Abstract
Socially contagious itch is ubiquitous in human society, but whether it exists in rodents is unclear. Using a behavioral paradigm that does not entail prior training or reward, we found that mice scratched after observing a conspecific scratching. Molecular mapping showed increased neuronal activity in the suprachiasmatic nucleus (SCN) of the hypothalamus of mice that displayed contagious scratching. Ablation of gastrin-releasing peptide receptor (GRPR) or GRPR neurons in the SCN abolished contagious scratching behavior, which was recapitulated by chemogenetic inhibition of SCN GRP neurons. Activation of SCN GRP/GRPR neurons evoked scratching behavior. These data demonstrate that GRP-GRPR signaling is necessary and sufficient for transmitting contagious itch information in the SCN. The findings may have implications for our understanding of neural circuits that control socially contagious behaviors.
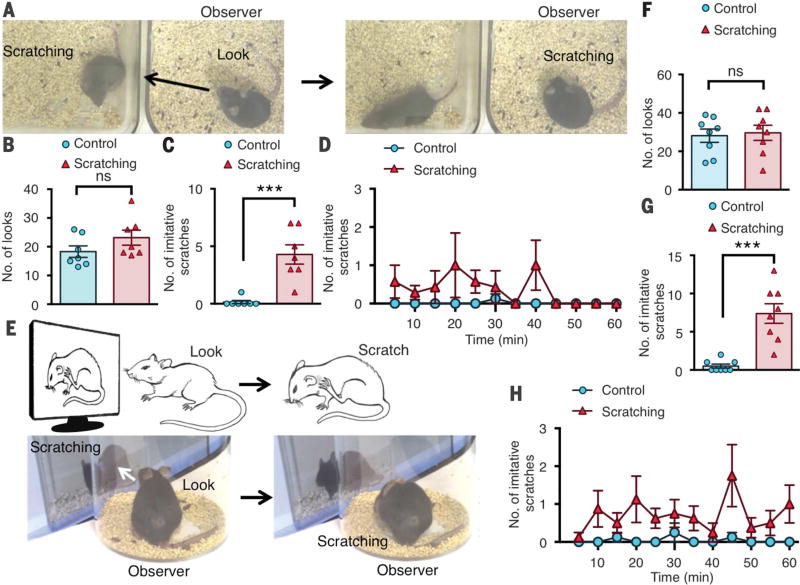
Socially contagious behaviors such as contagious itching/scratching and yawning are prevalent in humans (1, 2) and in highly social animals (3, 4). However, such behaviors have only been sporadically mentioned in the context of imitation and/or social learning (2, 5–7). The lack of experimental paradigms that permit recreation of socially contagious behaviors in a naturalistic environment without prior training or reward has hindered studies of such behaviors at the molecular and circuit levels (3, 5, 8). To test whether scratching is contagious in mice, we used mice with excessive spontaneous scratching because of chronic itch as the demonstrators (9) and naïve mice with no prior contact to mice with chronic itch in an adjacent home cage as the observers (Fig. 1A). Mice that did not scratch excessively were used as control demonstrators. Both observer groups showed similar numbers of looks, defined as a pause and look toward the demonstrator (18.3 ± 2 versus 23.1 ± 2.6 looks, P = 0.17) (Fig. 1B). Control observers displayed almost no coincidental look-and-scratch behavior (Fig. 1C). In contrast, mice displayed significantly increased scratching within 5 s after look behavior (movie S1), defined as imitative scratching, upon observation of demonstrators scratching (4.5 ± 0.8 versus 0.2 ± 0.2 look-and-scratches, P < 0.001) (Fig. 1, C and D, and fig. S1A).
Fig. 1. Mice display imitative scratching behavior.
(A) Representative screen shots of a mouse observing a conspecific scratching in an adjacent home cage followed by imitative scratching. (B) Mean number of look behaviors toward the control or scratching demonstrator. (C) Mean number of instances of look-and-scratch (imitative scratch) behavior by the observers watching the scratching demonstrator versus those watching the control demonstrator. (D) Time course (in 5-min intervals) of mean number of imitative scratches. (E) Images of look-and-scratch behavior and screen shots of a mouse observing a video of a con-specific scratching followed by imitative scratching. (F) Mean number of looks toward the on-screen control or scratching demonstrators. (G) Mean number of imitative scratches by the observers watching the on-screen scratching demonstrator versus those watching the control demonstrator. (H) Time course of mean number of imitative scratches. n = 7 or 8 mice per group. Unpaired t test in (B), (C), (F), and (G); ***P < 0.001; ns, not significant. Data are means ± SEM.
Next, we tested whether nonvisual cues such as auditory and olfactory cues are dispensable for imitative scratching by placing a mouse in a clear cylinder in front of a computer screen that displayed a conspecific with scratching behavior (Fig. 1E). As a control, a video of a mouse that ambulated without scratching was displayed. Comparable look behaviors between the control and scratching groups were detected (28.1 ± 3.5 versus 29.6 ± 4 looks, P = 0.78) (Fig. 1F and fig. S1B). The video screen paradigm recapitulated home-cage results, as mice observing the scratching demonstrator displayed characteristic imitative scratching behavior (movie S2), where-as the controls showed almost no coincidental look-and-scratch behavior (7.4 ± 1.3 versus 0.5 ± 0.3 look-and-scratches, P < 0.001) (Fig. 1, G and H, and fig. S1, C to G).
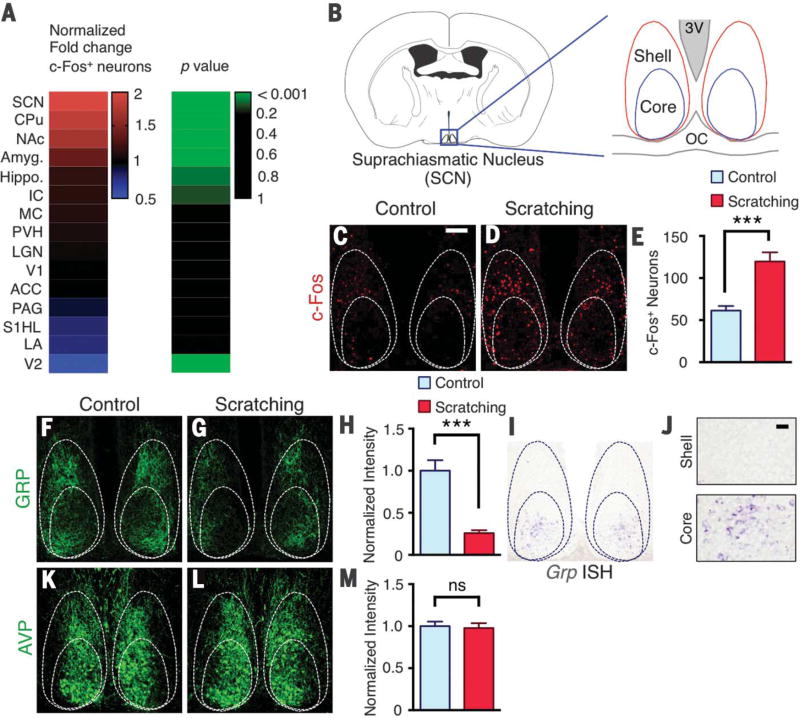
To evaluate the brain regions that are activated in the contagious itch test, we performed immunohistochemistry with antibody to c-Fos in the brains of mice that watched the scratching demonstrator video or watched the control ambulating mouse video. One hour after the contagious itch test, animals were perfused and processed. Mice that observed scratching were recorded and counted for imitative scratches to confirm contagious itch behaviors. Relative to the controls, mice that displayed contagious scratching exhibited significant increases in c-Fos expression in several brain regions (Fig. 2A). These regions include the suprachiasmatic nucleus (SCN), nucleus accumbens, caudate putamen, and amygdala. The majority of brain areas showed either comparable c-Fos expression between the two groups or low c-Fos signal (table S1). Located in the hypothalamus, the SCN is a master circadian pacemaker; it can be divided into core and shell regions (10, 11) (Fig. 2B). Relative to control mice, an approximate doubling of the number of c-Fos+ neurons was detected in the core and shell of mice with contagious itch behavior (59 ± 5 versus 120 ± 11, P < 0.001), with more c-Fos+ neurons in the shell area (Fig. 2, C to E). Because the SCN receives visual input directly or indirectly (11), we hypothesized that it could be one of the first relay circuits for mediating contagious itch behavior. This prompted us to examine the expression of GRP, a well-established neuropeptide marker in the SCN that has been implicated in photic modulation of circadian phase (10, 12, 13), as well as in dorsal root ganglion neurons that are required for transmission of itch information to GRPR in the spinal cord (14). Consistent with our recent study (15), we confirmed the specificity of the GRP antibody using Grp knockout (KO) mice (fig. S2). We found a notably reduced staining intensity of GRP in SCNs of mice with contagious itch behavior relative to controls (1 ± 0.1 versus 0.25 ± 0.04 normalized intensity, P < 0.001) (Fig. 2, F to H). Because immunohistochemistry largely revealed GRP-expressing fibers that arborize densely in the core and shell of the SCN where Grpr expression is detected (16), we used in situ hybridization to investigate whether Grp+ cells are located in the core area (15). Consistent with previous studies (10), Grp+ cell bodies were detected in the core, but not the shell, of the SCN (Fig. 2, I and J). In contrast, expression of arginine vasopressin (AVP) was not affected after the contagious itch test (P = 0.78) (Fig. 2, K to M).
Fig. 2. c-Fos and GRP expression in the suprachiasmatic nucleus of mice with contagious itch.
(A) Heat map showing comparison of normalized fold change in number of c-Fos+ neurons in select brain regions between mice observing ambulating (control) or scratching demonstrators. (B) Schematic of coronal brain section with suprachiasmatic nucleus (SCN) enlarged with core and shell regions, third ventricle (3V), and optic chiasm (OC) indicated. (C and D) c-Fos expression in SCN of mice observing control (C) and scratching (D) demonstrators. (E) Mean number of c-Fos neurons in SCN of control and scratching observer groups. (F and G) GRP immunohistochemistry in SCN of mice observing control (F) and scratching (G) videos. (H) Mean normalized intensity of GRP staining in SCN sections of control and scratching observer groups. (I) Image of Grp in situ hybridization (ISH) in SCN. (J) Higher-magnification image of Grp in situ hybridization in the shell and core, respectively. (K and L) AVP immunohistochemistry image in SCN of mice observing control (K) and scratching (L) demonstrators. (M) Mean normalized intensity of AVP staining in SCN sections of control and scratching observer groups. Scale bars, 100 µm [(C), (D), (F), (G), (I), (K), and (L)], 20 µm (J). n = 3 mice and 10 sections per group. Unpaired t test in (E), (H), and (M); ***P < 0.001. Data are means ± SEM.
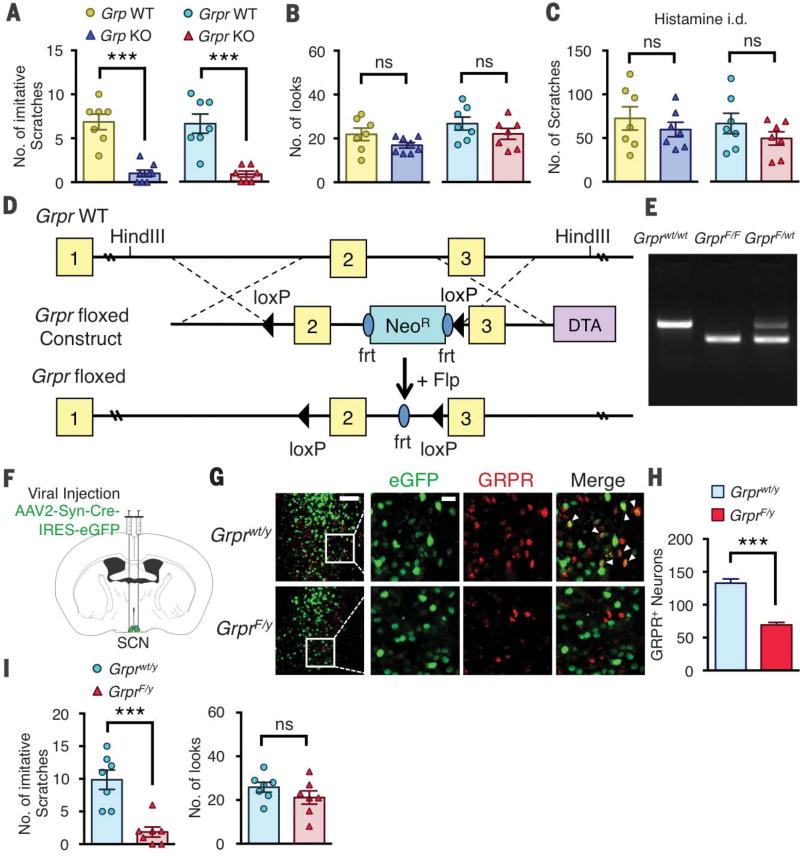
The apparent depletion of GRP in the SCN prompted us to ask whether GRP has a role in contagious itch transmission. We first evaluated contagious itch behavior in mice lacking Grp (Grp KO) or Grpr (Grpr KO) (9). Grp or Grpr KO mice showed almost no contagious scratching behavior relative to their respective wild-type littermates (7 ± 1 versus 1 ± 0.4 look-andscratches for Grp wild-type versus KO, 7 ± 1 versus 1 ± 0.3 look-and-scratches for Grpr wild-type versus KO, P < 0.001), despite normal look numbers (Fig. 3, A and B). The lack of imitative scratching behavior was not due to the failure of Grp or Grpr KO mice to scratch, because they still retained the ability to scratch in response to intradermal injection of histamine (Fig. 3C), consistent with previous reports that the role of GRP-GRPR signaling is largely restricted to nonhistaminergic itch (14, 17).
Fig. 3. The role of GRP-GRPR signaling in contagious itch.
(A) Mean number of imitative scratches in Grp wild-type (WT) and KO or Grpr WT and KO littermates. (B) Mean number of looks in Grp WT and KO or Grpr WT and KO littermates. (C) Mean number of scratches induced by intradermal (i.d.) injection of histamine (200 µg) in Grp WT and KO or Grpr WT and KO littermates. (D) Schematic of targeting strategy for Grpr floxed allele. (E) Gel image of genotype polymerase chain reaction results from Grprwt/wt, GrprF/F, and GrprF/wt littermates. (F) Coronal brain section illustrating bilateral injection of AAV2-Syn-Cre-IRES-eGFP into SCN of Grprwt/y or GrprF/y mice. (G) Images of GRPR and eGFP expression in SCN of AAV2-Syn-Cre-eGFP–injected Grprwt/y or GrprF/y mice. Arrowheads indicate GRPR-eGFP double-positive neurons. (H) Mean number of GRPR+ neurons in SCN of AAV2-Syn-Cre-eGFP–injected Grprwt/y or GrprF/y mice. (I) Mean number of imitative scratches and mean number of looks in AAV2-Syn-Cre-eGFP–injected Grprwt/y or GrprF/y mice. Scale bars in (G), 100 µm (left column), 20 µm (other images). n = 7 mice per group for behavior; n = 3 mice and 14 to 16 sections per group for imaging. Unpaired t test in (A) to (C), (H), and (I); ***P < 0.001. Data are means ± SEM.
To determine whether SCN GRPR is required for contagious itch, we generated mice harboring floxed Grpr (GrprF/y) (Fig. 3, D and E). To conditionally delete Grpr in the SCN, we stereotactically injected a viral vector encoding Cre recombinase and enhanced green fluorescent protein (AAV2-Syn-Cre-IRES-eGFP) bilaterally into the SCN of GrprF/y mice or control Grprwt/y mice (Fig. 3F). To verify the site of the injection and expression of Cre recombinase, we performed double immunohistochemistry. Many neurons coexpressed eGFP and GRPR in the SCN of Grprwt/y mice (Fig. 3G). In contrast, almost no GRPR expression was detected in neurons expressing CreeGFP (Fig. 3G). Relative to Grprwt/y mice, the number of SCN GRPR+ neurons was significantly reduced in GrprF/y mice (126 ± 7 versus 69 ± 3, P < 0.001) (Fig. 3H). Consistently, in situ hybridization also showed decreased Grpr expression in the SCN of GrprF/y mice relative to Grprwt/y mice (fig. S3, A and B). Whereas Grprwt/y mice showed normal contagious itch, GrprF/y mice showed almost no imitative scratching behaviors (9.9 ± 1.5 versus 1.9 ± 0.8 look-and-scratches, P < 0.001), despite normal look behavior (P = 0.34) (Fig. 3I).
We selectively ablated SCN GRPR neurons using bombesin-sap (BB-sap), a peptide conjugated toxin that kills GRPR neurons in the spinal cord (17, 18). After bilateral injection of BB-sap into the SCN (fig. S4A), immunohistochemicstry showed that BB-sap injection resulted in ablation of most SCN GRPR+ neurons (145 ± 5 versus 37 ± 4, P < 0.001) (fig. S4, B and C). The effects of BB-sap–induced Grpr neuronal ablation were also confirmed by in situ hybridization (fig. S5, A and B). Although control mice exhibited normal imitative scratching behavior as expected, mice treated with BB-sap in the SCN barely showed imitative scratching behavior (7.7 ± 1.8 versus 1.3 ± 0.6 look-and-scratches, P < 0.01) (fig. S4D). BB-sap treatment did not affect the number of look behaviors relative to controls (P = 0.32) (fig. S4E). There was no difference in acute itch behavior after histamine injection (P = 0.43) or spontaneous scratches (P = 0.86) in BB-sap SCN-treated mice relative to controls (fig. S4, F and G).
We asked whether chemogenetic silencing of Grp neuron activity could inhibit contagious itch. A viral vector expressing Cre-dependent Gi-coupled designer receptors exclusively activated by designer drugs (DREADDs), hM4Di (AAV5-EF1a-DIO-h4MDi-mCherry), or expressing AAV5-eGFP as a control was injected bilaterally into the SCN of Grp-Cre mice (fig. S4, H and I). After clozapine N-oxide injection, mice with h4MDi expression in Grp-Cre SCN neurons displayed almost no imitative scratching behavior, whereas eGFP control mice showed normal imitative behaviors (7.7 ± 1.4 versus 1.6 ± 0.6 look-and-scratches, P < 0.01) (fig. S4J). Look behavior was not significantly affected in the h4MDi-expressing Grp-Cre mice relative to controls (P = 0.16) (fig. S4K). Acute itch behavior after histamine injection was also not affected in h4MDi-expressing Grp-Cre mice relative to controls (P = 0.83) (fig. S4L).
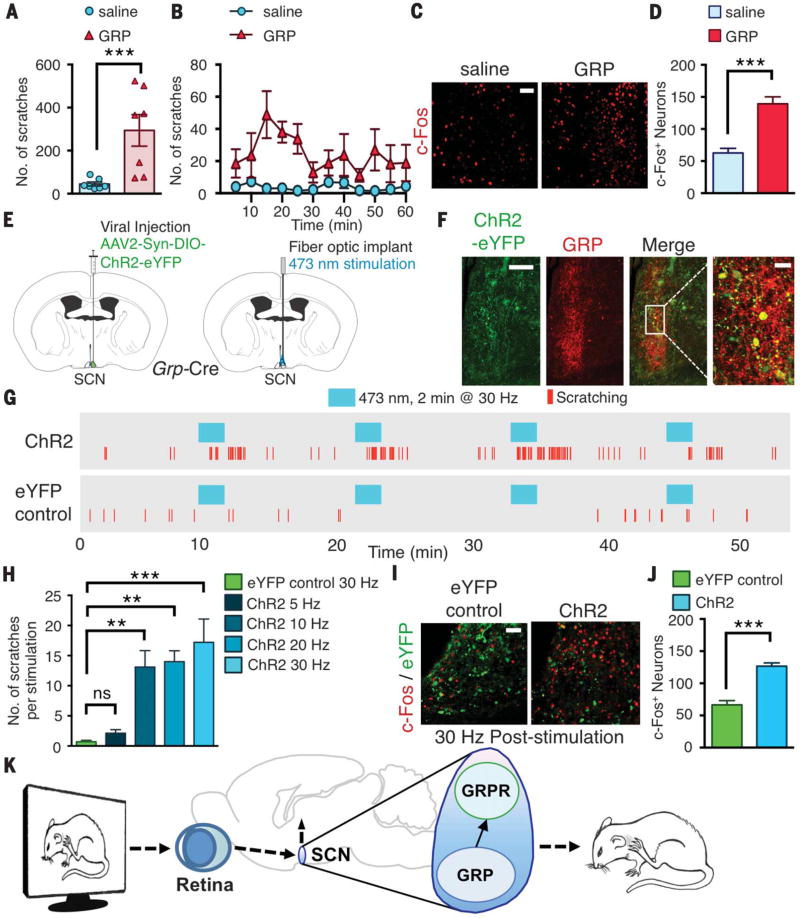
Next, we asked whether GRP transmits visual input–encoded itch information. If so, artificial activation of SCN GRPR neurons by GRP should evoke mice to scratch without visual stimuli. We unilaterally microinjected GRP (0.04 nmol per 200 nl) or saline into the SCN of mice through an internal cannula. Exogenous GRP elicited vigorous scratching that lasted ~1 hour, whereas mice injected with saline showed little scratching (294 ± 73 versus 44 ± 9 scratches, P < 0.01) (Fig. 4, A and B, and movie S3). The number of c-Fos+ neurons in the SCN was significantly increased in mice injected with GRP relative to controls (62 ± 7 versus 139 ± 10, P < 0.001) (Fig. 4, C and D). To further test whether robust scratching behaviors evoked by exogenous GRP could be attributed exclusively to GRPR devoid of other nonspecific effects, we microinjected GRP into the SCN of Grpr KO mice and found that Grpr KO mice showed little scratching behavior (fig. S6A). We also microinjected GRP into BB-sap SCN-treated mice that showed significantly fewer scratches relative to control mice (fig. S6B).
Fig. 4. Activation of GRP or GRPR neurons in the SCN evokes scratching behavior.
(A) Mean number of scratches induced by microinjection of saline or GRP (0.04 nmol per 200 nl) into SCN of mice. (B) Time course of scratching induced by saline or GRP into SCN. (C) Image of c-Fos+ neurons in SCN after injection of saline or GRP. (D) Mean number of c-Fos neurons in SCN after injection of saline or GRP. (E) Brain sections illustrating unilateral injection of AAV2-Syn-DIO-ChR2-eYFP into SCN of Grp-Cre mice and subsequent fiber-optic implantation. (F) Images of GRP and ChR2-eYFP expression in SCN after viral injection. (G) Representative raster plots of scratching induced by photostimulation (473 nM for 2 min at 30 Hz) of eYFP control or ChR2 mice. (H) Mean number of scratches induced by photostimulation at 30 Hz in eYFP control mice or at 5, 10, 20, or 30 Hz in ChR2 mice. (I) Image of eYFP and c-Fos expression in SCN after photostimulation at 30 Hz in eYFP control or ChR2 mice. (J) Mean number of c-Fos+ neurons in SCN of eYFP control or ChR2 mice. (K) Diagram illustrating role of GRP-GRPR signaling in SCN for visually induced contagious itch. Scale bars, 50 µm [(C) and (I)], 100 µm [(F), far left], 20 µm [(F), far right]. n = 6 or 7 mice per group for behavior; n = 3 mice and 9 sections per group for imaging. Unpaired t test in (A), (D), and (J); one-way analysis of variance with Tukey post hoc in (H); **P < 0.01, ***P < 0.001. Data are means ± SEM.
Next, we activated SCN Grp neurons optogenetically (19). The SCN of Grp-Cre mice was unilaterally injected with a viral vector encoding Cre-dependent channelrhodopsin (AAV2-Syn-DIO-ChR2-eYFP) or enhanced yellow fluorescent protein (eYFP) virus as a control, followed by fiber-optic implantation for light stimulation (Fig. 4E). Immunohistochemistry confirmed that ChR2-eYFP virus in the SCN was expressed in GRP+ neurons (Fig. 4F) but not in VIP+ neurons (fig. S6C). Upon photostimulation with blue light (wavelength 473 nm) for 2 min at 30 Hz (power 10 mW), ChR2 mice exhibited scratching responses (movie S4), whereas eYFP-control mice showed almost no evoked scratching (17.2 ± 3.9 versus 0.6 ± 0.4 scratches, P < 0.001) (Fig. 4G). We observed a frequency-dependent scratching response when Grp-ChR2 neurons were photostimulated with increasing frequencies of 10, 20, or 30 Hz (Fig. 4H). After 10 min of recovery, repeated stimulations in ChR2 mice still evoked scratching bouts, with ~75% of 10-, 20-, and 30-Hz stimulations showing responses, yet eYFP control mice displayed almost no scratching with repeated stimulation (fig. S5D). The mean onset of scratching occurred within 30 s to 1 min of stimulation for all frequencies, and all ChR2 mice tested showed evoked scratching behaviors (fig. S5E). The number of c-Fos neurons in the SCN increased after stimulation of Grp-ChR2–expressing neurons relative to eYFP controls (66 ± 6 versus 126 ± 5, P < 0.001) (Fig. 4, I and J).
We found that itch is contagious in mice. Our results identify a previously unknown function of SCN by revealing its critical role in contagious itch transmission. Using cell type–specific inhibition and activation, we discovered that SCN GRP/GRPR neurons are key conduits for receiving, integrating, and transmitting visually induced itch information. GRP depletion after the contagious itch test supported the idea that GRP is released from GRPergic fibers to activate GRPR neurons. Exogenous GRP-induced scratching behavior is completely abolished in Grpr KO mice. This underscores the specificity and sufficiency of GRP-GRPR signaling in mediating contagious itch behavior within intra-SCN circuits. SCN GRPR neuronsmay functionally be heterogeneous. Alternatively, SCN GRPR neurons could possess a dual function: one for photic modulation and another for itch. Spinal and SCN GRPR neural circuits represent the two parallel pathways for transmission of itch (14, 18), induced by cutaneous and visual stimuli, respectively. Whether they may converge on the same sensorimotor system to compute stereotyped scratching is not yet known.
Scratching behavior in mice was induced by mere observation of a conspecific scratching in a video; this finding provides evidence that contagious itch is not a formof empathy, which in mice appears to be restricted to familiars (20). Visual cue–induced scratching action could bemimicked by artificially stimulating SCN GRPR neurons, which suggests that an automatic activation of a cascade of neural circuits for itch, rather than a manifestation in a higher cognitive/affective capacity required for empathy, gives rise to contagious itch (21). The discovery of SCN GRPR circuits for itch permits in-depth dissection of the input and output circuitry for itch transmission; previous studies have suggested that GRP neurons receive non–image-forming photic information (10, 22). It will be important to probe how visual input that encodes scratching action activates SCN GRP neurons and how GRPR neurons compute the output information (Fig. 4K). Given that myriad neural circuits are likely modulated upon viewing the scratching motion, further research is necessary to elucidate the discrete neural circuits subserving visual transmission of scratching motion and/or motor control. It will also be of interest to determine whether SCN subcircuits may mediate other types of socially contagious behaviors, such as yawning or empathy for pain.
Observing someone else scratching themselves can make you want to do so. This contagious itching has been observed in monkeys and humans, but what about rodents? Yu et al. found that mice do imitate scratching when they observe it in other mice. The authors identified a brain area called the suprachiasmatic nucleus as a key circuit for mediating contagious itch. Gastrin-releasing peptide and its receptor in the suprachiasmatic nucleus were necessary and sufficient to transmit this contagious behavior.
Acknowledgments
We thank J. Yin for technical support and N. Shah for discussion. AAV5-EF1a-DIO-h4MDi-mCherry virus was kindly provided as a gift from M. R. Bruchas’ lab. Virus preparation was performed by M. Li of the Hope Center Viral Vectors Core at Washington University School of Medicine. The data reported in this manuscript are tabulated in the main paper and in the supplementary materials. Supported by NIH-NIDA T32 training grant 5T32DA007261-23 and the W. M. Keck Fellowship (D.M.B.) and by NIH grants 1R01AR056318-06, R21 NS088861-01A1, R01NS094344, R01 DA037261-01A1, and R56 AR064294-01A1 (Z.-F.C.).
Footnotes
Author contributions: Y.-Q.Y., D.M.B., Y.H., and X.-T.L. participated in experimental design, performed tests, and analyzed the data; Z.-F.C. conceived and supervised the project; Z.-F.C. wrote the manuscript; and D.M.B. and Y.-Q.Y. contributed to the writing.
SUPPLEMENTARY MATERIALS
www.sciencemag.org/content/355/6329/1072/suppl/DC1
Materials and Methods
Figs. S1 to S6
Table S1
Movies S1 to S4
References (23–27)
REFERENCES AND NOTES
- 1.Provine RR. Behav. Brain Sci. 2014;37:216–217. doi: 10.1017/S0140525X13002458. [DOI] [PubMed] [Google Scholar]
- 2.Morgan CL. Habit and Instinct. Edward Arnold; London: 1896. [Google Scholar]
- 3.Zentall TR. Anim. Cogn. 2006;9:335–353. doi: 10.1007/s10071-006-0039-2. [DOI] [PubMed] [Google Scholar]
- 4.Schut C, Grossman S, Gieler U, Kupfer J, Yosipovitch G. Front. Hum. Neurosci. 2015;9:57. doi: 10.3389/fnhum.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galef BG. In: Social Learning: Psychological and Biological Perspectives. Zentall TR, Galef BG Jr, editors. Psychology Press; 1988. pp. 3–22. [Google Scholar]
- 6.Heyes CM. Anim. Behav. 1993;46:999–1010. [Google Scholar]
- 7.Thorpe WH. Learning and Instinct in Animals. Methuen; 1963. [Google Scholar]
- 8.Whiten A, Ham R. Adv. Stud. Behav. 1992;21:239–283. [Google Scholar]
- 9.Zhao ZQ, et al. J. Clin. Invest. 2013;123:4769–4780. doi: 10.1172/JCI70528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrahamson EE, Moore RY. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 11.Herzog ED. Nat. Rev. Neurosci. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- 12.Gamble KL, Allen GC, Zhou T, McMahon DG. J. Neurosci. 2007;27:12078–12087. doi: 10.1523/JNEUROSCI.1109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara K, Tominaga K, Isobe Y, Inouye ST. J. Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun YG, Chen ZF. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 15.Barry DM, et al. Mol. Pain. 2016;12 doi: 10.1177/1744806916643724. 1744806916643724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karatsoreos IN, Romeo RD, McEwen BS, Silver R. Eur. J. Neurosci. 2006;23:1047–1053. doi: 10.1111/j.1460-9568.2006.04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao ZQ, et al. J. Neurosci. 2014;34:12402–12414. doi: 10.1523/JNEUROSCI.1709-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun YG, et al. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenno L, Yizhar O, Deisseroth K. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langford DJ, et al. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 21.Panksepp JB, Lahvis GP. Neurosci. Biobehav. Rev. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeGates TA, Fernandez DC, Hattar S. Nat. Rev. Neurosci. 2014;15:443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]