Abstract
There are several forms of brain-derived neurotrophic factor (BDNF), the precursor of BDNF, mature BDNF, and BDNF propeptide. They exert different effects through different transmembrane receptor signaling systems. Precursor of BDNF is enzymatically cleaved, either by intracellular or by extracellular proteases, to generate mature BDNF and its propeptide (BDNF propeptide). The aim of this study was to evaluate the potential molecular mechanisms that underlie the inhibition of glioma cell growth by the BDNF propeptide. To achieve this, we examined the expression of BDNF propeptide in C6 glioma cells. The 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide assay and the apoptosis assay were used to assess the effects of the BDNF propeptide on the growth and apoptosis of glioma cells. We found that the BDNF propeptide promoted C6 glioma cell apoptosis and decreased in-vitro cell growth. We also found using western blot that cleaved caspase3 and B cell lymphoma 2 (Bcl2)-associated X protein abundances increased, whereas Bcl2 abundance decreased. Our data suggest that the BDNF propeptide may have an inhibitory effect on glioma through activation of the caspase3 pathway.
Keywords: brain-derived neurotrophic factor propeptide, cell death, cell survival, glioma cell
Introduction
Gliomas are tumors derived from neuroepithelial tissue that typically arise in the brain or the spine. High-grade malignant glioma is the most common form of primary glioma in adults, with a median survival of 9–12 months after diagnosis. Treatment failure can arise because of invasive growth of glioma cells around normal brain tissue 1,2. This can make such tumors difficult to remove completely by surgery 2. It is therefore essential to further understand the biological characteristics of glioma cells.
Brain-derived neurotrophic factor (BDNF) is considered an oncogenic factor and is involved in tumorigenesis, tumor proliferation, and tumor survival. It is also associated with a poor prognosis in central nervous system (CNS) tumors 3–5. BDNF is synthesized as proBDNF, and endoproteolytically processed into mature brain-derived neurotrophic factor (mBDNF) and BDNF propeptide 6. There is increasing evidence to suggest that the counterbalance in the ratios of mBDNF and proBDNF may regulate glioma cell growth 7. However, the functions of BDNF propeptide in glioma cells are currently unknown. The aim of our study was to investigate whether the BDNF propeptide promotes C6 glioma cell apoptosis and decreased cell growth in vitro.
Materials and methods
C6 glioma cells culture
C6 glioma cells were grown in low-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Gaithersburg, Maryland, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 1% glutamate, and 1% penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
DNA construct and purification of the BDNF propeptide
A DNA sequence that encodes for the human BDNF propeptide up to the furin cleavage site was inserted between the NdeI and SpeI sites of a pTXB1 vector (New England Biolab, Ipswich, Massachusetts, USA). Recombinant BDNF propeptide was expressed in BL21 Escherichia coli and purified using an IMPACT (Intein Mediated Purification with an Affinity Chitin-binding Tag) kit according to the manufacturer’s protocol (cat. #E6901S; New England Biolab). The IMPACT system utilizes the inducible self-cleavage activity of protein splicing elements (termed inteins) to separate the target protein from the affinity tag. An extra histidine residue at the C-terminus was included to enhance cleavage of the intein affinity tag from the BDNF propeptide.
Cell viability assay
Cell viability was assessed using an 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma; St. Louis, Missouri, USA). Briefly, cells were plated in 96-well plates and treated with recombinant BDNF propeptide protein at various concentrations for 24 or 48 h (1, 5, 10, and 50 ng/ml) in serum-free media. Untreated cells served as a control. MTT assays were performed at both 24 and 48 h time-points. To minimize any variation among different assays, data were plotted using the optical density of the control wells set to 100% survival. Experiments were conducted in triplicate and repeated at least three times.
Cell apoptosis assay
C6 glioma cells were plated (15 000/well) in 96-well plates and cultured until 60–70% confluent. On the day of the experiment, cells were treated and prepared as described previously in serum-free DMEM. After the addition of the BDNF propeptide and a period of 24 h, C6 cells were fixed using 4% paraformaldehyde for 20 min and then stained with 6-diamidino-2-phenylindole (DAPI), which is a kind of specific dye for binding DNA. This dye does not have complete permeability. Once it overpasses cell membranes of normal cells, the blue fluorescence will be observed by fluorescent microscopy. With the process of apoptosis, the ability of permeability for dye is improved and the apoptotic cells will produce high blue fluorescence. At the same time, for normal cells, the round nucleus is stained uniformly and its margin is clear. However, for apoptotic cells, the margin of the nucleus is irregular and the condensed chromosome is easily stained. Cell images were collected for each sample (≤five fields/well) using a fluorescence microscope (Leica; Wetzlar, Hesse, Germany). The total number of nuclei was counted, in addition to those nuclei that showed apoptosis. The ratio of apoptotic nuclei to the total number of nuclei was then calculated. To minimize any variation among different assays, data were corrected against the control.
Western blot
For the antibody blocking, cells were pretreated with the antibody (4 μg/ml) for 30 min, followed by treatment with the BDNF propeptide for 24 h. The C6 cells were harvested with lysis buffer, vortexed, and centrifuged at 4°C at 13 000 rpm for 20 min. Proteins were separated on a 7.5–15% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was blocked with 5% milk in Tris-buffered saline for 1 h and then incubated with the primary antibodies against human proBDNF (1 : 2000, cat. #H10001; GeneCopoeia Inc., Rockville, Maryland, USA), cleaved caspase3 (1 : 1000, cat. #9664; Cell Signaling Technology; Danvers, Massachusetts, USA), B cell lymphoma 2-associated X protein (Bax) (1 : 1000, cat. #2772; Cell Signaling Technology), B cell lymphoma 2 (Bcl2) (1 : 1000, cat. #sc-7382; Santa Cruz Biotechnology; Dallas, Texas, USA), and β-actin (1 : 5000, cat. #A2228; Sigma-Aldrich, St Louis, Missouri, USA). After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody. Protein bands were identified using ECL. ECL-exposed films were digitized and densitometric quantification of immunoreactive bands was performed.
Statistical analysis
All quantitative data are expressed as mean±SD. Statistical analysis was carried out using one-way analysis of variance, followed by a two-tailed independent-samples t-test. Statistical analyses were carried out using SPSS 20 (SPSS Inc., Chicago, Illinois, USA), with a P value of less than 0.05 considered statistically significant.
Results
Expression of mBDNF and BDNF propeptide in C6 cells
Figure 1 A shows a schematic representation of the BDNF precursor, BDNF, and propeptide. The BDNF propeptide and BDNF (mature BDNF) are generated by enzymatic cleavage of proBDNF. Western blot showed the expression of BDNF propeptide in C6 cells (Fig. 1b). We also found that the BDNF propeptide was expressed in rat brain and spinal cord tissue. This indicated that the BDNF propeptide might play a physiological role.
Fig. 1.
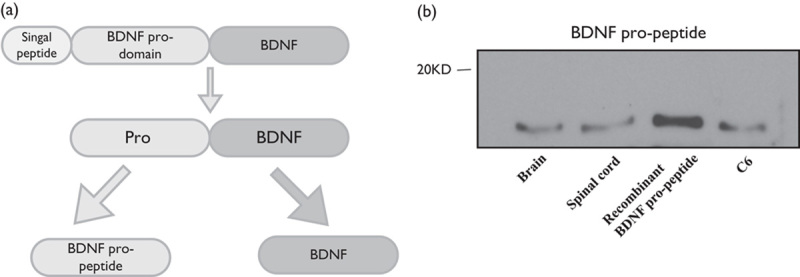
The expression of the BDNF propeptide in C6 glioma cells. Schematic representation of the BDNF precursor, BDNF, and propeptide. The BDNF propeptide and BDNF (mature BDNF) are indicated (a). Western blot analysis was used to examine the BDNF propeptide in cultured C6 glioma cells and mice brain and spinal cord tissue (b). BDNF, brain-derived neurotrophic factor.
The BDNF propeptide inhibits C6 glioma cell growth
Initially, we investigated whether the BDNF propeptide inhibited the growth of glioma cells. As shown in Fig. 2a, MTT assays indicated that treatment with exogenous BDNF propeptide for 24 h significantly decreased cell proliferation at doses of 10 and 50 ng/ml. 90 and 77.6% cells survived, respectively, after 10 and 50 ng/ml treatment of the BDNF propeptide. Therefore, BDNF propeptide inhibited cell proliferation in a dose-dependent manner. When cells were incubated with the BDNF propeptide for 48 h, BDNF propeptide levels as low as 5 ng/ml also suppressed C6 glioma cell growth . The survival rate for the cells that were treated for 48 h with 5, 10, and 50 ng/ml of the BDNF propeptide was 94, 82, and 77.3%, respectively.
Fig. 2.

Growth curve and apoptosis of C6 cells following treatment with a recombinant BDNF propeptide. Cells were incubated in serum-free medium, supplemented with the indicated concentrations of recombinant BDNF propeptide (1, 5, 10, and 50 ng/ml) in 96-well plates. Quantitative analysis of apoptosis of C6 cells following treatment with a recombinant BDNF propeptide at various concentrations (10 and 50 ng/ml). (a) MTT assays were performed after 24 and 48 h for each treatment. (b) Apoptosis assays were performed after 24 and 48 h for each treatment. (c) Morphology changes after treatment for 48 h with different concentrations of the BDNF propeptide. The white arrow shows apoptotic cells. Data are presented as the mean±SE. *P<0.05, **P<0.01 compared with the control group. BDNF, brain-derived neurotrophic factor; MTT, 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide.
BDNF propeptide promotes apoptosis of C6 glioma cells in vitro
The number of apoptotic cells was determined by morphologic observation using fluorescence microscopy following DAPI staining. DAPI staining is not a rigorous way to detect apoptosis, but it does indicate morphological change and may or may not be an indicator of cell death. Following the addition of recombinant BDNF propeptide (10 and 50 ng/ml) to the cultured C6 cells, apoptotic cells were significantly increased at 50 ng/ml at both the 24 and 48 h time-points. with death rates of 26 and 36%, respectively (Fig. 2b and c). Furthermore, we observed a significant increase in apoptosis with 10 ng/ml of the recombinant BDNF propeptide in serum-free medium at the 48 h time-point with a death rate of 14% (Fig. 2b).
The BDNF propeptide promotes apoptosis through the caspase pathway
Imbalances between prosurvival proteins (i.e. Bcl2) and proapoptotic proteins (i.e. Bax) can lead to apoptotic pathway activation 8–10. Therefore, the protein levels of Bcl2 and Bax were investigated in the cell lines. These results indicated that protein abundance of the antiapoptotic protein Bcl2 was decreased in recombinant BDNF propeptide-treated cells, whereas the abundance of the proapoptotic protein Bax was increased (Fig. 3a). When cells were treated with the BDNF propeptide antibody plus the BDNF propeptide, the protein levels were reversed. The BDNF propeptide antibody alone exerts no effect on Bax, Bcl2, and caspase3 expression. The quantitative values of band intensity for cleaved caspase3, Bcl2, and Bax between the BDNF propeptide-treated and untreated cells were determined to be 1.96±0.28 versus 1; 0.58±0.07 versus 1 and 1.87±0.25 versus 1, respectively (P<0.01; Fig. 3b summary data). These results suggested that the upregulation of Bax and the downregulation of Bcl2 may be responsible for activation of the apoptotic pathway.
Fig. 3.
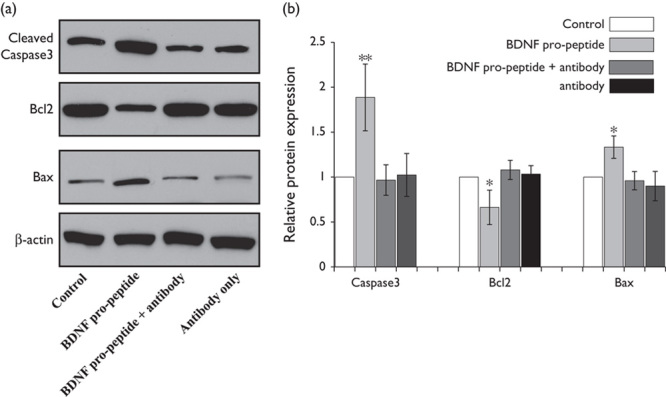
The expression levels of cleaved caspase3, Bcl2, and Bax in the C6 cells upon BDNF propeptide treatment. Caspase3, Bcl2, and Bax protein were examined using western blot analysis; BDNF propeptide treatment increases caspase3 and Bax protein. Bcl2 protein was decreased upon BDNF propeptide treatment for 24 h. The BDNF propeptide antibody (4 μg/ml) was added to sequester the BDNF propeptide. The expression of caspase3, Bax, and Bcl2 was reversed by the BDNF propeptide antibody plus the BDNF propeptide (lane 3) and antibody treatment only (lane4) (a). The quantitative analysis of gray intensity was carried out. Data are presented as the mean±SE. *P<0.05, **P<0.01 compared with the control group (b). Bcl, B cell lymphoma 2; Bax, B cell lymphoma 2-associated X protein; BDNF, brain-derived neurotrophic factor.
Discussion
Like other neuropeptides, BDNF (synthesized as proBDNF) is proteolytically converted into an active form (mBDNF) and a propeptide (BDNF propeptide) 11. It has been reported that both mBDNF and the BDNF propeptide are present at roughly equimolar ratios in the hippocampus, and both are 10-fold more abundant than proBDNF 12. The functions of mBDNF and proBDNF on different neuronal processes are well studied, although the presence and possible functions of BDNF propeptide in the brain have been reported only recently. These include studies showing the presence of the BDNF propeptide in rodent brains and membrane depolarization induced by BDNF propeptide secretion in dissociated neuronal cultures. In addition, the BDNF propeptide has been shown to induce acute growth cone retraction and facilitate long-term depression 8–10. Our study shows a novel biological role for the BDNF propeptide beyond that of aiding the folding of BDNF. In the present study, we found that both mBDNF and the BDNF propeptide are expressed by glioma cells. Using a C6 glioma cell model, we have also found that exogenous recombinant BDNF propeptide can inhibit cell growth and promote apoptosis in a dose-dependent manner. This indicates that endogenous BDNF propeptide can inhibit glioma cell proliferation and survival.
Previous studies have shown that Bax and Bcl2 proteins are involved in the apoptosis pathway. Apoptosis is mediated through a caspase cascade 13 and the ability of cells to undergo apoptosis is determined by interactions between prosurvival proteins (i.e. Bcl2) and proapoptotic proteins (i.e. Bax) 14. Several studies have also shown that Bcl2 overexpression is associated with resistance to conventional radiation and chemotherapeutic agents in many tumor cell types, including malignant glioma cells 15,16. This resistance is because of Bcl2 binding to Bax, thereby inhibiting Bax activation 17. In the present study, we found that BDNF propeptide reduced Bcl2 protein abundance and increased Bax protein abundance. Our study shows the critical role of the caspase3 pathway in BDNF propeptide-induced apoptosis. These findings are supported by the fact that the BDNF propeptide induced an increase in cleaved caspase3 protein abundance.
Conclusion
We have uncovered a novel biological effect on cultured glioma cells of the BDNF propeptide. Our study suggests that the BDNF propeptide may exert an inhibitory effect on glioma through activation of the caspase3 pathway.
Acknowledgements
This work was supported by a grant from Renmin Hospital of Wuhan University (20873999).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol 1994; 14:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephan H, Zakrzewski JL, Bölöni R, Grasemann C, Lohmann DR, Eggert A. Neurotrophin receptor expression in human primary retinoblastomas and retinoblastoma cell lines. Pediatr Blood Cancer 2008; 50:218–222. [DOI] [PubMed] [Google Scholar]
- 3.Artico M, Bronzetti E, Pompili E, Ionta B, Alicino V, D’Ambrosio A, et al. Immunohistochemical profile of neurotrophins in human cranial dura mater and meningiomas. Oncol Rep 2009; 21:1373–1380. [DOI] [PubMed] [Google Scholar]
- 4.Barde YA. Trophic factors and neuronal survival. Neuron 1989; 2:1525–1534. [DOI] [PubMed] [Google Scholar]
- 5.Bartkowska K, Turlejski K, Djavadian RL. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol Exp (Wars) 2010; 70:454–467. [DOI] [PubMed] [Google Scholar]
- 6.Zhou XF, Song XY, Zhong JH, Barati S, Zhou FH, Johnson SM. Distribution and localization of pro-brain-derived neurotrophic factor-like immunoreactivity in the peripheral and central nervous system of the adult rat. J Neurochem 2004; 91:704–715. [DOI] [PubMed] [Google Scholar]
- 7.Hamel W, Westphal M, Szönyi E, Escandón E, Nikolics K. Neurotrophin gene expression by cell lines derived from human gliomas. J Neurosci Res 1993; 34:147–157. [DOI] [PubMed] [Google Scholar]
- 8.Mizui T, Ishikawa Y, Kumanogoh H, Lume M, Matsumoto T, Hara T, et al. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc Natl Acad Sci USA 2015; 112:E3067–E3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B, Yang C, Ren Q, Zhang JC, Chen QX, Shirayama Y, Hashimoto K. Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. Eur Arch Psychiatry Clin Neurosci 2016; 266:765–769. [DOI] [PubMed] [Google Scholar]
- 10.Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun 2013; 4:2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: Recent advances and challenges. Dev Neurobiol 2010; 70:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol 2012; 196:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev 2003; 17:2481–2495. [DOI] [PubMed] [Google Scholar]
- 14.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007; 26:1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol 2006; 3:388–398. [DOI] [PubMed] [Google Scholar]
- 16.Renault TT, Teijido O, Antonsson B, Dejean LM, Manon S. Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): keep your friends close but your enemies closer. Int J Biochem Cell Biol 2013; 45:64–67. [DOI] [PubMed] [Google Scholar]
- 17.Ju H, Li X, Li H, Wang X, Wang H, Li Y, et al. Mediation of multiple pathways regulating cell proliferation, migration, and apoptosis in the human malignant glioma cell line U87MG via unphosphorylated STAT1: laboratory investigation. J Neurosurg 2013; 118:1239–1247. [DOI] [PubMed] [Google Scholar]


