INTRODUCTION
Mesial temporal lobe epilepsy (MTLE) is the most common cause of medication-resistant epilepsy, and decades of targeted drug discovery in epilepsy have not decreased the percentage of patients with MTLE suffering disabling seizures. For these patients, resective surgical treatment, as shown in a recent meta-analysis, leads to approximately 75% of patients becoming seizure free.1 The adverse surgical effects of open resection, whether anterior temporal lobectomy (ATL) or selective amygdalohippocampectomy (SAH), may be well tolerated in most circumstances. In the absence of other options, it has been emphasized that the benefits of seizure freedom outweigh the adverse effects, resulting in overall improvement in quality of life.2 Nevertheless, both ATL and SAH have significant effects on neurocognitive function(s), some related to the mesial resection (eg, declarative memory impairment), but some more related to the surgical approach to the mesial structures, that is, anterior-lateral temporal resection, division, or retraction during ATL or SAH.3–5 Cognitive declines related to the approach (collateral damage) impair naming and verbal learning (dominant hemisphere) or object recognition and figural learning (nondominant hemisphere). These impairments can be impactful and permanent, although the seizures become a distant memory. The potential of alternative, minimally invasive stereotactic procedures to accomplish the therapeutic goal of preventing seizures in the absence of such collateral damage may therefore increase the quality of life in surgical MTLE patients compared with traditional open resective procedures.
Stereotactic approaches to the mesial temporal lobe hold the promise of target ablation in the absence of collateral damage by avoiding injury as a result of the approach to the mesial temporal structures (Table 1). Several techniques have been explored over the last several decades.
Table 1.
Stereotactic ablation techniques
| Technique | Pros | Cons |
|---|---|---|
| SRFA | Reduced collateral damage Immediate benefit Low cost |
Temperature monitored only at tip Best results with string electrode not available in United States |
| Stereotactic radiosurgery (SRS) | Noninvasive | Delayed benefit after initial increase in seizures/risk of sudden unexpected death from epilepsy Potential radiation injury, dose limitations High cost |
| Laser interstitial thermal therapy (LITT) | Minimal collateral damage Immediate benefit Near real-time magnetic resonance thermography guides therapy and confirms ablation zone |
High cost/disposables |
With respect to stereotactic radiofrequency ablation (SRFA):
Parrent and Blume6 achieved a rate of 27% (4 of 15) seizure freedom in a subgroup of patients with many confluent lesions within the amygdala and hippocampus from a transtem-poral approach, orthogonal to the hippocampal long axis, spanning 21.5 mm (mean) of the hippocampus.
More recently, Liscak and colleagues7 working in the Czech Republic reported seizure-free rates of 78%, comparable with open resection, by using an occipital approach along the axis of the hippocampus and a string electrode that provides more extensive lesions radial to that axis. Longer (35 mm mean) and wider ablation zones, resulting from 16 to 38 lesions (mean 25), likely contributed to the increased success rate.
Noting the recently reported successes with SRFA, we explored the use of laser interstitial thermal therapy (LITT) for MTLE. During LITT, laser light is delivered fiber-optically into tissue (interstitial) via a stereotactic approach, where photonic energy causes local heating and thermocoagulation.8,9 Several critical advances have made modern LITT platforms into elegant and powerful neurosurgical tools (Box 1). The most critical advance is the ability to use MRI thermometry to measure not only the temperature at the device tip (the only temperature monitored in radiofrequency [RF] ablation) but also the temperature of tissue any distance from the tip during heating, thus providing near real-time confirmation of the ablation zone relative to off-target structures.
Box 1. Laser interstitial thermal therapy.
Optical fiber with diffusing tip (10 or 3 mm)
Cooling cannula to control thermal spread within tissue and protect device tip
984-nm diode laser causes rapid heating of water within tissue
Magnetic resonance thermography reads temperature change in all voxels
Six safety points to automatically shut off laser if temperature limits exceeded
Irreversible damage zone estimate at each voxel based on Arrhenius equation
The mechanism of heating, that is, photonic, is different from RF ablation, but the effects of temperature on tissue are the same (Fig. 1). However, with LITT, magnetic resonance (MR) thermography allows temperature monitoring beyond the laser tip, whereas in RF the thermocouple provides only temperature adjacent to the device tip. This technology allows protection of off-target tissue at risk in a way that is not possible with standard use of RF ablation. At present, 2 LITT devices are available in the United States that offer overlapping but distinctive features, only one of which (Visualase, Medtronic, Louisville, CO) we have used in our studies.
Fig. 1.
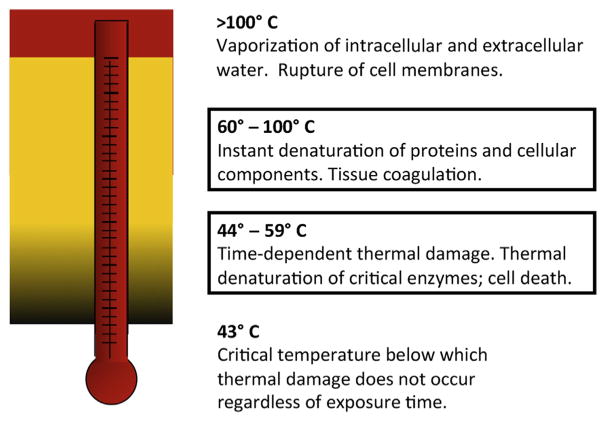
Effects of temperature on tissues. Both laser ablation and RF ablation have effects on tissues as a function of temperature. Lower than 44ºC, tissue effects are absent irrespective of time. Damage is time dependent between 44ºC and 59ºC; damage occurs as a function of time and temperature as predicted by the Arrhenius equation. This is the important temperature range for controllable lesions using LITT. At 60ºC or greater, tissue coagulation is instantaneous; these temperature effects occur close to the laser fiber diffuser tip. Instant vaporization occurs greater than 100ºC; the system is generally set to turn off automatically if the temperature near the tip reaches 90ºC to prevent damage to the tip.
PATIENT EVALUATION OVERVIEW
Patients undergoing LITT for thermocoagulation of the mesial temporal structures, which we termed stereotactic laser amygdalohippocampotomy (SLAH),10 undergo the standard workup of patients being considered for epilepsy surgery, and our criteria are similar to those for the recommendation of open resective surgery (Box 2). In this article, that evaluation is reviewed as it pertains specifically to SLAH. Our decision tree is described later; extensive discussion of indications for mesial temporal lobe surgery is beyond the scope of this article.
Box 2. Criteria for candidacy for stereotactic laser amygdalohippocampotomy.
Semiology: dyscognitive seizures consistent with mesial temporal onset, ± aura (typically smell, epigastric sensation, fear, déjà vu)
MRI: mesial temporal sclerosis positive (MTS +) or negative (MTS −)
PET: temporal lobe hypometabolism lateralized or greater on the same side as electroencephalography
Long-term video electroencephalographic monitoring: localization to anterior temporal region (eg, F7/T1 or F8/T2)
-
Neuropsychological testing: the following refers to normally organized memory function
MTS + or MTS −: domain-specific memory decline present on side of anticipated ablation
-
In absence of domain-specific memory decline referable to side of ablation:
MTS +: acceptable. If memory loss is asymmetric to contralateral side, intracarotid amobarbital test (ie, Wada test) is considered
-
MTS −:
Absence of visuospatial memory decline for nondominant ablation acceptable for ablation
Absence of verbal memory decline for dominant-side surgery: consider also nonablative surgical options (eg, responsive neurostimulation)
Intracranial electroencephalography (iEEG): in setting of ambiguity as to seizure onset zone from noninvasive studies alone, onsets with iEEG referable to ipsilateral mesial temporal lobe and absence of contralateral onsets
History
A careful history of medication usage is sufficient to identify medication-resistant epilepsy, defined as “failure of adequate trials of two tolerated, appropriately chosen, and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.”11 A history of typical mesial temporal lobe seizure semiology (dyscognitive) with or without aura is key; semiology suggestive of other onset zone(s) (eg, nighttime motor seizures suggesting frontal onsets) warrants additional investigation.
MRI Studies
MRI is sufficient to classify the presence or absence of mesial temporal sclerosis (MTS) and also to detect/exclude confounding lesions such as focal cortical dysplasias, secondary cortical gliosis, or other lesions that may indicate dual disease, a situation not uncommon in the temporal pole region.12
Metabolic and Blood Flow Imaging
Metabolic imaging (ie, fluorodeoxyglucose-PET [FDG-PET]) is not mandatory in the setting of unilateral MTS and concordant long-term video electroencephalographic [EEG] monitoring (LTVM; see later discussion). It is corroborative when bilateral MTS is present and should be concordant with LTVM; discrepancy should prompt consideration of intracranial EEG (iEEG) monitoring. We require FDG-PET unilateral hypometabolism in non-MTS cases, which, when concordant with LTVM, may allow proceeding directly to SLAH without iEEG monitoring in nondominant cases. However, we do maintain a low threshold for iEEG in non-MTS cases. We do not routinely require subtractive ictal/interictal single-photon emission computed tomography (CT), receptor imaging, or magneto-encephalography, except in diagnostically challenging cases.
Electrographic Studies
LTVM with scalp electrodes is often sufficient to recognize the seizure onset zone in the anteromesial temporal lobe and allows for qualitative and quantitative analysis of interictal epileptiform discharges. In situations in which the seizure onset is obscured or when questions of false lateralization or bilateral onsets arise, iEEG monitoring is used. We typically use depth electrodes inserted orthogonally to provide lateral and mesial temporal coverage. Additional depth electrodes may be required to investigate other regions of interest. In certain cases, percutaneous foramen ovale electrodes may be sufficient to clarify onset laterality but should not be used alone when there is a question of lateral versus mesial or temporal versus extratemporal onsets. We insert depth electrodes through anchor bolts using standard stereotactic techniques (eg, stereotactic head frame or robotic articulating arm) to minimize the skin opening. In anticipation of the possibility of a minimally invasive therapeutic procedure, we make every attempt to similarly provide a minimally invasive diagnostic procedure (ie, minimizing the use of subdural strip and grid electrodes when possible).
Neuropsychometric Evaluation
All patients undergo extensive preoperative evaluation by specialized neuropsychologists. These tests are used both to contribute to confirmation of the onset zone as reflected in relative areas of neurocognitive weakness (eg, material specific memory dysfunction) and also to prognosticate regarding potential loss of function after various surgical approaches, including SLAH.13 Results with respect to cognitive domains at risk also contribute to procedural decision making, that is, destructive versus nondestructive (neuromodulatory) therapeutic procedures. Further, change from baseline after surgery is informative both academically and clinically and guides referral for cognitive rehabilitation when indicated. Intracarotid amobarbital testing or the Wada test is performed on a case-by-case basis, as described later.14
PHARMACOLOGIC TREATMENT OPTIONS
Patients who are candidates for surgical procedures in epilepsy must be determined to be medication resistant, that is, they have tried at least 2 appropriately chosen drugs, appropriately used, and have not become seizure free.11 After 2 failed antiepileptic drug (AED) trials, Brodie and colleagues15–17 found that a third trial achieved only 8% and a fourth trial 4% long-term seizure freedom. However, this finding was recently reevaluated in a larger cohort, in which 18.5% and 16.5% were made seizure free with a third and fourth AED, respectively, and decreased to 0% after 5 or 6 previous AED failures.18 This re-evaluation of seizure intractability warrants consideration in terms of surgical options. In the setting of MTS in which the seizure-free rate is high and the risk low after both ablative and resective surgery, surgery may be considered earlier, that is, after 2 drug failures.19 Conversely, when the chance of seizure freedom after surgery is possibly lower, such as in the absence of MTS, and risk is correspondingly higher in the setting of preserved neurocognitive functions, surgical treatment with SLAH or resection might be delayed until after a more exhaustive regimen of AEDs or neuromodulation (discussed later) have been attempted.18
NONPHARMACOLOGIC TREATMENT OPTIONS
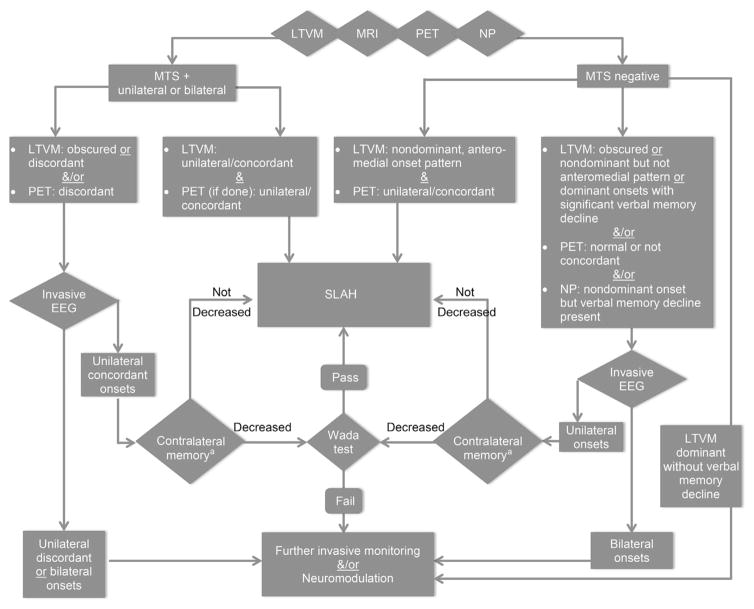
For patients with MTLE, there are other, albeit less effective, treatment alternatives to open resection and laser ablation. Both vagus nerve stimulation (VNS) and responsive neurostimulation (RNS) are approved by the US Food and Drug Administration, and anterior thalamic deep brain stimulation is approved in many countries and likely pending in the United States.20,21 These are options for patients considered poorer surgical (ie, resection or ablation) candidates, but none achieves the greater rates of seizure freedom associated with destructive surgery. We thus reserve neuromodulation for patients with MTLE in whom (1) onsets are bilateral or (2) onsets are unilateral from the dominant mesial temporal lobe and in whom verbal memory function is intact and conferred by that hemisphere. In particular, this situation occurs typically in the setting of the hippocampus that appears normal on MRI. When verbal memory is preserved in patients with dominant-side MTS, this domain is presumed to be conferred by the contralateral hemisphere and such patients remain ablation candidates. If clarification of this situation is sought, an asymmetric Wada test result showing relative weakness of the targeted versus the nontargeted side is reassuring (see later discussion and Fig. 2).
Fig. 2.
Decision tree for SLAH in patients with MTLE. All patients undergo comprehensive diagnostic evaluation for epilepsy, including history, seizure semiology, MRI, fluorodeoxyglucose-PET, LTVM, and neuropsychological testing (NP). Other diagnostic tests are performed as indicated. Therapeutic decision making occurs during comprehensive epilepsy surgery conference. This decision tree is a general guide to determine surgical candidacy and may vary from case to case depending on individual patient information. a Depicted is the indication for Wada testing for the purpose of determining contralateral memory performance, that is, nondominant associated visual memory when the focus is on the dominant side, and vice versa. However, Wada testing is almost always performed with bilateral carotid amobarbital injections, to also determine ipsilateral memory performance that may factor into decision making as well.
SURGICAL TREATMENT OPTIONS
The decision tree regarding surgical options (see Fig. 2) begins with candidacy for a mesial temporal resective or ablative procedure as determined by a comprehensive epilepsy team based on the diagnostic tests outlined earlier, the most pivotal factor being the presence or absence of MTS on MRI.
Patients with Mesial Temporal Sclerosis
MRI evidence of MTS has been recognized as an important factor in selecting patients with MTLE for resective surgery,1 and seizure freedom may be lower in patients in the absence of MTS.22 Our results, although limited with respect to patients who do not have MTS, support similarly higher rates of seizure freedom after SLAH in patients with MTS.10 Thus, patients with unilateral MTS and video EEG scalp monitoring providing electrographic evidence of unilateral ictal onsets confined to the anteromesial temporal lobe are excellent candidates for SLAH, with the following caveats:
The presence of contralateral ictal onsets or in-terictal spikes (a risk factor for surgical failure in open resection patients as well22) prompts invasive monitoring to rule out bilateral onsets or false lateralization. In the setting of welldocumented bilateral ictal temporal onsets, we generally offer RNS23 as a first-line procedure, considering unilateral SLAH for palliation if this is not feasible/desirable.
FDG-PET, if performed, should show concordant or predominantly ipsilateral temporal hypometabolism.
We use the intracarotid amobarbital (Wada) test to probe the ability of the contralateral hemisphere to support memory only in the presence of evidence that it is compromised, such as when there is more than mild visual memory loss (nondominant hemisphere function) in a dominant-side onset case, and vice versa. In the setting of a failed Wada test (inability of the contralateral hemisphere to support memory function), if there is a question of cross flow to the contralateral internal carotid artery, we may repeat the test with an ipsilateral injection in the posterior cerebral artery (so-called selective Wada) but otherwise give strong consideration to a neuromodulation procedure (eg, VNS or RNS) rather than SLAH, irrespective of side.
As with resection, in the setting of patients with MTS, the potential for memory decline is mitigated by the damage to the mesial structures already manifested both on imaging and in memory testing. Nevertheless, patients are cautioned about risk of some noticeable decline being possible for dominant-side SLAH. In the absence of preoperative verbal memory decline in the setting of dominant-side MTS, it is presumed that there exists some contralateral mediation of verbal memory. In this setting, a strongly asymmetric Wada test showing ipsilaterally poor performance is reassuring, but risk of verbal memory decline with SLAH is not completely excluded and patients are thus counseled. Although our preliminary data suggest less impact on memory of SLAH compared with open resection, this conclusion awaits analysis of a larger group.
It has become our routine to offer dominant SLAH as the only first-line option for patients with MTS because of the significant potential for collateral damage and associated naming deficits associated with open resective approaches to the dominant side.24 In those patients who do not achieve seizure freedom, reablation is considered if delayed postoperative MRI shows persistent hippocampal or amygdalar remnants, but open resection may be considered as well.
Patients Without Mesial Temporal Sclerosis
Patients with MTLE without MTS may be at higher risk for failure after open temporal lobe surgery.25 It is conceivable that a more focal procedure such as SLAH results in inferior rates of seizure freedom, but this is by no means certain, because in many cases, invasive recordings show onsets originating from the hippocampus in patients without MTS. Conversely, neurocognitive issues sway us to consider SLAH in patients with MTLE without MTS over open resection for 2 reasons: (1) patients without MTS are similarly (or more so) at risk for collateral damage from open procedures than are patients with MTS; and (2) memory is generally more preserved in patients without MTS; selective ablation of the amygdalohippocampal complex (relatively preserving entorhinal/parahippocampal cortex) is certainly not more deleterious to memory, and may be less so, compared with open resection. However, the seizure and neurocognitive outcomes in these groups remain to be determined. In our initial series, only 1 of 4 patients without MTS maintained seizure freedom for 6 months10 and 2 years (R. Gross, J. Willie, unpublished observations, 2015). However, 1 of the patients who is not seizure free has a reduction in seizures greater than 85% and although 9 seizures localized unilaterally during preoperative LTVM, post-SLAH LTVM captured a contralateral seizure only. Another patient became seizure free for greater than 12 months after repeat SLAH targeting residual amygdalohippocampal tissue. Thus, this small sample size may underestimate the effectiveness of SLAH on the operated side even in patients without MTS.
Our present approach, therefore, involves offering SLAH to patients without MTS with onsets on the nondominant side in whom loss of visual memory function has been considered less consequential. However, because emerging data suggest underappreciation for at-risk temporal lobe functions (eg, recognition of familiar faces, landmarks, and animals; theory of mind and other more subtle aspects of social processing), we do discuss potential at-risk functions with our patients.5,24,26,27 If a patient desires the most expeditious pathway to seizure freedom, we perform nondominant open resection if desired. If seizure freedom is not obtained after SLAH, repeat ablation or open resection is offered; LTVM is repeated only if there is a change of semiology or if there is a significant decrease in seizure frequency, suggesting the possibility that unrecognized contralateral seizures may account for the recurrence. On the other hand, we do not offer SLAH as a first-line measure to dominant onset patients without MTS unless there has already been significant loss of verbal memory, because such patients are at significant risk to memory in the setting of possible decreased chance of benefit; such patients are considered for neuromodulation. In the setting of dominant onset patients without MTS with more than mild verbal memory decline, and for patients who do not want neuromodulation and are willing to assume the risk of memory dysfunction on intensive preoperative counseling, we do offer SLAH and do not offer resection because of the risk to naming function of the latter. Open resection is offered only as a salvage measure if SLAH is not effective. One patient without MTS in our initial report (hippocampal T2 signal changes only) did not become seizure free with repeat ablation but did with subsequent ATL, whereas a more recent patient did not become seizure free after open SAH after failed SLAH. Such patients remain challenging. Routinely performing invasive monitoring with depth electrodes may be advisable in the evaluation for SLAH in patients without MTS. In our more extended series, 3 of 4 patients without MTS that localized to the hippocampus with depth electrodes have become seizure free (R. Gross, J. Willie, unpublished observations, 2015).
STEREOTACTIC LASER AMYGDALOHIPPOCAMPECTOMY: PROCEDURE
Stereotactic Planning
Stereotactic planning may be performed on any navigation workstation and can be performed ahead of time. A volumetric T1-weighted sequence with gadolinium contrast enhancement is necessary and can be supplemented with a T2 volumetric series.
Choose an initial target point in the center of the pes hippocampus, that is, dentate gyrus
Choose entry point in center of hippocampus at coronal level between the lateral mesence-phalic sulcus and the tectal plate
-
Extend the entry point to the skull; adjust the trajectory to:
Avoid cerebral veins and deep arterial branches
Avoid the ventricle (if possible); this usually involves an approach inferior to the occipital horn of the ventricle
Avoid the choroid plexus (if possible)
Keep the trajectory in the center of the hippocampus (dorsoventrally and mediolaterally)
Extend the target point along the existing trajectory to just anterior to the amygdala
Stereotactic Insertion of Laser Fiber Assembly
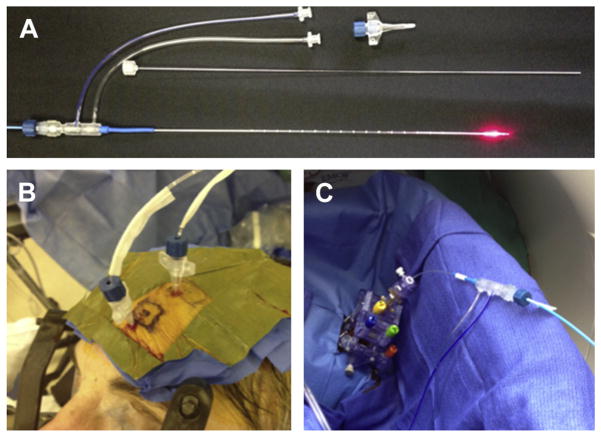
Several techniques are available to insert the laser fiber assembly; our experience has been with the Visualase system (Medtronic, Louisville, CO) (Fig. 3). Any stereotactic approach may be used that maximizes stereotactic accuracy, including frame-based systems, frameless systems, or robot-assisted systems. However, a high level of accuracy is required because of the need to be centered within the hippocampus (coronally) and the constraints imposed by the need for accuracy all along the longitudinal trajectory.
Fig. 3.
Hardware components for SLAH using Visualase laser thermal therapy system using different ste-reotactic methods. (A) Fifteen-Watt 980-nm diode laser energy is directed along a 400-μm core silica optical fiber that terminates in a circumferential diffusing tip (red). This fiber optic is housed within a 1.65-mmdiameter saline-cooled polycarbonate cooling cannula (bottom). A threaded plastic bone anchor (top right) and stiffening stylet (middle) are used to stereo-tactically deliver the device to brain structures. (B) Stab incisions and 3.2-mm twist drill holes are made using a stereotactic headframe. Anchor bolts are threaded into twist holes under stereotactic control; Visualase laser applicators are passed through bolts, secured, and flagged with sterile adhesive strips. (C) Alternative direct real-time MRI-guided placement of Visualase laser applicator via an MRI guidance miniframe (SmartFrame, MRI Interventions, Irvine, CA) within an MRI suite. ( From Willie JT, Tung JK, Gross RE. MRI-guided stereotactic laser ablation. Chapter 16. In: Golby AJ, editor. Image-guided neurosurgery. Boston: Academic Press; 2015. p. 380; with permission.)
The laser fiber assembly can be inserted through a stereotactically implanted anchor bolt in the operating room (OR) using a frame or frameless system (see Fig. 3). In this implementation, the following steps are taken:
-
Insertion through anchor bolt in OR
Affix stereotactic frame. We perform this procedure under general anesthesia because it is unwise to perform procedures in awake epileptic patients unless necessary. This strategy avoids needing to intubate a patient with the frame in place; this could also be done with laryngeal mask anesthesia.
Imaging. Obtain volumetric contrast-enhanced MRI or contrast-enhanced CT merged with historical MRI.
Plan trajectory. As described earlier.
Positioning. Position supine with head flexed and maximally elevated (near sitting position) and anchor frame to table base unit. Minimal hair clipping followed by preparation and draping.
Stereotactic twist drill craniostomy. Use minimal stab incision.
Radiological control. Align C-arm fluoroscopy or intraoperative CT scanner.
Insert anchor bolt. Insert rod through frame bushing and then through the anchor bolt under fluoroscopic or CT control to make sure rod, and thus the anchor bolt, accurately achieves stereotactic target.
Insert cooling cannula. Remove rod and insert cooling cannula with inner stylet under guidance.
Insert fiber optic with diffusor tip. Remove stylet and insert optical fiber and secure. We use a 10-mm diffuser tip.
Remove frame arc but leave base ring in place.
Transport to the MRI suite. Patient positioned supine on the table with head turned to protect the laser assembly.
Confirm cannula accuracy and obtain scan planes for temperature mapping. If cannula is not accurately placed, the patient must be returned to the OR for repositioning.
Our alternative technique uses an MRI targeting platform in the MRI suite or intraoperative MR. This technique (1) allows the best three-dimensional radiologic targeting control and (2) avoids the need for transport of the patient during the procedure.
-
Insertion using MRI targeting platform. We use the Clearpoint system (MRI Interventions, Irvine, CA) (see Fig. 3).
General anesthesia. General anesthesia is established in the MRI suite or OR.
Positioning. The patient is placed prone on the MRI table on bolsters and padded. The head is flexed and secured in skull pins. Clip hair, prepare, and drape. Affix a fiducial grid over the likely entry site, essentially 2 to 3 cm above the pinna, halfway between the pinna and the inion.28
Imaging. Volumetric T1 (eg, magnetization-prepared rapid gradient echo [MPRAGE]) with contrast.
Plan trajectory. As described earlier using Clearpoint workstation.
Affix scalp-mount Smartframe base and tower. This apparatus is centered over the entry point noted by the Clearpoint software and projected onto the skin using the fiducial grid.
Cannula alignment. This procedure is performed iteratively using serial slab MRI volumes guided by the software.
Twist drill craniotomy. Use minimal stab incision.
Insert ceramic stylet. Targeting accuracy is confirmed with a ceramic stylet with minimal image artifact inserted to target.
Insert cooling cannula. Replace ceramic with cooling cannula with stylet inserted to prescribed depth.
Insert optical fiber with diffusor tip. Remove stylet and insert optical fiber and secure. We use a 10-mm diffuser tip.
Confirm cannula accuracy and obtain scan planes for temperature mapping.
Laser Ablation with MRI Thermography
Scan planes. Up to 3 scan planes (generally at least para-axially and parasagittally along the axis of the cannula) can be monitored with temperature mapping (T-maps) during the procedure. Additional planes increase the time between temperature updates. Although an additional coronal plane may be useful for monitoring the optic tract, new coronal scan planes would be necessary for each lesion along the longitudinal axis of the hippocampus.
Attach optical fiber to laser power source; attach irrigation tubing. Confirm return flow of the irrigation before using laser.
Choose safety monitoring points. The temperature at up to 6 points can be monitored. Typically 3 high points are chosen near the diffuser tip to ensure the temperature does not exceed 9ºC to protect the tip. Three points are chosen on the brainstem, thalamus and optic tract to automatically turn off laser if temperature exceeds 45ºC to 50ºC.
Low-power LITT. The laser is activated at 30% power (4.5 W) to verify the location of the diffuser tip; this can be translated in or out as needed. We begin the ablation at the anterior border of the amygdala.
Create ablation. We typically create the first few overlapping ablations at 65% to 70% power (Fig. 4, Video 1). Each lesion is carried out until the pixels indicating the irreversible damage zone plateau, typically after 2 but no more than 3 minutes at a time. The cannula is then retracted 5 to 8 mm and overlapping ablations are serially performed as far back, but no further than, the tectal plate. For the posterior ablations, 60% power is sufficient and protects the optic radiation which is vulnerable here, as the diffuser tip may exit the hippocampus, and due to the narrowness of the lateral ventricle at this point.
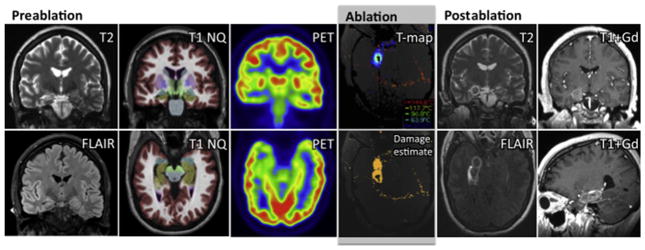
Postablation imaging. The immediate effects of the ablation are apparent on diffusion-weighted imaging, fluid-attenuated inversion recovery, T2, and gadolinium-enhanced T1 (eg, MPRAGE) imaging, the last of which shows the area of blood-brain barrier breakdown at the periphery of the lesion (see Fig. 4).
Remove laser assembly, remove frame, single ligature of skin.
Fig. 4.
Imaging associated with a case of SLAH for right MTS. Preablation diagnostic MRI (coronal T2 and fluid-attenuated inversion recovery [FLAIR], first column) show typical features of right hippocampal atrophy and mildly increased T2 and FLAIR intensity. Coronal and axial T1 with NeuroQuant (CorTechs Laboratory, San Diego, CA) analysis (second column) with colorized mesial temporal structures (amygdala in blue, hippocampus in brown) further exhibit right MTS. Coronal and axial FDG-PET images (third column) emphasize reduced hypome-tabolism of right mesial temporal structures. Visualase workstation screenshots during SLAH procedure (fourth column) show real-time axial MR gradient-based T-map during LITT in amygdala region (above) and combined irreversible damage estimate encompassing mesial temporal structures at time of procedure completion (below). Immediate postablation MR images show features after LITT including T2 (coronal) and FLAIR (axial) hypointense rings (fifth column), and peripheral contrast enhancement surrounding T1 hypointensity (coronal/sagittal gadolinium [Gd]-contrasted T1 images, sixth column).
Postablation Management
The patient is typically monitored for 1 night on the floor unit. No intensive care unit monitoring is required in the absence of a complication.
Steroids are administered for 1 to 2 weeks. Transient headache is a common complaint after the procedure; this may be related to in-flammogens released by the lesion coming in contact with the dura (eg, tentorial edge) but could also be related to local mass effect.
Discharge on the day after surgery is typical.
Antiepileptic medication is continued without change. We taper medications to some degree (eg, decreasing dosage[s] or eliminating a medication) if the patient remains seizurefree at 6 months but never discontinue medications completely until 2 years.
Postoperative imaging beyond that acquired at the completion of the procedure is obtained only in the event of seizure recurrence.
TREATMENT RESISTANCE
Even in highly selected patients (eg, MTS), open resection is associated with a ~25% failure rate.1 Some failed patients can be made seizure free by repeat surgery.29 When patients are not seizure free after SLAH, we evaluate delayed MRI for remnant hippocampus/amygdala (although we never attempt full ablation of the most superior aspect of the amygdala because of continuity with basal ganglia and proximity to the optic tract). If remnants are present, these are targeted with a repeat procedure. Typically, remnant cortex is either mesial (subiculum) or lateral pes hippocampus or posterior body. Whereas entorhinal cortex (ECtx) and parahippocampal gyrus (PHG) are typically resected during open amygdalohippocampectomy, we do not target these in our initial procedure, and it remains uncertain whether PHG should be ablated during repeat procedures. Because the preservation of this region conceivably could account in part for better neurocognitive outcome after SLAH compared with open resection (particularly memory functions), we have generally elected not to target ECtx/PHG in our dominant-sided reablations if there is persistent mesial temporal cortex that might account for failure but have performed more maximal ablations on the nondominant side. However, the precise neuro-cognitive consequences of repeated and more extensive ablations remain to be determined with more patients. Of 5 reablated patients with sufficient follow-up, 3 have become seizure free for 6 months or more. Conversely, only 1 of 3 patients who underwent ATL after SLAH became seizure free; 1 of these 3 patients had recurrent ipsilateral, contralateral, and psychogenic nonepileptic seizures, 1 had normal MRI, and the other had another possible ictal focus. This finding serves to emphasize that reasons for failure to control seizures may be assorted and multifactorial after both open resection and laser ablative surgery.
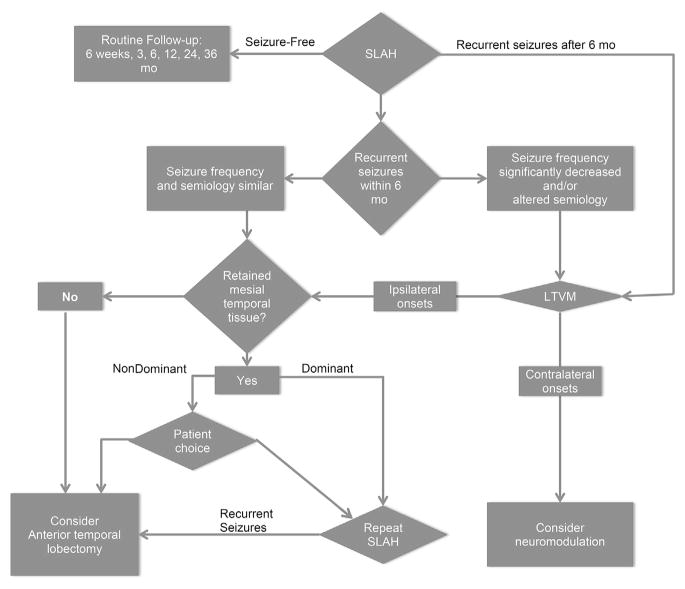
Our approach to recurrent seizures after SLAH is as follows (Fig. 5). Some seizures in the acute perioperative period (~6 weeks) do not necessarily indicate failure (winding-down phenomenon). However, persistent seizures after 2 months have invariably indicated failure and, in our experience, no such patient has experienced an extended seizure-free interval after this point. Thus, we evaluate patients for a follow-up procedure if recurrent seizures are present after 4 to 6 months. If seizures are of the same semiology as preoperatively, then further LTVM is not required before repeat SLAH. If there is persistent hippocampal or amygdalar tissue (uncus), then repeat ablation is offered. We offer open resection as an alternative in the case of seizure recurrence in patients after nondominant ablation. However, in the setting of dominant temporal lobe seizure recurrence, we avoid open resection if there is remnant tissue that may be ablated during a repeat procedure, because of the tangible risk of naming deficits with either dominant-sided ATL or SAH. Moreover, if we consider an open resection on the dominant side, we perform LTVM first to ensure that the recurrent seizures are still coming from the side of the previous ablation. Neurocognitive assessment and MRI are repeated before the patient undergoes a repeat procedure.
Fig. 5.
Decision tree for further surgical treatment in non–seizure-free patients after SLAH. This is a guide to our decision making process in patients who are not seizure free after SLAH. Decision making may vary in a case-by-case manner depending on individual information and preferences.
COMPLICATIONS
Complications (Table 2) from SLAH are related to the stereotactic approach, that is, brain penetration leading to hemorrhage and thermal effects on nearby structures. In 49 SLAH including 6 repeat procedures, we have experienced 2 tract hemorrhages: 1 small acute subdural hemorrhage and 1 temporo-occipital lobar hematoma associated with a transient visual field defect. Thermal injury can affect nearby visual pathways. One patient early on in the series experienced a subtotal hemianopia most likely related to superior cannula deviation as seen on fluorography; there was no enhancement within the visual pathways to indicate thermal lesion. Two other patients experienced a non-disabling superior quadrantanopia noted on clinical exam and field testing; however, we do not routinely perform postoperative visual field testing unless a patient is noted to have a field deficit on clinical confrontation testing. We believe the occurrence of a superior quadrantanopia can occur if a lesion is made too far posterolateral where the trajectory can encounter the optic radiation. Another location for thermal injury involves cranial nerves 3 and 4 if the lesion is made more medially near the tentorium. We have experienced 1 partial transient third nerve palsy with aggressive uncal ablation and 1 partial transient fourth nerve palsy with a repeat procedure to reablate remnant medial tissue at the subiculum/ECtx. Both partial deficits responded rapidly to steroids.
Table 2.
Complications of stereotactic laser amygdalohippocampotomy
| Complication | Incidence in 49 Procedures (6 Reoperations) (n) (%) |
|---|---|
| Hemorrhage | 2 (4.1) |
| Visual field deficit (total) | 4 (8.2) |
| Transient (superior quadrantanopia) | 1 (2.0) |
| Persistent | 3 (6.1) |
| Homonymous heminanopia | 1 (2.0) |
| Superior quadrantanopia | 2 (4.1) |
| Cranial nerve deficit (transient) | 2 (4.1) |
| CN3 | 1 (2.0) |
| CN4 | 1 (2.0) |
EVALUATION OF OUTCOME AND LONG-TERM RECOMMENDATIONS
Engel seizure classification scale is used to report outcome at 1 year after surgery. We have previously reported that 7 of our first 13 patients (54%) were seizure free at 6 months.10 All remained seizure free at 1 year follow-up, except 1 patient having a single seizure only with rapid taper of lacosamide. All 7 remained seizure free at median 25 months (15–36 months) with the following exceptions: 1 patient had a single seizure with a dose of meperidine; 1 patient had 2 seizures with rapid taper of levatiracetam and concurrent hyponatremia; and 1 patient had 8 seizures over 4 months after discontinuing all of his seizure medications and binge drinking alcohol. In the last patient, control was regained with medications and he has been seizure free for an additional 12 months. Also, 1 patient from that initial series underwent repeat SLAH, resulting in seizure freedom for 1 year. Thus, the seizure-free rate at 12 months in this initial cohort has improved to 61.5%. Critically, 6 of 9 patients with MTS (67%) and 2 of 4 patients without MTS (50%) from the initial cohort are seizure free. The data from our larger series of 32 patients at 12 months follow-up are likewise consistent with these results (R. Gross, J. Willie, unpublished observations, 2015).
SUMMARY AND DISCUSSION
Open temporal lobe surgery for MTLE, having been performed in various forms for more than a half-century, is well established and effective. Class 1 evidence showed that 64% of operated patients became seizure free after ATL30 and meta-analyses of numerous series of ATL and SAH1 indicated that on average 75% of patients became seizure free for at least 1 year. Further, ATL and SAH are well-tolerated procedures that improve quality of life.31 Nevertheless, we and others have shown that open resection is associated with previously unrecognized neurocognitive deficits beyond that related purely to the amygdalohippocampectomy,5 as a result of collateral damage to the temporal lobe during the approach to the mesial temporal structures. This situation may be avoided with less invasive stereotactic procedures (see Table 1), but LITT has additional practical advantages over the alternatives.
We have shown in our initial series that SLAH is effective in eliminating seizures for follow-up periods of 15 to 36 months. As with open resection, superior results are obtained in patients with MTS (~70% seizure free thus far), rivaling the results from ATL and SAH. Patients failing to meet radiographic criteria for MTS are less likely to remain seizure free, but our experience with this group is too small to draw any conclusions, and these patients may go on to have additional procedures if indicated. However, we think it is important to continue to offer SLAH to patients without MTS for reasons discussed.
There are 2 areas in which SLAH offers distinct advantages over, and for these reasons may supplant, open resective surgery for MTLE as a first-line procedure for medication-resistant patients. First and foremost is the reduction of collateral damage to the lateral temporal structures, both gray and white matter, sparing patients from unnecessary neurocognitive deficits. We do not wish to underestimate the overriding importance of improvements in quality of life from seizure freedom after ATL/SAH. Nevertheless, neurocognitive deficits can remain bothersome and even disabling, especially in the dominant temporal lobe. Even when seizures are a distant memory, persistent deficits are not. Achieving seizure freedom and maximizing cognitive function by SLAH offers patients a distinct win/win. Second, as a so-called minimally invasive procedure, with a 1-stitch incision, SLAH is better tolerated and desirable to patients than even keyhole open resection, with no craniotomy and extracranial tissue injury. Postoperative intensive care admission/observation is obviated, and hospitalizations are brief. Many patients can return to work within days of SLAH.
Although it is clear to us that SLAH is the preferred treatment of patients with MTS and MTLE because of rates of seizure freedom comparable with open resection, the path remains less obvious for patients without MTS. Perhaps an isolated amygdalohippocampal ablation is too selective in the setting of uncertainty as to the onset region in these patients. However, in as much as the seizure network in this increasingly significant group of patients is poorly understood, a potentially superior procedure should not be withheld because of assumptions. Given the certain minimization of cognitive deficits, we believe it is important to explore the role of SLAH even in patients without MTS. It is for this reason that we also do not presuppose that such patients initially require more widespread ablations to include the PHG or the temporal pole. Perhaps we will learn that this factor is useful in the course of time, but to presuppose this may commit us to a larger surgery with greater deficits and preclude the opportunity to determine the minimum necessary substrate for achieving seizure freedom in various patient populations. The answer to the debate over “less is more” versus “more is more” is that “just right is more.” The chance to iteratively enlarge the ablation/resection allows us to answer this question while we still have equipoise and while not subjecting patients to excessive risk. However, decisions are made in partnership with patients and caregivers. In particular cases, circumstances may be such (eg, limited social or economic resources) that we may have only 1 opportunity to intervene and provide the patient with a chance to be seizure free. If such patients can be identified prospectively (“we can always go back” is not always true), a resection rather than an ablation may be on balance more appropriate even on the dominant side.
The aforementioned considerations may have health care economic impacts. There clearly are savings from avoidance of intensive care unit observation as is routine after a craniotomy, and with nearly all patients being discharged on the morning after SLAH. Moreover, surgical time in the OR is decreased, and complications and discomfort related to craniotomy are eliminated. Conversely, there are increased technology expenses related to the disposable laser fiber assembly, the capital expense of the laser and computer workstation, and the opportunity cost of appropriating a diagnostic MRI scanner for interventional procedures that are more time consuming and potentially less profitable. The overall balance sheet with respect to these considerations is a topic we are pursuing.
SLAH has the potential to have even greater impact, related to its minimally invasive nature, by bringing more people with medication-resistant MTLE (and other forms of focal epilepsy) to surgical treatment. Epilepsy surgery use has lagged for decades, including for MTLE,32 despite class 1 evidence supporting its effectiveness and safety since 2001.30 This situation is related to reluctance by both patients and referring neurologists because of the invasiveness and side effects of open surgery. We have performed laser ablation on epileptic patients who have clearly stated that they would not consider open resection but were amenable to laser ablation. In this regard, comparison of seizure-free rates after SLAH to open resection captures only part of the picture; rather, for patients who accept this procedure but refuse open resection, the true comparator is best medical therapy, with its 8% chance of seizure freedom.30 If one of the impacts of laser ablation is to bring more patients with epilepsy to surgery, with downstream benefits of decreasing medical complications (including sudden unexpected death from epilepsy) and increasing socioeconomic status, it will be a profound and persistent benefit to the community with epilepsy.
Supplementary Material
KEY POINTS.
Stereotactic laser amygdalohippocampotomy (SLAH) is a minimally invasive approach to the treatment of medication-resistant mesial temporal lobe epilepsy that accomplishes ablation of the seizure focus with real-time magnetic resonance thermal mapping.
Seizure-free rates in early series suggest that SLAH approaches the effectiveness of open resection for patients with mesial temporal sclerosis (MTS).
SLAH avoids neurocognitive adverse effects of open resection on naming (dominant side) and object recognition (nondominant side).
Although early data suggest more preserved memory function after SLAH, further research is required. Thus, patients with relatively preserved memory in the absence of MTS are offered SLAH only after careful considerations of risks, benefits, and alternative procedures, including neuromodulation.
Secondary benefits of SLAH include decreased length of stay, elimination of intensive care unit stay, reduced procedure-related discomfort, and improved access to surgical treatment for patients less likely to consider an open resective procedure.
Footnotes
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.nec.2015.08.004.
Disclosures: Funding was provided to Emory University by way of a clinical study agreement from Visualase, which developed products related to the research described in this article. In addition, R.E. Gross served as a consultant to Visualase and Medtronic and received compensation for these services. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. D.L. Drane receives funding from the NIH/NINDS (K02 NS070960), which provides support for his work.
References
- 1.Josephson CB, Dykeman J, Fiest KM, et al. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013;80(18):1669–76. doi: 10.1212/WNL.0b013e3182904f82. [DOI] [PubMed] [Google Scholar]
- 2.Mohammed HS, Kaufman CB, Limbrick DD, et al. Impact of epilepsy surgery on seizure control and quality of life: a 26-year follow-up study. Epilepsia. 2012;53(4):712–20. doi: 10.1111/j.1528-1167.2011.03398.x. [DOI] [PubMed] [Google Scholar]
- 3.Helmstaedter C. Cognitive outcomes of different surgical approaches in temporal lobe epilepsy. Epileptic Disord. 2013;15(3):221–39. doi: 10.1684/epd.2013.0587. [DOI] [PubMed] [Google Scholar]
- 4.Baxendale S, Thompson PJ, Sander JW. Neuropsychological outcomes in epilepsy surgery patients with unilateral hippocampal sclerosis and good pre-operative memory function. Epilepsia. 2013;54(9):131–4. doi: 10.1111/epi.12319. [DOI] [PubMed] [Google Scholar]
- 5.Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56:101–13. doi: 10.1111/epi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parrent AG, Blume WT. Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe. Epilepsia. 1999;40(10):1408–16. doi: 10.1111/j.1528-1157.1999.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 7.Liscak R, Malikova H, Kalina M, et al. Stereotactic radiofrequency amygdalohippocampectomy in the treatment of mesial temporal lobe. Acta Neurochir (Wien) 2010;152(8):1291–8. doi: 10.1007/s00701-010-0637-2. [DOI] [PubMed] [Google Scholar]
- 8.Bown SG. Phototherapy in tumors. World J Surg. 1983;7(6):700–9. doi: 10.1007/BF01655209. [DOI] [PubMed] [Google Scholar]
- 9.Curry DJ, Gowda A, McNichols RJ, et al. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012;24(4):408–14. doi: 10.1016/j.yebeh.2012.04.135. [DOI] [PubMed] [Google Scholar]
- 10.Willie JT, Laxpati NG, Drane DL, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74(6):569–84. doi: 10.1227/NEU.0000000000000343. discussion: 584–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 12.Chabardes S, Kahane P, Minotti L, et al. The temporopolar cortex plays a pivotal role in temporal lobe seizures. Brain. 2005;128(Pt 8):1818–31. doi: 10.1093/brain/awh512. [DOI] [PubMed] [Google Scholar]
- 13.Drane DL. Neuropsychological evaluation of the epilepsy surgical candidate. In: Barr WB, Morrison C, editors. Handbook on the neuropsychology or epilepsy. New York: Springer; 2015. pp. 87–122. [Google Scholar]
- 14.Loring DW, Meador KJ, Lee GP, et al. Amobarbital effects and lateralized brain function: the Wada test. New York: Springer-Verlag; 1992. [Google Scholar]
- 15.Kwan P, Brodie MJ. Refractory epilepsy: mechanisms and solutions. Expert Rev Neurother. 2006;6(3):397–406. doi: 10.1586/14737175.6.3.397. [DOI] [PubMed] [Google Scholar]
- 16.Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol. 2006;13(3):277–82. doi: 10.1111/j.1468-1331.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- 17.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 18.Schiller Y, Najjar Y. Quantifying the response to antiepileptic drugs: effect of past treatment history. Neurology. 2008;70(1):54–65. doi: 10.1212/01.wnl.0000286959.22040.6e. [DOI] [PubMed] [Google Scholar]
- 19.Engel J. Early surgical therapy for drug-resistant temporal lobe epilepsy. a randomized trial. JAMA. 2012;307(9):922. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics. 2014;11(3):508–26. doi: 10.1007/s13311-014-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Sharan AD. Neurostimulation for the treatment of epilepsy: a review of current surgical interventions. Neuromodulation. 2013;16(1):10–24. doi: 10.1111/j.1525-1403.2012.00501.x. discussion: 24. [DOI] [PubMed] [Google Scholar]
- 22.Bell ML, Rao S, So EL, et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2009;50(9):2053–60. doi: 10.1111/j.1528-1167.2009.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrell MJ RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 24.Drane DL, Ojemann GA, Aylward E, et al. Category-specific naming and recognition deficits in temporal lobe epilepsy. Neuropsychologia. 2008;46(5):1242–55. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wassenaar M, Leijten FS, Egberts TC, et al. Prognostic factors for medically intractable epilepsy: a systematic review. Epilepsy Res. 2013;106(3):301–10. doi: 10.1016/j.eplepsyres.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Crane J, Milner B. Do I know you? Face perception and memory in patients with selective amygdalohippocampectomy. Neuropsychologia. 2002;40(5):530–8. doi: 10.1016/s0028-3932(01)00131-2. [DOI] [PubMed] [Google Scholar]
- 27.Drane DL, Ojemann GA, Ojemann JG, et al. Category-specific recognition and naming deficits following resection of a right anterior temporal lobe tumor in a patient with atypical language lateralization. Cortex. 2009;45(5):630–40. doi: 10.1016/j.cortex.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, LaRiviere MJ, Laxpati N, et al. Extraventricular long-axis cannulation of the hippocampus: technical considerations. Neurosurgery. 2014;10(Suppl 2):325–32. doi: 10.1227/NEU.0000000000000320. discussion: 332–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germano IM, Poulin N, Olivier A. Reoperation for recurrent temporal lobe epilepsy. J Neurosurg. 1994;81(1):31–6. doi: 10.3171/jns.1994.81.1.0031. [DOI] [PubMed] [Google Scholar]
- 30.Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 31.Spencer SS, Berg AT, Vickrey BG, et al. Health-related quality of life over time since resective epilepsy surgery. Ann Neurol. 2007;62(4):327–34. doi: 10.1002/ana.21131. [DOI] [PubMed] [Google Scholar]
- 32.Englot DJ, Ouyang D, Garcia PA, et al. Epilepsy surgery trends in the United States, 1990–2008. Neurology. 2012;78(16):1200–6. doi: 10.1212/WNL.0b013e318250d7ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.