sensing modalities.
Simultaneous Electrochemical Sensing
Electrochemical measures on their own include interesting challenges. First, the typical currents measured are quite small, requiring careful instrumentation design. But, also they are dependent on carefully controlling the potential drop at the electrode surface. This is then typically done by measuring the electrode potential with respect to a reference electrode – which will have its own interface potential. Measurement errors occur then when this reference potential varies, for example because current is driven through it, or if the potential within the skin between electrodes varies. To make multiple parallel electrochemical measurements along with both bio-potential recording requires circuit and system designs that minimize current through the reference electrodes, suitably amplifies the small currents and removes additional artifacts from the reference potential variations.
B. Electrode DC Offset
Unlike electrochemical sensing, which is fundamentally current sensing, bio-potential sensing system should consider the DC potential in the measurement electrodes. A voltage known as the half-cell potential develops across the skin and electrode interface appears as DC offset in bio-potential sensing system. This offset voltage can be up to ±300 mV and may saturate the preamplifier; thus limit the maximum gain of the amplifier unless DC offset rejection circuitry is implemented in the analog front-end of the system.
The typical front-end gain of commercially available ICs (for example, ADS129x from Texas Instruments, Inc. and ADAS1000 from Analog Devices, Inc.), which do not have DC offset rejection circuitry, is only 24V/V although the amplitude of bio-signals of interests ranges 1 μV to 5 mV. Instead, they have a large dynamic range so as to digitally remove DC offset after digitization. Therefore, the system, consists of those commercial IC chipsets, should have passive high pass filters using discrete resisters and capacitors between electrodes and the analog front-end to reject the DC offset if further digital signal processing is not available. This circuitry, however, has quite high input-referred noise and degrades the common mode rejection ratio (CMRR) characteristics of the system.
Alternatively, active AC-coupling approaches have been introduced [5, 6], which use AC-coupled capacitive feedback with fast-recovery circuits to reset the proper DC levels after input overload. However, these designs typically use single-ended amplifier and measures signal with respect to common reference. As a result, the CMRR of the system becomes limited and often cannot meet the CMRR requirement as it must use single-ended input analog-digital converter as well, while differential analog signal processing is preferred to achieve high CMRR characteristics.
C. Motion Artifact
Every bio-signal monitoring sensor suffers from motion artifacts because the system shares the ground with the human body of which the ground is floating and sensitive to motion. Since the artifacts from the floating ground are common mode inputs to the front-end amplifier, the artifacts can be easily rejected at the output of the amplifier. However, the weak contact between the electrodes and skin can also induce motion artifacts because the electrode-skin impedance is subject to change by motion. Wrist-worn devices should be taken more special attention in terms of motion artifacts because the wrist is one of the most frequent-moving organs in the human body.
In practical systems, motion artifacts in PPG devices are more severe than those in ECG devices. ECG requires strong electrode-skin contacts and the recording systems are equipped with electrodes that give us good electrode-skin contacts. Whereas, wrist-worn PPG sensors rely on the optical reflection signals and the reflected signals are inevitably recorded with motion artifacts. Thus, one has to extract heart rate or oxygen concentration from a weaker signal that corrupted by a larger motion artifacts.
Over the years, there are a number of methods proposed for PPG heart rate monitoring under different types and magnitudes of motion, from involuntary finger motion, to walking or jogging, and further to intense exercise such as running at maxima speed of the subject [8, 9]. Accelerometer signal is often used to provide additional information for separating motion and heart rate signals. A large amount of existing algorithms belong to the category of noise filtering or cancellation. One of commonly accepted methods is the adaptive noise cancellation (ANG) [9], especially when the motion is strong and persistent such as during exercise. The main idea of ANG is to use a noise reference, e.g. the accelerometer signal in the context, to estimate the linear additive noise by enforcing the assumption that the resulting output signal should not be correlated with the estimated noise signal. Notably, the motion related signals are time varying; hence the frequency components are constantly changing that noise cannot be cancelled using a single non-adaptive filter. ANG has been used on PPG heart rate monitoring during motion, yielding decent accuracies. Others has proposed methods based on the assumption that, motion and heart rate has distinct properties in frequency domain, and large frequency components of the PPG signal can then be classified as motion or heart rates [7, 10].
III. Proposed Solutions
A. Multi-Modal Circuit Development
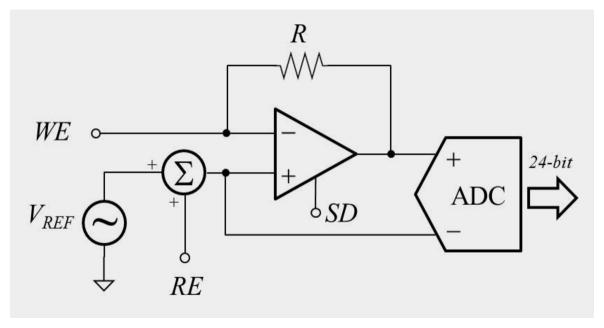
As described in the previous section, one of key challenges in bio-instrumentation is to implement the ability to do multi-modal sensing – especially, simultaneous skin-electrode impedance measurement and electrochemical sensing. We can do this by placing a potentiostat integrated with analog front-end circuitry as shown in Figure 1. The basic design requires an Op-amp, plus a summing amplifier between the reference measurement (RE) and a program bias (VREF). In the electrochemical sensing mode, the Op-amp drives current through the resistor and working electrode (WE) in order to maintain the WE potential at the sum of the bias plus reference potentials, and the ADC effectively measures the voltage across (and the current through) the resistor. To measure impedance, the bias potential VREF will be driven at various frequencies. Then, the WE will be virtually driven to the same potential as the VREF potentials. The impedance of WE can be calculated by straightforward signal processing.
Figure 1.
Simplfied schematic diagram of the potentiostat
B. Electrode DC Offset Rejection
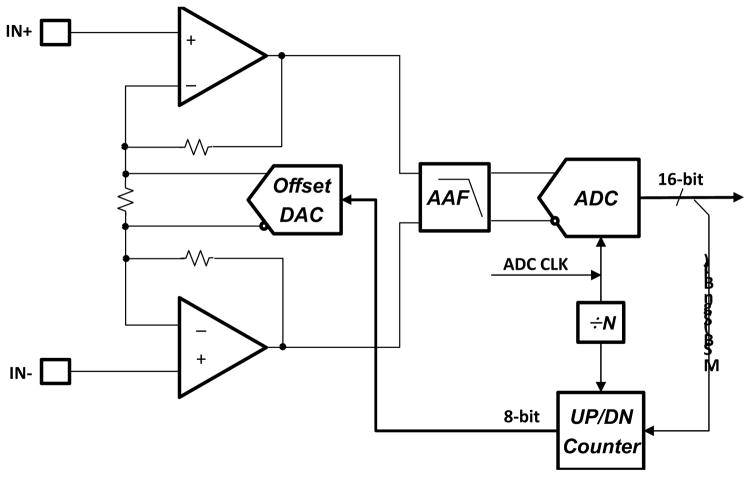
Figure 2 shows a schematic diagram of the proposed DC offset cancellation circuit. The circuit consists of an instrumentation amplifier with a gain of 100V/V, connected to a SAR (successive approximation register) ADC that outputs 16 digital bits. The MSB of an offset binary ADC is used as a sign bit and a counter is used to count the number of times the signal crosses zero.
Figure 2.
Simplfied block diagram of the DC offset rejection circuit
If there is DC offset in the system, the signal to the ADC spends more time above zero (in the case of a positive offset) than it does below zero. This is used to advance the counter connected to a digital-to-analog converter (DAC) in such a way so as to cancel the DC offset. The output of the amplifier will come out of saturation as the offset cancellation loop nears the final electrode offset. When a predetermined time passes, the counter output is frozen, at this time; the output of the counter has the correct amount of bits so as to cancel the offset at the input. The loop has reached its desired value and can be turned off or its update rate reduced so as to track a very slow frequency fluctuation. In this loop the counter acts as an Integrator with a time-constant of the update rate.
The loop bandwidth is dependent on the bandwidth of the Instrumentation amplifier and the update rate of the counter. When the system is attempting to first acquire the offset, it is run at a much faster rate, after a predetermined time, the update rate is slowed down so that it is much slower than the lowest frequency signal of interest. In the case of ECG, that would be in the sub-zero hertz.
C. Motion Artifact Removal Algorithm
To improve the accuracy of the heart rate estimation based on wrist PPG, it should be taken into account the fact that motion signal is highly correlated with heart rate during exercises, such as running. The motion artifact of wrist PPG during running comes mainly from swinging arms, causing the wrist device to have different contact pressure against the skin, and causing the PPG signal to vary. The displacement also caused by motion may also lead to ambient light being captured by the sensors. As the running speed varies, the speed of arm swinging also varies accordingly, as well as the heart rate. Due to the correlation, if one eliminates the motion signal, inevitably the resulting heart rate signal will also be partially eliminated. Instead of cancelling or removing the motion signal, and then estimate the heart rate, we propose to treat the problem as a joint estimation problem or a signal separation problem, and then classify the resulting signals as motion or heart rate, such that correlations between signal sources are considered in the framework.
Also, we focus on developing representations in which inferences such as estimation, separation, and classification are easy. Such representations allow the use of inference methods with fewer free parameters such that have better generalizability. Also lower computational complexity inference methods can be used, which is particularly important in the context of mobile wrist devices with limited computing resources. Notably, the representation itself should also be low complexity for the same reason.
IV. Results
A. Simultaneous Electro-Chemical Sensing
The potentiostat depicted in Figure 1 has been realized to demonstrate the concept in Gluckman’s Lab. The WE is connected to Enzyme-based lactate sensor (Pinnacle Technology, Laurence, KS) and VREF is applied with redox potential of 0.6V. The WE successfully measures the different concentration of lactate solution as shown in Figure 3.
Figure 3.
Output of the instrumentation amplifier shown in Figure 1. The WE was manually dipped in the lactate solution with different concentrations.
B. DC Offset Rejection Simulation Results
The DC offset rejection circuit has been designed with Global Foundries’ 65nm CMOS fabrication technology. The proposed circuit shows rail-to-rail input with low input referred noise characteristics (1.65 μVRMS at 40Hz), whereas the CMRR is only dependent on the internal resistor mismatches. The differential ADC has been also designed to have large CMRR and low-power consumption of 50 μW.
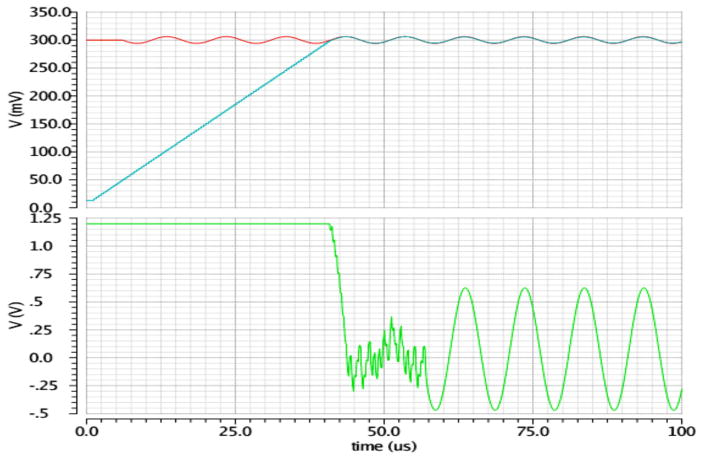
Figure 4 shows the simulation results of the DC offset rejection circuit. The upper figure shows the desired input signal (red) that is 10 mV in amplitude, 100 KHz in frequency and with undesired 300 mV DC offset. The blue trace presents the output of the offset DAC, shown in Figure 2. The green trace (bottom figure) shows the output of the instrumentation amplifier (gain: 100V/V), saturated till the offset DAC catches up with the electrode offset (around 40 μsec). After the amplifier comes out of saturation, the offset DAC starts tracking (attenuating) the desired signal. At 60 μsec the loop is turned off and the desired signal emerges at the output of the amplifier, unaffected by the mostly cancelled electrode offset. Alternatively, the update rate of the loop can be drastically reduced (by changing the clocking rate of the loop) to track a lower frequency offset that is usually created by motion artifacts. There is also the option of periodically turning on the offset cancellation loop when clipping is detected in the ADC.
Figure 4.
Simulation results of the instrumentation amplifier with digitally-assisted DC offset rejection
C. PPG Heart Rate
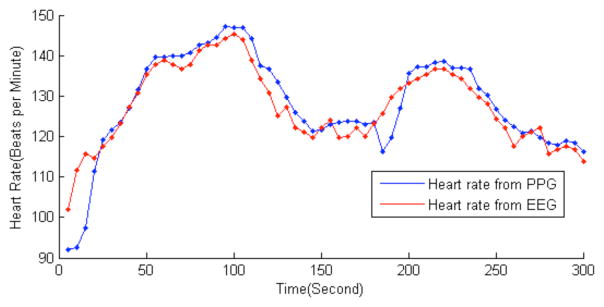
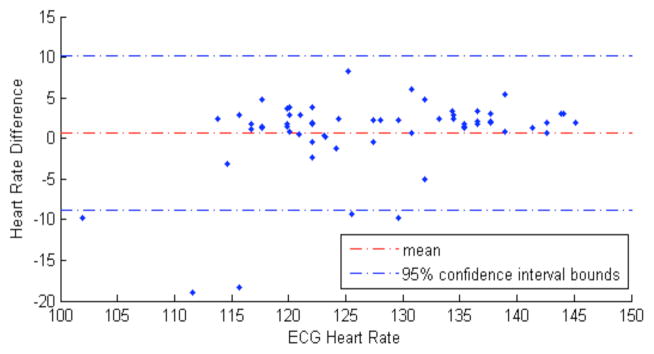
Figure 5 shows the estimated heart rate using a green LED-based (wavelength: 525 nm) PPG sensor. The subject is instructed to run at a speed that the subject is accustomed to, and to change to walking in-between two running periods. The mean error is 3.3 beats per minute, defined as the average absolute difference: mean (|PPG_HR-ECG_H R|). The Bland-Altman plot, illustrated in Figure 6, depicts the degree of agreement of heart rate measured by the PPG and the ECG sensors, as a function of ECG heart rate as x-axis reference. Notably, larger error is observed when the subject went from walking to running, e.g. at the beginning and at the 200th second in Figure 5, and lower ECG heart rate regions in Figure 6. This is also observed in other literatures that the performance of the algorithm generally degrades in changing states due to irregular arm motions [7, 10].
Figure 5.
Comparison of estimated heart rate based on PPG and ECG during exercise
Figure 6.
The Bland-Altman plot for analyzing agreements between PPG and ECG heart rates
V. Conclusion
This paper presents the challenges and solutions in wearable bio-signal sensing systems. The multi-modal sensing from wrist will enables to monitor many interesting physiological signals while to maintain users’ comport. Further development efforts are being currently undertaken to implement the solutions in the single IC chip with low power low complexity microcontroller for controls and signal processing including motion artifact removal.
Contributor Information
Insoo Kim, Pennsylvania State University, University Park, PA, and he is now with the Samsung Research America – Dallas, Richardson, TX 75082 USA.
Po-Hsiang Lai, Samsung Research America – Dallas, Richardson, TX, USA.
Ryan Lobo, Samsung Research America – Dallas, Richardson, TX, USA.
Bruce J. Gluckman, Pennsylvania State University, University Park, PA 16802 USA.
References
- 1.Pantelopoulos A, Bourbakis NB. A Survey on Wearable Sensor-Based Systems for Health Monitoring and Prognosis. IEEE Trans Systems, Man, and Cybernetics-Part C: applications and Reviews. 2010 Jan;40(1) [Google Scholar]
- 2.Paradiso R, Loriga G, Taccini N. A wearable health care system based on knitted integrated sensors. IEEE Trans Information Technology in Biomedicine. 2005 Sep;9(3) doi: 10.1109/titb.2005.854512. [DOI] [PubMed] [Google Scholar]
- 3.Milenković A, Otto C, Jovanov E. Wireless sensor networks for personal health monitoring: Issues and an implementation. Computer Communications. 2006 Aug;29(13–14) [Google Scholar]
- 4.Asada HH, Shaltis P, Reisner A, Rhee S, Hutchinson RC. Mobile Monitoring with Wearable Photoplethysmgrapic Biosensors. IEEE EMBS magazine. 2013 May-Jun; doi: 10.1109/memb.2003.1213624. [DOI] [PubMed] [Google Scholar]
- 5.Galian W, Hafkemeyer KM, Tomasik JM, Wagner F, Krautschneider WH, Schroeder D. IEEE EUROCON 2009. St. Petersburg, Russia: May, 2009. Highly Sensitive Biomedical Amplifer with CMRR Calibration and DC-Offset Compensation. [Google Scholar]
- 6.Huang S, Zhang J, Wang L. IEEE BioCAS 2013. Rotterdam, Netherlands: Nov, 2013. A 6.7 μW CMOS Bioamplifier for Active Electrode with DC Rejection. [Google Scholar]
- 7.López-Silva SM, Giannetti R, Dotor ML, Silveira JP, Golmayo D, Miguel-Tobal F, Bilbao A, Canales MG, Escudero PM. Heuristic algorithm for photoplethysmographic heart rate tracking during maximal exercise test. J of Med Biol Eng. 32(3) [Google Scholar]
- 8.Lee HW, Lee JW, Jung WG, Lee GK. The periodic moving average filter for removing motion artifacts from PPG signals. Int J Control Automation Systems. 5:701–706. [Google Scholar]
- 9.Widrow B, Glover JR, McCool JM, Kaunitz J, William CS, Hearn RH, Zeidler JR, Dong ED, Goodlin R. Adaptive noise cancelling: Principles and applications. Proc IEEE. 1975 Dec;63(12):1692–1716. [Google Scholar]
- 10.Fukushima1 H, Kawanaka H, Bhuiyan MdS, Oguri K. Estimating Heart Rate using Wrist-type Photoplethysmography and Acceleration sensor while running. 34th Annual International Conference of the IEEE EMBS; San Diego, California USA. 2012; [DOI] [PubMed] [Google Scholar]