Supplemental Digital Content is available in the text
Abstract
BACKGROUND
Limited information exists about the epidemiology and outcome of surgical patients at increased risk of postoperative pulmonary complications (PPCs), and how intraoperative ventilation was managed in these patients.
OBJECTIVES
To determine the incidence of surgical patients at increased risk of PPCs, and to compare the intraoperative ventilation management and postoperative outcomes with patients at low risk of PPCs.
DESIGN
This was a prospective international 1-week observational study using the ‘Assess Respiratory Risk in Surgical Patients in Catalonia risk score’ (ARISCAT score) for PPC for risk stratification.
PATIENTS AND SETTING
Adult patients requiring intraoperative ventilation during general anaesthesia for surgery in 146 hospitals across 29 countries.
MAIN OUTCOME MEASURES
The primary outcome was the incidence of patients at increased risk of PPCs based on the ARISCAT score. Secondary outcomes included intraoperative ventilatory management and clinical outcomes.
RESULTS
A total of 9864 patients fulfilled the inclusion criteria. The incidence of patients at increased risk was 28.4%. The most frequently chosen tidal volume (VT) size was 500 ml, or 7 to 9 ml kg−1 predicted body weight, slightly lower in patients at increased risk of PPCs. Levels of positive end-expiratory pressure (PEEP) were slightly higher in patients at increased risk of PPCs, with 14.3% receiving more than 5 cmH2O PEEP compared with 7.6% in patients at low risk of PPCs (P < 0.001). Patients with a predicted preoperative increased risk of PPCs developed PPCs more frequently: 19 versus 7%, relative risk (RR) 3.16 (95% confidence interval 2.76 to 3.61), P < 0.001) and had longer hospital stays. The only ventilatory factor associated with the occurrence of PPCs was the peak pressure.
CONCLUSION
The incidence of patients with a predicted increased risk of PPCs is high. A large proportion of patients receive high VT and low PEEP levels. PPCs occur frequently in patients at increased risk, with worse clinical outcome.
TRIAL REGISTRATION
The study was registered at Clinicaltrials.gov, number NCT01601223.
This article is accompanied by the following Invited Commentary:
Haller G, Walder B. Postoperative pulmonary complications - Still room for improvement. Eur J Anaesthesiol 2017; 34:489–491.
Introduction
Mechanical ventilation with positive pressure can cause overdistension as well as repetitive opening and collapse of lung units, which can induce or worsen existing lung injury.1 In critically ill patients with acute respiratory distress syndrome (ARDS) who need ventilatory support, ventilation strategies that use lower tidal volumes (VT) have been found to be beneficial.2 Recent studies show that critically ill patients without ARDS who require mechanical ventilation could benefit from this strategy.3,4 Ventilation strategies that use higher levels of positive end-expiratory pressure (PEEP) have also been found to improve outcome in patients with ARDS.5 Worldwide, these findings have led to significant changes in ventilation practice in critically ill patients.6,7
Theoretically, surgical patients with uninjured lungs could also benefit from intraoperative ventilation strategies that use low VT and higher PEEP levels.8 Indeed, three recent randomised controlled trials in patients scheduled for major abdominal surgery showed that intraoperative ventilation with low VT as part of a lung-protective ventilation strategy reduced the occurrence of postoperative pulmonary complications (PPCs).9–11 The debate on the best PEEP level during intraoperative ventilation, however, is ongoing.12,13 Notably, one meta-analysis of recent randomised controlled trials suggests that it is intraoperative VT restriction rather than an increase in PEEP level that was responsible for the benefits observed.14
Attempts to improve outcomes in surgical patients by preventing PPCs may be a more effective strategy than treating PPCs once they occur.15 With an estimated worldwide number of surgical procedures more than 234 million each year, even a small reduction in the incidence of PPCs could have a significant effect.16 Epidemiologic data suggest that PPCs are rarely present shortly after surgery, but in a subset of patients at increased risk, PPCs develop over a period of days with considerable impact on outcome.17,18 Although this particular group of patients at increased risk of PPCs would benefit most from lung-protective ventilation, the current management of lung ventilation in these patients is unknown. Neither is it known if ventilation strategies differ between patients at high or low risk of PPCs.
Therefore, we undertook the ‘Local ASsessment of VEntilatory management during General Anaesthesia for Surgery’ (LAS VEGAS) study to determine the incidence of patients at increased risk of PPCs, and to compare ventilation management and outcomes in patients at increased risk of PPCs with patients at low risk of PPCs.
Methods
Study design
The LAS VEGAS study was an international, multicentre, prospective cross-sectional study. The study protocol was first approved by the ethical committee of the Academic Medical Center, Amsterdam, the Netherlands (W12_190#12.17.0227). Surgical patients were enrolled over a period of 7 consecutive days between 14 January and 4 March 2013. National coordinators selected the exact period during which data were collected for the study in their respective countries.
Study sites were recruited through the Clinical Trial Network of the European Society of Anaesthesiology (ESA), providing access to a large network of anaesthesiologists. The participating hospitals represented a convenient sample of those that initially agreed to participate in the study. Each site was then required to seek approval to implement this protocol from their respective institutional review boards and, if required, to obtained written informed consent from individual patients or their legal representatives.
The ESA assisted in developing the electronic case record forms and hosted the electronic database, but had no influence on the study design, conduct, data analysis and interpretation, or on the final reporting.
Quality control
National coordinators assisted local coordinators to ensure that the study was performed according to the ‘International Conference on Harmonisation (Good Clinical Practice)’ guidelines.19 Local coordinators arranged regulatory approvals, supervised local researchers, and assured the integrity of the data and its timely collection. Patient data were entered into a password secured, web-based electronic case record form (OpenClinica, Boston, Massachusetts, USA) and was anonymised before entry. Two rounds of extensive data cleaning were performed before the start of data analysis to check for outliers and possible invalid data. Local investigators were queried on incorrect data, then asked to verify the data in the patient records, and correct the electronic form.
Inclusion and exclusion criteria
All adult patients receiving invasive ventilation (via either an endotracheal tube or supraglottic device) during general anaesthesia for elective or non-elective surgery were included. Patients were excluded from participation if they were aged less than 18 years, or scheduled for pregnancy-related surgery, surgical procedures outside the operating room, or procedures involving cardiopulmonary bypass. Data from patients undergoing thoracic surgery, or who required one-lung ventilation during surgery, and those who had received ventilation at any time in the previous 30 days were collected, but excluded from the current analysis.
Data collection
Centres with large patient numbers, defined as more than 180 surgical procedures per week, could request and were allowed after consent from the Steering Committee, to randomly select either 25 or 50% of their eligible patients for inclusion using the ALEA software (ALEA Version 2.2; NKIAVL, Amsterdam Netherlands). The randomisation procedure is further described in the Supplemental Digital Material.
Based on the literature, we collected baseline characteristics and preoperative risk factors that help to identify patients at risk of PPCs.20–22 During the intraoperative period we collected data on intraoperative ventilator settings and vital parameters hourly, and recorded intraoperative events possibly related to mechanical ventilation. PPCs were observed and collected daily from the day of surgery (day 0) until discharge from hospital or postoperative day 5, whichever came first. Length of hospital stay and in-hospital mortality was collected by examination of patient records at postoperative day 28.
Definitions
The risk of PPCs was based on preoperative data and defined retrospectively by the ‘Assess Respiratory Risk in Surgical Patients in Catalonia risk score for PPCs’ (ARISCAT score). For the purpose of this study, moderate-risk and high-risk groups (ARISCAT scores 26 to 44 and ≥45, respectively) were combined into a group called ‘increased risk of PPCs’ (ARISCAT score ≥26) (eTable 1).17,18 Of note, clinicians providing care were not informed a priori on use of the ARISCAT score for stratification of patients.
Intraoperative events included episodes of hypoxia (SpO2 < 92%), use of lung recruitment manoeuvres (ventilation strategies aimed to restore aeration of the lungs); airway pressure reduction (ventilation strategies aimed to lower peak and plateau pressure), presence of expiratory flow limitation (expiratory flow higher than zero at end-expiration as suggested by visual analysis of the expiratory gas flow curve), hypotension (systolic arterial blood pressure <90 mmHg for 3 min or longer), use of vasoactive drugs (any given to correct hypotension), and new arrhythmias (atrial fibrillation, sustained ventricular tachycardia, supraventricular tachycardia, or ventricular fibrillation).
PPCs were defined as unplanned supplementary oxygen (oxygen administered due to PaO2 < 8 kPa or SpO2 < 90% in room air, but excluding oxygen supplementation given as standard care, e.g. directly after arrival in the postanaesthetic care unit), respiratory failure (PaO2 < 8 kPa or SpO2 < 90% despite oxygen therapy, or a need for noninvasive positive pressure ventilation (NIPPV); unplanned new or prolonged invasive mechanical ventilation (after discharge from the operating room), ARDS (defined according to the Berlin definition of ARDS),23 pneumonia (presence of a new or progressive radiographic infiltrate and at least two of three clinical features; fever >38°C or >100.4°F, leucocytosis or leukopenia (WBC count >12 000 cells μl−3 or <4000 cells μl−3 and purulent secretions), and pneumothorax (air in the pleural space with no vascular bed surrounding the visceral pleura on the chest radiograph).
Outcomes
The primary outcome was the incidence of patients at increased risk of PPC. Secondary outcomes included ventilatory management, namely VT (ml kg−1 predicted body weight, PBW), level of PEEP (cmH2O), VT-PEEP combinations, number of intraoperative events, number of PPCs developing in the first 5 postoperative days, length of hospital stay, and in-hospital mortality. A composite endpoint was calculated for the PPCs observed from the day of surgery (day 0) until hospital discharge or postoperative day 5, whichever came first. Each adverse pulmonary event was recorded on days 1 to 5, and was scored ‘YES’ as soon as the event occurred on either ward or intensive care unit. If the event was present on subsequent days, it was not scored again.
Analysis plan
Part of the statistical analysis plan was published previously in this journal.24 We planned to include only data from centres that had more than 95% of complete and reliable data with regard to VT size (i.e. in ml and in ml kg−1 PBW) and PEEP levels.
Patients were stratified into groups based on the retrospectively applied ARISCAT score: preoperative low risk (ARISCAT score <26) or increased risk of PPCs (ARISCAT score ≥26).17 The proportion of patients at increased risk of PPCs was calculated by dividing the number of patients with increased preoperative risk of PPCs by the total number of patients. The number of patients at increased risk of PPCs per surgical procedure over the study period was calculated by dividing the number of patients with increased risk of PPCs divided by the number of total surgical procedures performed in this cohort. The ventilatory data, which were collected hourly, were first averaged for each patient before being included in the whole population data analysis. The data are presented for the whole population and for patients at low versus increased risk for PPCs. Length of hospital stay and in-hospital mortality was censored at postoperative day 28.
The distributions of combinations of VT size and PEEP level, VT size and respiratory rate, and VT size and peak pressure level, are presented in scatterplots. Cut-offs of 8 ml kg−1 PBW for VT, 5 cmH2O for PEEP, 20 cmH2O for peak pressure, and 14 bpm for respiratory rate were chosen to form the matrices. These cut-offs were based on widely accepted values of each variable, or according to normal daily practice. The driving pressure, defined as plateau pressure (Pplat) minus the PEEP level, was analysed following the same analysis plan as for the other ventilatory parameters. The driving pressure analysis was only possible in patients in whom the Pplat was collected. VT size and driving pressure level combinations were plotted in one extra scatterplot, in which the median driving pressure (12 cm H2O) was used as a cut-off to build the matrix.
Finally, we compared ventilator settings in patients who did and did not develop PPCs. A multivariable model was built to quantify the net effect of intraoperative ventilation settings on the occurrence of PPCs, while controlling for other demographic and perioperative data.20–22
In one post-hoc analysis we restricted the composite endpoint of PPCs to severe PPCs, by ignoring ‘unplanned supplementary oxygen’. In a second post-hoc analysis, in an attempt to provide more insight into the effects of stratification using the three original ARISCAT risk groups, we analysed the data according to the original boundaries, that is, ARISCAT score less than 26, 26 to 44, and at least 45.
We strictly followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for observational studies (provided in the supplemental digital material, pp. 11 to 13).
Statistical analysis
All variables were tested for normality using Kolmogorov–Smirnov–Lilliefors. Hourly collected variables, including VT size, PEEP level, peak and plateau pressure levels, respiratory rate, oxygen fraction of inspired air (FiO2), are presented as medians with their interquartile ranges. VT size is presented as an absolute volume (ml) and volume normalised for PBW (ml kg−1 PBW). The PBW was calculated as 50 + 0.91 × (height [cm] − 152.4) for men, and 45.5 + 0.91 × (height [cm] − 152.4) for women.23 Parametric data are presented as means (with standard deviations), whereas non-parametric data are presented as medians [with the 25th and 75th percentiles]. To clarify missing data in the calculations, we report the number of cases with specific outcome data (n) along with the total number of relevant cases (N) for all variables. We reported the n/N on all variables, to be transparent on reporting of missing data.
Proportions are compared using χ2 or Fisher exact tests and continuous variables are compared using the t test or Wilcoxon rank-sum test, as appropriate. Adjustments for multiple comparisons were not performed for the preoperative and intraoperative characteristics. Kaplan–Meier estimates of the cumulative probability of development of PPCs and survival were performed. We used log-rank tests to compare survival distributions in patients at low risk or increased risk of PPCs. Patients discharged from the hospital before the end of follow-up at day 28 were assumed alive and without complications at this time point.
To build the multivariable model, independent variables were selected from the demographic and perioperative data according to biologic plausibility and when a P value less than 0.2 was found in the univariable analysis. Peak pressure, plateau pressure, and driving pressure had high collinearity; therefore, only peak pressure was entered into the model, as plateau pressure had missing values. Effects were expressed as an average odds ratio (OR) with 95% confidence interval (95% CI). In the model, statistical significance was set at a P value < 0.05. Statistical significance was considered to be at P < 0.05. All analyses were performed with R version 3.1 (http://www.R-project.org/).
Results
Participating centres and patients
Of 219 centres that expressed an initial interest in participating in the LAS VEGAS study, 73 (in seven countries) were unable to obtain formal approval from their local institutional review board in time, or had other reasons not to participate (Fig. 1). The 146 hospitals taking part in the study were recruited from 30 different countries (eTable 2). The list of countries, participating centres, and their respective numbers of included patients are presented in eTable 2. Hospital characteristics of participating centres are given in eTable 3. Only two centres used the randomisation program to reduce the number of patients – one to reduce the number of patients to 50% and one to reduce the number of patients to 25% of eligible patients.
Fig. 1.
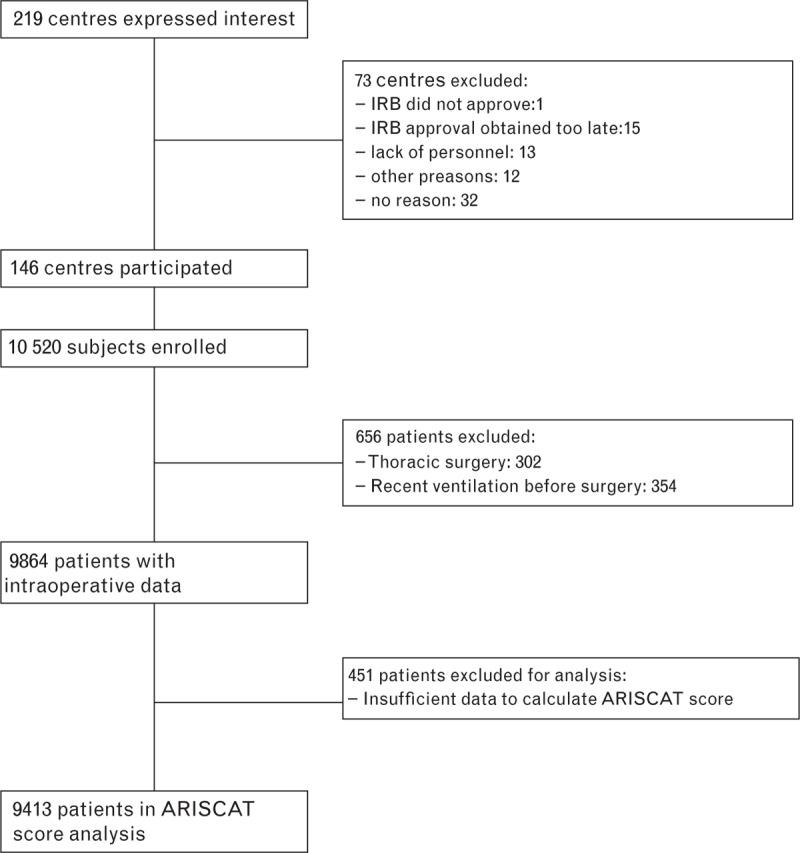
Flow chart: Data collection and selection of centres and patients. Two centres used the optional randomisation program to reduce the number of patients: one centre reduced the patient numbers by 50% (excluding 75 patients) and another centre by 75% (excluding 307 patients). ARISCAT, Assess Respiratory Risk in Surgical Patients in Catalonia; IRB, institutional review board.
In total 10 520 patients requiring intraoperative ventilation were enrolled. After exclusion of patients undergoing one-lung ventilation and patients who had received mechanical ventilation before surgery, 9864 patients of the complete cohort were available for analysis. Sufficient data to calculate the ARISCAT score retrospectively were available in 9413. Patient and surgical characteristics are shown in Table 1 and eTable 4.
Table 1.
Patient and surgical baseline characteristics within each group
| Variable | All patients | Low risk of PPCs | Increased risk of PPCs |
| Male sex (%) | 45.0 (4439/9864) | 43.6 (2937/6743) | 48.5 (1294/2670) |
| Age (years) (%) | 53.0 [39.0 to 66.0] | 50.0 [36.0 to 63.0] | 62.0 [50.0 to 72.0] |
| ≤50 | 45.0 (4440/9861) | 52.1 (3510/6742) | 25.5 (680/2669) |
| 51 to 80 | 51.0 (5033/9861) | 46.1 (3111/6742) | 65.2 (1741/2669) |
| >80 | 3.9 (388/9861) | 1.8 (121/6742) | 9.3 (248/2669) |
| BMI (kg m−2) | 26.2 [23.4 to 30.0] | 26.0 [23.2 to 29.7] | 26.8 [23.7 to 30.7] |
| ASA physical status classification system | |||
| ASA 1 | 30.6 (3013/9840) | 36.3 (2445/6734) | 14.0 (373/2663) |
| ASA 2 | 48.2 (4743/9840) | 49.1 (3305/6734) | 47.0 (1252/2663) |
| ASA 3 | 19.3 (1903/9840) | 13.8 (929/6734) | 34.5 (919/2663) |
| ASA 4 | 1.8 (173/9840) | 0.8 (53/6734) | 4.3 (115/2663) |
| ASA 5 | 0.1 (8/9840) | 0.0 (2/6734) | 0.2 (4/2663) |
| Functional status | |||
| Non dependent | 92.4 (9105/9858) | 94.7 (6385/6739) | 86.5 (2308/2669) |
| Partially dependent | 6.3 (621/9858) | 4.3 (291/6739) | 11.5 (307/2669) |
| Totally dependent | 1.3 (132/9858) | 0.9 (63/6739) | 2.0 (54/2669) |
| ARISCAT score | 15.0 [3.0 to 26.0] | 11.0 [3.0 to 16.0] | 34.0 [31.0 to 41.0] |
| <26 | 71.6 (6743/9413) | 100.0 (6743/6743) | - |
| 26 to 44 | 23.5 (2215/9413) | - | 83.0 (3315/2670) |
| >44 | 4.8 (455/9413) | - | 17.0 (455/2670) |
| Preoperative SpO2 (%) | 98.0 [96.0 to 99.0] | 98.0 [97.0 to 99.0] | 97.0 [95.0 to 98.0] |
| ≥96 | 83.4 (7254/8698) | 90.2 (5450/6043) | 65.9 (1609/2440) |
| 91 to 95 | 15.3 (1331/8698) | 9.8 (591/6043) | 29.5 (721/2440) |
| ≤90 | 1.3 (113/8698) | 0.0 (2/6043) | 4.5 (110/2440) |
| Preoperative anaemia (Hb ≤ 10 g dl−1) | 4.0 (329/8265) | 1.0 (53/5573) | 10.5 (265/2528) |
| Chronic comorbidity – a patient can have more than one comorbidity | |||
| Metastatic cancer | 4.0 (392/9864) | 1.8 (124/6743) | 9.7 (260/2670) |
| Chronic kidney dysfunction | 3.1 (310/9864) | 2.0 (137/6743) | 6.1 (162/2670) |
| COPD | 6.0 (596/9864) | 4.8 (322/6743) | 9.6 (256/2670) |
| Heart failure | 5.9 (585/9864) | 4.6 (313/6743) | 9.6 (255/2670) |
| Obstructive sleep apnoea | 2.1 (205/9864) | 2.0 (132/6743) | 2.5 (68/2670) |
| Neuromuscular diseaseb | 0.9 (88/9864) | 1.0 (66/6743) | 0.8 (21/2670) |
| Liver dysfunction | 1.0 (102/9864) | 0.8 (54/6743) | 1.6 (43/2670) |
| Surgical procedure – a patient can have more than one type of surgical procedure | |||
| Lower GI | 11.1 (1096/9864) | 6.9 (466/6743) | 21.7 (579/2670) |
| Upper GI, hepatobiliary, pancreas | 13.8 (1357/9864) | 11.2 (753/6743) | 21.3 (568/2670) |
| Vascular surgerya | 3.1 (309/9864) | 2.9 (197/6743) | 3.7 (99/2670) |
| Aortic surgery | 0.6 (64/9864) | 0.3 (18/6743) | 1.7 (45/2670) |
| Neurosurgery, head and neck | 20.3 (2006/9864) | 3.1 (1558/6743) | 12.8 (342/2670) |
| Urological and kidney | 8.7 (858/9864) | 6.7 (455/6743) | 13.8 (368/2670) |
| Gynaecological | 11.6 (1141/9864) | 10.9 (733/6743) | 12.9 (345/2670) |
| Endocrine surgery | 2.0 (194/9864) | 2.4 (159/6743) | 1.1 (30/2670) |
| Transplant | 0.3 (34/9864) | 0.1 (5/6743) | 1.0 (28/2670) |
| Plastic, cutaneous, breast | 10.5 (1037/9864) | 13.1 (885/6743) | 4.2 (113/2670) |
| Bone, joint, trauma, spine | 16.2 (1595/9864) | 18.6 (1253/6743) | 9.6 (255/2670) |
| Other procedure | 5.9 (585/9864) | 7.2 (483/6743) | 3.0 (79/2670) |
| Surgical technique – a patient can have more than one type of surgical procedure | |||
| Open abdominal surgery | 18.0 (1773/9864) | 7.6 (512/6743) | 44.8 (1195/2670) |
| Laparoscopic surgery | 17.6 (1737/9864) | 16.0 (1082/6743) | 22.0 (587/2670) |
| Laparoscopic assisted surgery | 1.7 (167/9864) | 1.1 (73/6743) | 3.4 (90/2670) |
| Peripheral surgery | 18.5 (1827/9864) | 22.2 (1500/6743) | 8.2 (218/2670) |
| Other | 44.9 (4427/9864) | 53.3 (3594/6743) | 23.5 (628/2670) |
| Urgency of surgeryc | |||
| Elective | 88.9 (8765/9862) | 91.1 (6141/6742) | 84.2 (2248/2670) |
| Urgent | 8.6 (845/9862) | 7.5 (508/6742) | 10.8 (288/2670) |
| Emergency | 2.6 (252/9862) | 1.4 (93/6742) | 5.0 (134/2670) |
| Duration of surgery (min)d | 73.0 [42.0 to 125.0] | 60.0 [35.0 to 95.0] | 131.0 [75.0 to 199.0] |
| Duration of anaesthesia (min)e | 103.0 [66.0 to 160.0] | 90.0 [60.0 to 128.0] | 170.0 [107.0 to 246.0] |
Data are presented as median [LQ to UQ] or % (n/N); low versus increased risk of PPCs, according to the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score (<26 versus ≥26, respectively). ASA, American Society of Anaesthesiology; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; Hb, haemoglobin; LQ, lower quartile; UQ, upper quartile; n, number with characteristic; PPC, postoperative pulmonary complication; N, number in group or subgroup; SpO2, peripheral oxygen saturation.
aVascular surgery is carotid endarterectomy, aortic surgery and peripheral vascular taken together.
bNeuromuscular disease affecting the respiratory system.
cUrgency of surgery: elective: surgery that is scheduled in advance because it does not involve a medical emergency, urgent: surgery required within <48 h, emergency: nonelective surgery performed when the patient's life or well being is in direct jeopardy.
dDuration of surgery is the time between skin incision and closure of the incision.
eDuration of anaesthesia is the time between start of induction and tracheal extubation or discharge from operation room if mechanical ventilation continued.
Incidence of patients at increased risk of postoperative pulmonary complications
Patients at increased risk of PPCs represented 2670 of 9413 patients ventilated for surgery or 28 cases per 100 surgical procedures over one week among all types of procedure. Patients undergoing transplant surgery or aortic surgery had the highest incidence of PPCs of all types of surgical procedures (Table 2).
Table 2.
Surgical procedure and incidence of postoperative pulmonary complications
| Surgical procedure | Incidence of PPC | Incidence of severe PPC |
| Lower GI | 16.1 (177/1096) | 6.5 (71/1096) |
| Upper GI, hepatobiliary, pancreas | 13.0 (177/1357) | 5.0 (68/1357) |
| Vascular surgerya | 11.9 (37/309) | 3.9 (12/309) |
| Aortic surgery | 20.3 (13/64) | 10.9 (7/64) |
| Neurosurgery, head and neck | 7.7 (154/2006) | 1.9 (38/2006) |
| Urological and kidney | 11.4 (98/858) | 2.4 (21/858) |
| Gynaecological | 9.2 (105/1141) | 1.4 (17/1141) |
| Endocrine surgery | 10.3 (20/194) | 3.6 (7/194) |
| Transplant | 38.2 (13/34) | 5.9 (2/34) |
| Plastic, cutaneous, breast | 6.7 (69/1037) | 1.6 (17/1037) |
| Bone, joint, trauma, spine | 10.3 (165/1595) | 1.8 (29/1595) |
| Other procedure | 7.9 (46/585) | 1.9 (11/585) |
All data are presented as proportion, % (n/N); a patient could have had more than one type of surgical procedure within one operation (e.g. neurosurgery and trauma). GI, gastrointestinal; n, number with characteristic; N, number in group or subgroup; PPC, postoperative pulmonary complication.
aVascular surgery is carotid endarterectomy, aortic surgery and peripheral vascular taken together.
Intraoperative ventilation characteristics
The most frequently chosen VT was 500 ml, which corresponds to a VT between 7.2 and 9.1 ml kg−1 PBW. Patients at increased risk of PPCs received higher VT (ml kg−1 PBW) than those at low risk of PPCs, but the differences were of minimal clinical significance (Table 3, Fig. 2a). VT was >8 ml kg−1 PBW in 43% of patients at increased risk of PPCs vs. 40% of patients at low risk of PPCs. PEEP levels were 5 cmH2O or less in most patients, and the most frequently chosen PEEP levels were 0 or 5 cmH2O. Compared with patients at low risk of PPCs, patients at increased risk of PPCs received higher PEEP levels but again, this difference was of minimal clinical significance (Table 3, Fig. 2b).
Table 3.
Intraoperative ventilation characteristics
| All patients | Low risk of PPCs | Increased risk of PPCs | P | |
| Ventilation mode | ||||
| Volume control | 70.1 (6816/9717) | 69.0 (4589/6649) | 72.7 (1910/2629) | <0.001 |
| Pressure control | 16.2 (1571/9717) | 17.5 (1161/6649) | 13.6 (358/2629) | |
| Pressure support or spontaneous | 1.1 (104/9717) | 1.3 (87/6649) | 0.6 (16/2629) | |
| Othera | 12.6 (1226/9717) | 12.2 (812/6649) | 13.1 (345/2629) | |
| Airway type | ||||
| Endotracheal tube | 81.8 (8064/9857) | 77.0 (5194/6742) | 95.5 (2550/2670) | <0.001 |
| Nasotracheal tube | 1.3 (127/9857) | 1.4 (97/6742) | 0.8 (21/2670) | |
| Supraglottic device | 15.9 (1570/9857) | 20.6 (1386/6742) | 2.7 (72/2670) | |
| Other | 1.0 (96/9857) | 1.0 (65/6742) | 1.0 (27/2670) | |
| Tidal volumes (ml) | 500.0 [455.0 to 558.5] | 500.0 [454.0 to 553.0] | 500.0 [460.0 to 562.5] | 0.017 |
| Tidal volumes ml kg−1 PBW | 8.1 [7.2 to 9.1] | 8.1 [7.2 to 9.1] | 8.2 [7.4 to 9.2] | 0.001 |
| Tidal volumes ml kg−1 ABW | 6.7 [5.8 to 7.7] | 6.7 [5.8 to 7.7] | 6.7 [5.8 to 7.6] | 0.354 |
| PEEP (cmH2O) | 3.5 [0.0 to 5.0] | 3.0 [0.0 to 5.0] | 4.5 [2.0 to 5.0] | <0.001 |
| Respiratory rate (bpm) | 12.0 [12.0 to 13.0] | 12.0 [12.0 to 13.0] | 12.0 [12.0 to 13.0] | 0.205 |
| Minute ventilation (ml min−1) | 6000 [5000 to 6769] | 6000 [5185 to 6816] | 6000 [4979 to 6870] | 0.003 |
| Ppeak (cmH2O) | 17.5 [15.0 to 21.0] | 17.0 [14.5 to 20.0] | 19.0 [16.0 to 22.0] | <0.001 |
| Pplat (cmH2O) | 15.5 [13.0 to 18.5] | 15.0 [13.0 to 18.0] | 17.0 [14.0 to 20.0]) | <0.001 |
| Driving pressure (cmH2O) | 12.0 [10.0 to 15.0] | 12.0 [10.0 to 15.0] | 13.0 [10.0 to 16.0] | <0.001 |
| Cdyn (ml cm−1 H2O) | 34.8 [28.1 to 42.8] | 35.4 [28.6 to 43.5] | 33.5 [27.1 to 41.1] | <0.001 |
| Cq.stat. (ml cm−1 H2O) | 41.7 [33.4 to 51.4] | 42.3 [34.3 to 52.0] | 40.0 [32.0 to 50.0] | <0.001 |
| Recruitment manoeuvre performed | 9.8 (965/9813) | 8.5 (570/6719) | 12.9 (343/2656) | <0.001 |
| FiO2 | 0.52 [0.45 to 0.70] | 0.54 [0.47 to 0.72] | 0.50 [0.45 to 0.60] | <0.001 |
| <0.40 | 7.1 (699/9808) | 6.5 (440/6727) | 9.0 (240/2660) | <0.001 |
| ≥0.40 to <0.60 | 51.7 (5068/9808) | 48.9 (3288/6727) | 60.5 (1609/2660) | |
| ≥0.60 to <0.80 | 29.5 (2895/9808) | 32.0 (2156/6727) | 22.5 (599/2660) | |
| ≥0.80 | 11.7 (1146/9808) | 12.5 (843/6727) | 8.0 (212/2660) | |
| SpO2% | 99.0 [98.0 to 100.0] | 99.0 [98.0 to 100.0] | 99.0 [98.0 to 100.0] | 0.198 |
| ≥96 | 97.6 (9583/9817) | 97.8 (6571/6721) | 97.0 (2573/2652) | 0.040 |
| >90 to <96 | 2.3 (222/9817) | 2.1 (140/6721) | 2.9 (77/2652) | |
| ≤90 | 0.1 (12/9817) | 0.1 (10/6721) | 0.1 (2/2652) | |
| EtCO2-kPa | 4.5 [4.1 to 4.9] | 4.5 [4.1 to 4.9] | 4.4 [4.0 to 4.8] | <0.001 |
Low versus increased risk of PPCs, according to the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score (<26 versus ≥26, respectively).
Data are presented as median [LQ to UQ] or % (CI); χ2 for categorical variables and Mann-Whitney for continuous variables.
aOther (e.g. high frequency oscillatory ventilation, jet ventilation, synchronised intermittent mandatory ventilation (SIMV). ABW, actual body weight; PBW, predicted body weight, calculated as: 50 + (0.91 × (cm height – 152.4)) for males and 45.5 + [0.91 × (cm height − 152.4)] for females; Cdyn, calculated dynamic respiratory compliance, calculated as [tidal volume/(Ppeak − PEEP)]; Cq.stat, static respiratory compliance; FiO2, fractional inspired oxygen; LQ, lower quartile; UQ, upper quartile; n, number with characteristic; N, number in group or subgroup; Ppeak, peak pressure; Pplat, Plateau pressure; PEEP, positive end-expiratory pressure; SpO2, peripheral oxygen saturation; EtCO2, expiratory carbon dioxide.
Fig. 2.
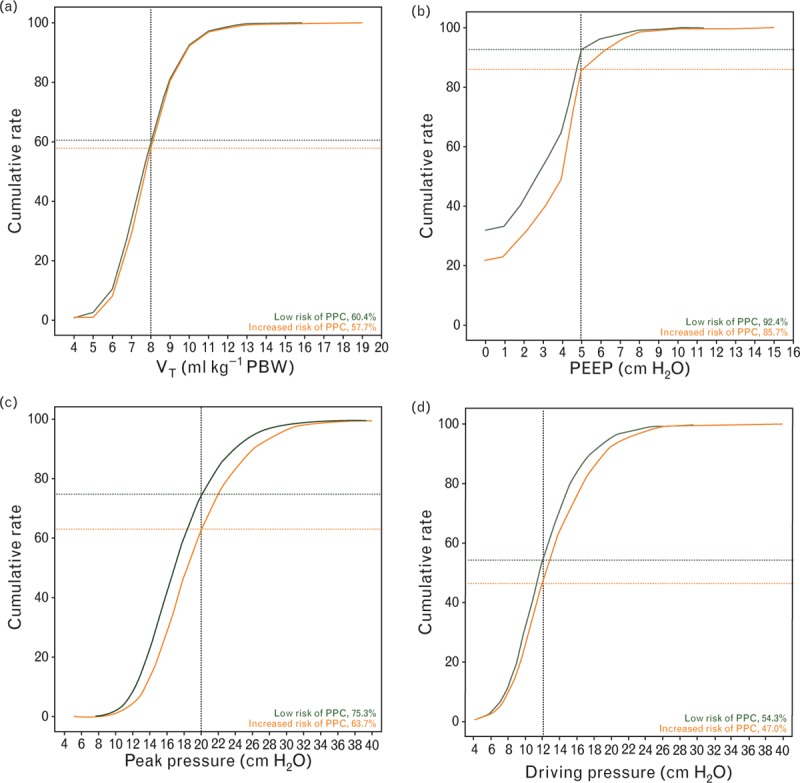
Ventilation parameters in patients at increased vs. patients at low risk of PPCs. (a) Cumulative frequency distribution of tidal volume; (b) cumulative frequency distribution of positive end-expiratory pressure; (c) cumulative distribution of peak pressure; (d) cumulative distribution of driving pressure. PBW, predicted body weight; PEEP, positive end-expiratory pressure; PPC, postoperative pulmonary complications; VT, tidal volume.
Anaesthetists generally used volume-controlled ventilation and the pressure support mode or combined modes of ventilation were seldom used (Table 3). Patients at increased risk of PPCs were ventilated at similar respiratory rates, but were ventilated with higher peak pressures (Table 3, Fig. 2c). Recruitment manoeuvres were applied more often in patients at increased risk of PPCs. The driving pressure was only calculable in patients in whom the plateau pressure was reported (60% of all patients). Driving pressure levels were higher in patients at increased risk of PPCs, but again the difference from patients at low risk was minimal (Table 3, Fig. 2d).
Distributions of combinations of ventilation settings are presented in Fig. 3. A VT 8 ml kg−1 or less PBW in combination with PEEP levels more than 5 cmH2O was used in a minority of patients, and was not different between the two risk groups. The combination of low respiratory rates with high VT was more often used in patients at increased risk of PPCs (Fig. 3d).
Fig. 3.
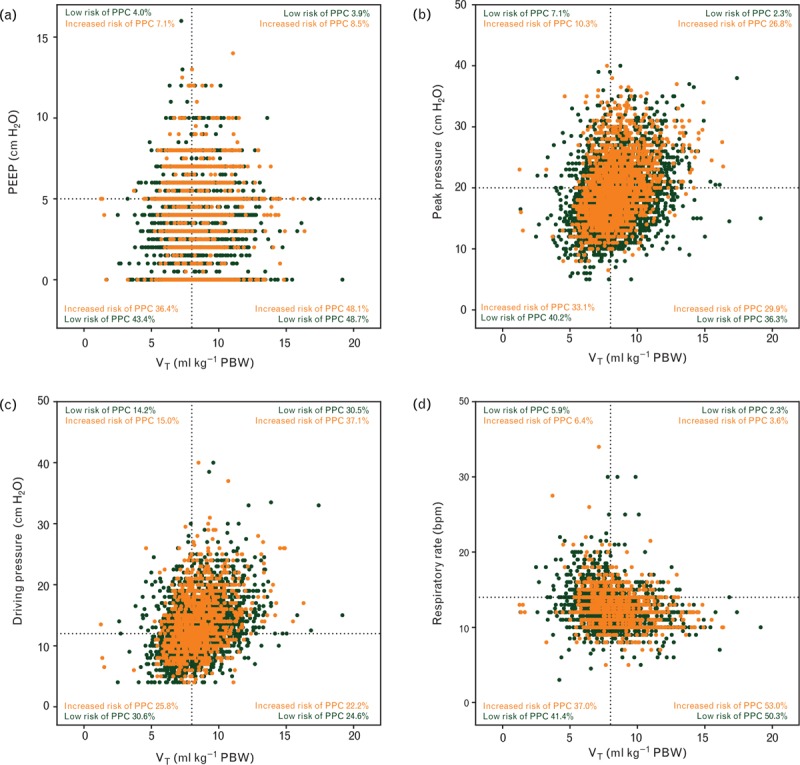
Scatterplots showing distribution of (a) tidal volume with positive end-expiratory pressure combinations; (b) tidal volume with peak pressure; (c) tidal volume with driving pressure; (d) tidal volume with respiratory rate in patients at increased vs. patients at low risk of PPCs. bpm, breaths per minute; PBW, predicted body weight; PEEP, positive end-expiratory pressure; PPC, postoperative pulmonary complications; VT, tidal volume.
Patient outcomes
Patients at increased risk of PPCs more frequently developed intraoperative events (35.3 versus 23.7%, RR 2.01 (95% CI 1.83 to 2.20), P < 0.001) and PPCs (19.2 versus 7.0%, RR 3.16 (95% CI 2.76 to 3.61), P < 0.001) (Tables 4 and 5). The most frequent intraoperative event was hypotension (Table 4). The most frequent PPCs were unplanned supplemental oxygen, followed by respiratory failure, and need for invasive mechanical ventilation (Table 5). Severe PPCs, defined as total PPCs excluding unplanned supplemental oxygen, occurred in 2.8% of all patients and in 14.5 versus 1.6% (RR 3.98 (95% CI 3.09 to 5.12), P < 0.001), of patients at increased versus low risk of PPCs, respectively (eTable 5). Patients at increased risk of PPCs had higher in-hospital mortality rates (1.7 versus 0.2%, RR 8.07 (95% CI 4.32 to 15.08), P < 0.001) and longer lengths of hospital stay (4 [1 to 7] versus 1 [0 to 3] days, P < 0.001) (Table 5, Fig. 4a to c).
Table 4.
Intraoperative events
| Variable | All patients | Low risk of PPCs | Increased risk of PPCs | Relative Risk (95% CI) | P |
| Any de-saturation | 3.9 (387/9844) | 3.3 (219/6736) | 5.6 (150/2658) | 1.78 (1.44 to 2.20) | <0.001 |
| Unplanned recruitment manoeuvre | 3.4 (332/9837) | 2.5 (171/6731) | 5.6 (148/2657) | 2.26 (1.81 to 2.83) | <0.001 |
| Ventilatory pressure reduction | 2.9 (282/9830) | 2.2 (147/6730) | 4.6 (122/1651) | 2.16 (1.69 to 2.76) | <0.001 |
| Expiratory flow limitation | 0.5 (52/9786) | 0.4 (25/6703) | 0.9 (24/2635) | 2.45 (1.40 to 4.31) | 0.001 |
| Hypotension | 26.6 (2617/9845) | 23.7 (1594/6737) | 35.3 (939/2659) | 1.76 (1.60 to 1.94) | <0.001 |
| Vasoactive drugs | 22.4 (2208/9845) | 18.5 (1246/6737) | 33.7 (897/2659) | 2.24 (2.03 to 2.48) | <0.001 |
| New arrhythmias | 0.6 (60/9838) | 0.4 (24/6732) | 1.2 (32/2657) | 3.41 (2.00 to 5.79) | <0.001 |
Low versus increased risk of PPCs, according to the Assess Respiratory Risk in Surgical Patients in Catalonia risk (ARISCAT) score (<26 versus ≥26, respectively).
Data are presented as % (n/N). Comparison of differences within a subgroup is performed by using the t-test for continuous variables and χ2 for categorical variables.
CI, confidence interval; n, number with characteristic; PPC, postoperative pulmonary complication; N, number in group or subgroup.
Definitions of intraoperative events: Any de-saturation, defined as the occurrence of SpO2 <92%; unplanned recruitment manoeuvre, ventilation strategies aimed at restoring lung aeration; ventilation pressure reduction, ventilation strategies aimed at lowering peak and/or plateau pressures; expiratory flow limitation, defined as expiratory flow higher than zero at end-expiration as suggested by visual analysis of the expiratory gas flow curve; hypotension, defined as systolic arterial pressure <90 mmHg for 3 min or longer; need for vasoactive drugs, defined as any vasoactive drug given to correct hypotension; new-onset arrhythmias, defined as new onset of atrial fibrillation, sustained ventricular tachycardia, supraventricular tachycardia, or ventricular fibrillation.
Table 5.
Patient outcomes
| Variable | All patients | Low risk of PPCs | Increased risk of PPCs | Relative Risk (95% CI) | P |
| Postoperative pulmonary complications | |||||
| Total PPCsa | 10.4 (1004/9697) | 7.0 (467/6675) | 19.2 (505/2632) | 3.16 (2.76 to 3.61) | <0.001 |
| Unplanned supplemental O2b | 8.5 (826/9697) | 5.8 (390/6675) | 15.5 (408/2632) | 2.96 (2.55 to 3.42) | <0.001 |
| Respiratory failure | 1.6 (156/9697) | 0.9 (60/6675) | 3.4 (90/2632) | 3.90 (2.81 to 5.43) | <0.001 |
| Invasive MV | 1.1 (107/9697) | 0.6 (41/6675) | 2.3 (61/2632) | 3.84 (2.58 to 5.72) | <0.001 |
| ARDS | 0.1 (9/9697) | 0.0 (1/6675) | 0.3 (8/2632) | 20.35 (2.54 to 162.76) | <0.001 |
| Pneumonia | 0.4 (40/9697) | 0.1 (10/6675) | 1.1 (28/2632) | 7.17 (3.48 to 14.77) | <0.001 |
| Pneumothorax | 0.1 (13/9697) | 0.1 (8/6675) | 0.2 (4/2632) | 1.27 (0.38 to 4.23) | 0.697 |
| Postoperative outcome | |||||
| Length of hospital stay | 1.0 [0.0 to 4.0] | 1.0 [0.0 to 3.0] | 4.0 [1.0 to 7.0] | - | <0.001 |
| In-hospital mortality | 0.6 (56/8973) | 0.2 (13/6163) | 1.7 (41/2445) | 8.07 (4.32 to 15.08) | <0.001 |
| Hospital-free daysc | 26.0 [23.0 to 27.0] | 26.0 [24.0 to 27.0] | 23.0 [21.0 to 26.0] | - | <0.001 |
Low versus increased risk of PPCs, according to the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score (<26 versus ≥26, respectively).
Data are presented as proportion, % (n/N) or median [LQ − UQ].
Comparison of differences within a subgroup is performed by using the t-test for continuous variables and χ2 for categorical variables.
ARDS, acute respiratory distress syndrome; CI, confidence interval; LOS, length of hospital stay; LQ, lower quartile; MV, mechanical ventilation; N, number in group or subgroup; n, number with characteristic; NIV, noninvasive ventilation by mask or helmet; PPCs, Postoperative pulmonary complications; PPCs: on day 1 to 5 were scored YES as soon as the event occurred on either ward or intensive care unit; UQ, upper quartile.
aTotal PPCs: one patient could present with multiple PPCs but was scored only once (YES or NO principle).
bunplanned supplementary O2: supplemental oxygen administered due to PaO2 <8 kPa or SpO2 <90% in room air, excluding oxygen supplementation given as standard care (e.g. directly after arrival in the Post Anaesthesia Care Unit.
cHospital-free days when discharged and alive at day 28.
Fig. 4.
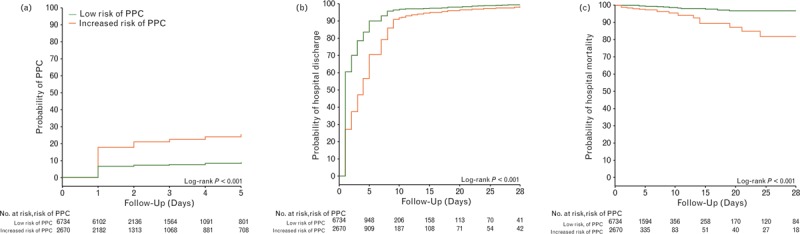
Outcome in patients at increased vs. patients at low risk of PPCs. (a) Probability of development of PPCs; (b) probability of hospital discharge; and (c) probability of in-hospital mortality. PPC, postoperative pulmonary complications.
Multivariable model to quantify the net effect of intraoperative ventilation settings
Ventilation practice in patients who did and who did not develop PPCs is presented in Table 6. The only intraoperative variables associated with the occurrence of PPCs were the peak pressure [OR 1.03 (95% CI 1.01 to 1.06), P = 0.013] and SpO2 [OR 0.84 (95% CI 0.78 to 0.91), P < 0.001]. Preoperative variables associated with increased risk of PPCs were age, American Society of Anaesthesiology (ASA) status, obstructive sleep apnoea, and emergency or urgent surgery. Restricting the PPC endpoint to more severe PPCs (by ignoring unplanned supplementary oxygen) did not change these results for either peak pressure [OR 1.04 (95% CI 1.0 to 1.08), P = 0.012] or SpO2 [OR 0.84 (95% CI 0.75 to 0.94), P = 0.002]. Preoperative variables associated with increased risk of severe PPCs were sex, metastatic cancer, obstructive sleep apnoea, emergency or urgent surgery, and laparoscopic surgery (Table 7).
Table 6.
Univariable and multivariable analyses using PPC as outcome
| Univariable analyses | Multivariable analyses | ||||||
| PPC (n = 1004) | No PPC (n = 8693) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Ventilatory parameters | |||||||
| Tidal volume (ml kg−1 PBW) | 8.0 [7.2 to 9.1] | 8.1 [7.2 to 9.1] | 0.425 | 1.01 (0.96 to 1.05) | 0.694 | 0.97 (0.90 to 1.05) | 0.501 |
| PEEP (cmH2O) | 4 [2 to 5] | 3 [0 to 5] | <0.001 | 1.12 (1.09 to 1.15) | <0.001 | 1.02 (0.97 to 1.08) | 0.343 |
| Peak pressure (cmH2O) | 18 [16 to 22] | 17 [15 to 21] | <0.001 | 1.05 (1.04 to 1.06) | <0.001 | 1.03 (1.01 to 1.06) | 0.013 |
| Plateau pressure (cmH2O)a | 17 [14 to 20] | 15 [13 to 18] | <0.001 | 1.07 (1.05 to 1.09) | <0.001 | - | - |
| Driving pressure (cmH2O)a | 13 [10 to 16] | 12 [10 to 15] | 0.002 | 1.04 (1.02 to 1.06) | <0.001 | - | - |
| FiO2 (%) | 50 [45 to 65] | 51 [45 to 70] | <0.001 | 0.99 (0.99 to 0.99) | <0.001 | 1.00 (0.99 to 1.01) | 0.152 |
| Respiratory rate (bpm) | 12 [12 to 13] | 12 [12 to 13] | 0.485 | 1.01 (0.98 to 1.04) | 0.628 | 0.99 (0.92 to 1.06) | 0.752 |
| Patient characteristics | |||||||
| Male sex | 468/1004 (46.6) | 3887/8693 (44.7) | 0.251 | 1.08 (0.95 to 1.23) | 0.252 | - | - |
| Age (years) | 61 [47 to 71] | 53 [39 to 65] | <0.001 | 1.02 ((.02 to 1.03) | <0.001 | 1.02 (1.01 to 1.02) | <0.001 |
| ≤50 | 301/1004 (30.0) | 4025/8690 (46.3) | |||||
| 51 to 80 | 628/1004 (62.5) | 4357/8690 (50.1) | <0.001 | - | - | - | - |
| >80 | 75/1004 (7.5) | 308/8690 (3.5) | |||||
| BMI (kg m−2) | 26.9 [23.9 to 30.5] | 26.2 [23.3 to 29.9] | <0.001 | 1.02 (1.01 to 1.04) | <0.001 | 1.00 (0.99 to 1.02) | 0.739 |
| ASA | 2 [2 to 3] | 2 [1 to 2] | <0.001 | 1.74 (1.60 to 1.89) | <0.001 | 1.36 (1.11 to 1.66) | 0.003 |
| 1 | 188/1000 (18.8) | 2745/8674 (31.6) | |||||
| 2 | 441/1000 (44.1) | 4245/8674 (48.9) | |||||
| 3 | 332/1000 (33.2) | 1547/8674 (17.8) | <0.001 | - | - | - | - |
| 4 | 38/1000 (3.8) | 130/8674 (1.5) | |||||
| 5 | 1/1000 (0.1) | 7/8674 (0.1) | |||||
| Functional status | |||||||
| Independent | 863/1002 (86.1) | 8090/8689 (93.1) | <0.001 | 1 (Reference) | 1 (Reference) | ||
| Partially dependent | 120/1002 (12.0) | 489/8689 (5.6) | 2.30 (1.86 to 2.84) | <0.001 | 1.26 (0.85 to 1.86) | 0.249 | |
| Totally dependent | 19/1002 (1.9) | 110/8689 (1.3) | 1.62 (0.99 to 2.65) | 0.055 | 1.31 (0.70 to 2.46) | 0.394 | |
| Smoker | 190/1003 (18.9) | 2058/8690 (23.7) | <0.001 | 0.75 (0.64 to 0.89) | <0.001 | 0.93 (0.76 to 1.15) | 0.516 |
| ARISCAT score | 26 [15 to 40] | 15 [3 to 26] | <0.001 | 1.04 (1.03 to 1.04) | <0.001 | 1.01 (1.00 to 1.02) | 0.058 |
| <26 | 467/972 (48.0) | 6208/8335 (74.5) | |||||
| 26 to 44 | 385/972 (39.6) | 1799/8335 (21.6) | <0.001 | - | - | - | - |
| >44 | 120/972 (12.3) | 328/8335 (3.9) | |||||
| Preoperative SpO2 (%) | 97 [95 to 99] | 98 [96 to 99] | <0.001 | 0.88 (0.86 to 0.91) | <0.001 | 0.98 (0.93 to 1.03) | 0.405 |
| ≥96 | 659/902 (73.1) | 6482/7656 (84.7) | |||||
| 91 to 95 | 208/902 (23.1) | 1101/7656 (14.4) | <0.001 | - | - | - | - |
| ≤ 90 | 35/902 (3.9) | 73/7656 (1.0) | |||||
| Preoperative anaemia | 62/897 (6.9) | 256/7247 (3.5) | <0.001 | 2.03 (1.52 to 2.70) | <0.001 | 1.56 (0.97 to 2.53) | 0.068 |
| Chronic comorbidity | |||||||
| Metastatic cancer | 89/1004 (8.9) | 299/8693 (3.4) | <0.001 | 2.73 (2.13 to 3.49) | <0.001 | 1.25 (0.89 to 1.76) | 0.193 |
| Chronic kidney dysfunction | 59/1004 (5.9) | 248/8693 (2.9) | <0.001 | 2.13 (1.59 to 2.85) | <0.001 | 1.25 (0.87 to 1.79) | 0.227 |
| COPD | 94/1004 (9.4) | 494/8693 (5.7) | <0.001 | 1.71 (1.36 to 2.16) | <0.001 | 1.34 (0.92 to 1.95) | 0.132 |
| Heart failure | 86/1004 (8.6) | 488/8693 (5.6) | <0.001 | 1.57 (1.24 to 2.00) | <0.001 | 0.95 (0.68 to 1.33) | 0.783 |
| Obstructive sleep apnoea | 39/1004 (3.9) | 166/8693 (1.9) | <0.001 | 2.08 (1.46 to 2.96) | <0.001 | 2.45 (1.42 to 4.23) | 0.001 |
| Neuromuscular diseaseb | 12/1004 (1.2) | 75/8693 (0.9) | 0.290 | 1.39 (0.75 to 2.57) | 0.292 | - | - |
| Liver dysfunction | 9/1004 (0.9) | 88/8693 (1.0) | 0.726 | 0.88 (0.44 to 1.76) | 0.727 | - | - |
| Surgical characteristics | |||||||
| Urgency of surgeryc | |||||||
| Elective | 836/1004 (83.3) | 7806/8691 (89.8) | <0.001 | 1 (Reference) | 1 (Reference) | ||
| Urgency | 125/1004 (12.5) | 691/8691 (8.0) | 1.69 (1.38 to 2.07) | <0.001 | 1.62 (1.14 to 2.29) | 0.007 | |
| Emergency | 43/1004 (4.3) | 194/8691 (2.2) | 2.07 (1.48 to 2.90) | <0.001 | 3.01 (1.64 to 5.53) | <0.001 | |
| Type of incision | |||||||
| Peripheral | 454/1004 (45.2) | 5026/8693 (57.8) | <0.001 | 1 (Reference) | 1 (Reference) | ||
| Abdominal | 550/1004 (54.8) | 3667/8693 (42.2) | 1.66 (1.46 to 1.89) | <0.001 | 1.05 (0.73 to 1.52) | 0.777 | |
| Type of surgery | |||||||
| Nonlaparoscopic | 821/1004 (81.8) | 7004/8693 (80.6) | 0.360 | 1 (Reference) | 0.361 | - | - |
| Laparoscopic | 183/1004 (18.2) | 1689/8693 (19.4) | 0.92 (0.78 to 1.09) | ||||
| Duration of surgery (min)d | 109 [60 to 180] | 70 [0 to 119] | <0.001 | 1.00 (1.00 to 1.00) | <0.001 | 1.00 (0.99 to 1.01) | 0.265 |
| Duration of anaethesia (min)e | 145 [93 to 230] | 100 [65 to 154] | <0.001 | 1.00 (1.00 to 1.00) | <0.001 | 1.00 (0.99 to 1.01) | 0.270 |
| Intraoperative characteristics | |||||||
| Tube type | |||||||
| Endotracheal | 885/1003 (88.2) | 7045/8688 (81.1) | 1 (Reference) | 1 (Reference) | |||
| Nasotracheal | 12/1003 (1.2) | 114/8688 (1.3) | 0.83 (0.46 to 1.52) | 0.563 | 1.42 (0.40 to 5.01) | 0.589 | |
| Supra-glottic | 94/1003 (9.4) | 1445/8688 (16.6) | 0.52 (0.42 to 0.64) | <0.001 | 0.78 (0.54 to 1.12) | 0.174 | |
| Other | 12/1003 (1.2) | 84/8688 (1.0) | 1.14 (0.62 to 2.09) | 0.679 | 0.86 (0.31 to 2.40) | 0.775 | |
| Ventilation mode | |||||||
| VCV | 705/991 (71.1) | 5994/8564 (70.0) | 1 (Reference) | 1 (Reference) | |||
| PCV | 185/991 (18.7) | 1368/8564 (16.0) | 0.005 | 1.15 (0.97 to 1.37) | 0.112 | 1.13 (0.85 to 1.49) | 0.395 |
| Assisted | 44/991 (4.4) | 578/8564 (6.7) | 0.65 (0.47 to 0.88) | 0.007 | 0.90 (0.45 to 1.82) | 0.774 | |
| Otherf | 57/991 (5.8) | 624/8564 (7.3) | 0.78 (0.59 to 1.03) | 0.079 | 0.93 (0.56 to 1.53) | 0.772 | |
| SpO2 (%) | 99 [98 to 100] | 99 [98 to 100] | <0.001 | 0.87 (0.83 to 0.91) | <0.001 | 0.84 (0.78 to 0.91) | <0.001 |
| EtCO2 (kPa) | 4.5 [4.1 to 4.9] | 4.5 [4.1 to 4.8] | 0.083 | 1.00 (0.99 to 1.01) | 0.273 | - | - |
| Transfusion of PRBC | 84/1004 (8.4) | 237/8693 (2.7) | <0.001 | 3.26 (2.52 to 4.22) | <0.001 | 1.51 (0.95 to 2.41) | 0.081 |
Data are presented as median [LQ − UQ] or n/N (%); driving pressure = plateau pressure-PEEP.
aPlateau pressure and driving pressure were only reported and calculable in 60% of all patients. Peak pressure, plateau pressure, and driving pressure had high collinearity, therefore only peak pressure was entered into the model, as plateau pressure had missing values.
ASA, American Society of Anaesthesiology; ARISCAT, Assess Respiratory Risk in Surgical Patients in Catalonia risk score; bpm, breaths per minute; CI, confidence interval; COPD, Chronic Obstructive Pulmonary Disease; EtCO2: expired carbon dioxide; FiO2, inspired fraction of oxygen; LQ, lower quartile; OR: odds ratio; PBW, predicted body weight; PEEP, positive end-expiratory pressure; PPC, composite endpoint of postoperative pulmonary complications; PRBC, packed red blood cells; SpO2, peripheral oxygen saturation; UQ, upperquartile.
bNeuromuscular disease affecting the respiratory system.
cUrgency of surgery: elective: surgery that is scheduled in advance because it does not involve a medical emergency, urgent: surgery required within <48 h, emergency: nonelective surgery performed when the patient's life or well being is in direct jeopardy.
dDuration of surgery is the time between skin incision and closure of the incision.
eDuration of anaesthesia is the time between start induction and extubation or discharge from operation room if mechanical ventilation remained.
fOther (e.g. high frequency oscillatory ventilation, jet ventilation, synchronised intermittent mandatory ventilation).
Table 7.
Univariable and multivariable analyses using severe PPC as outcome
| Univariable analyses | Multivariable analyses | ||||||
| Severe PPC (n = 1004) | No PPC (n = 8693) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Ventilator settings | |||||||
| Tidal volume (ml kg−1 PBW) | 8.0 [7.2 to 8.9] | 8.1 [7.2 to 9.1] | 0.580 | 1.01 (0.94 to 1.10) | 0.722 | 0.96 (0.84 to 1.11) | 0.621 |
| PEEP (cmH2O) | 4 [2 to 5] | 3 [0 to 5] | <0.001 | 1.11 (1.06 to 1.17) | <0.001 | 0.95 (0.88 to 1.02) | 0.153 |
| Peak pressure (cmH2O) | 18 [16 to 22] | 17 [15 to 21] | <0.001 | 1.05 (1.03 to 1.07) | <0.001 | 1.04 (1.01 to 1.08) | 0.012 |
| Plateau pressure (cmH2O)a | 17 [15 to 20] | 15 [13 to 18] | <0.001 | 1.06 (1.03 to 1.09) | <0.001 | - | - |
| Driving pressure (cmH2O)a | 13 [11 to 16] | 12 [10 to 15] | 0.001 | 1.04 (1.01 to 1.08) | 0.009 | - | - |
| FiO2 (%) | 50 [42 to 60] | 51 [45 to 70] | 0.001 | 0.99 (0.98 to 0.99) | <0.001 | 1.01 (0.99 to 1.02) | 0.080 |
| Respiratory rate (bpm) | 12 [12 to 13] | 12 [12 to 13] | 1.03 (0.98 to 1.09) | 0.204 | 1.00 (0.91 to 1.11) | 0.944 | |
| Patient characteristics | |||||||
| Male sex | 152/270 (56.3) | 4203/9427 (44.6) | <0.001 | 1.60 (1.25 to 2.04) | <0.001 | 0.036 | |
| Age (years) | 59 [45 to 69] | 53 [40 to 66] | <0.001 | 1.02 (1.01 to 1.02) | <0.001 | 0.99 (0.99 to 1.01) | 0.825 |
| ≤ 50 | 88/270 (32.6) | 4238/9424 (45.0) | |||||
| 51 to 80 | 164/270 (60.7) | 4821/9424 (51.2) | <0.001 | - | - | - | - |
| >80 | 18/270 (6.7) | 365/9424 (3.9) | |||||
| BMI (kg m−2) | 26.1 [23.2 to 29.9] | 26.2 [23.4 to 30.0] | 0.998 | 1.02 (0.99 to 1.04) | 0.065 | 1.00 (0.98 to 1.03) | 0.726 |
| ASA | 2 [2 to 3] | 2 [1 to 2] | <0.001 | 2.00 (1.69 to 2.29) | <0.001 | 1.14 (0.87 to 1.49) | 0.353 |
| 1 | 49/269 (18.2) | 2884/9405 (30.7) | |||||
| 2 | 101/269 (37.5) | 4585/9405 (48.8) | |||||
| 3 | 101/269 (37.5) | 1778/9405 (18.9) | <0.001 | - | - | - | - |
| 4 | 17/269 (6.3) | 151/9405 (1.6) | |||||
| 5 | 1/269 (0.4) | 7/9405 (0.1) | |||||
| Functional status | |||||||
| Independent | 224/270 (83.0) | 8729/9421 (92.7) | <0.001 | 1 (Reference) | 1 (Reference) | ||
| Partially dependent | 36/270 (13.3) | 573/9421 (6.1) | 2.45 (1.70 to 3.52) | <0.001 | 1.26 (0.69 to 2.29) | 0.451 | |
| Totally dependent | 10/270 (3.7) | 119/9421 (1.3) | 3.27 (1.69 to 6.33) | <0.001 | 1.70 (0.59 to 4.85) | 0.324 | |
| Smoker | 60/270 (22.2) | 2188/9423 (23.2) | 0.701 | 0.94 (0.71 to 1.26) | 0.702 | - | - |
| ARISCAT score | 27 [16 to 41] | 15 [3 to 26] | <0.001 | 1.04 (1.04 to 1.05) | <0.001 | 1.01 (0.99 to 1.03) | 0.165 |
| <26 | 104/260 (40.0) | 6571/9047 (72.6) | |||||
| 26 to 44 | 115/260 (44.2) | 2069/9047 (22.9) | <0.001 | - | - | - | - |
| >44 | 41/260 (15.8) | 407/9047 (4.5) | |||||
| Preoperative SpO2 (%) | 97 [95 to 99] | 98 [96 to 99] | 0.010 | 0.93 (0.89 to 0.97) | <0.001 | 0.99 (0.91 to 1.08) | 0.842 |
| ≥96 | 180/242 (74.4) | 6961/8316 (83.7) | |||||
| 91 to 95 | 48 242 (19.8) | 1261/8316 (15.2) | <0.001 | - | - | - | - |
| ≤90 | 14 242 (5.8) | 94/8316 (1.1) | |||||
| Preoperative anaemia | 25/259 (9.7) | 293/7885 (3.7) | <0.001 | 2.77 (1.80 to 4.25) | <0.001 | 1.83 (0.89 to 3.75) | 0.098 |
| Chronic co-morbidity | |||||||
| Metastatic cancer | 38/270 (14.1) | 350/9427 (3.7) | <0.001 | 4.25 (2.97 to 6.08) | <0.001 | 2.16 (1.28 to 3.62) | 0.004 |
| Chronic kidney dysfunction | 16/270 (5.9) | 291/9427 (3.1) | 0.008 | 1.98 (1.18 to 3.32) | <0.001 | 1.15 (0.64 to 2.05) | 0.643 |
| COPD | 35/270 (13.0) | 553/9427 (5.9) | <0.001 | 2.39 (1.66 to 3.44) | <0.001 | 1.50 (0.90 to 2.51) | 0.118 |
| Heart failure | 28/270 (10.4) | 546/9427 (5.8) | 0.001 | 1.88 (1.26 to 2.81) | 0.002 | 1.32 (0.75 to 2.31) | 0.327 |
| Obstructive sleep apnoea | 18/270 (6.7) | 187/9427 (2.0) | <0.001 | 3.53 (2.14 to 5.82) | <0.001 | 3.53 (1.72 to 7.26) | 0.001 |
| Neuromuscular diseaseb | 3/270 (1.1) | 84/9427 (0.9) | 0.705 | 1.25 (0.39 to 3.98) | 0.706 | - | - |
| Liver dysfunction | 3/270 (1.1) | 94/9427 (1.0) | 0.852 | 1.12 (0.35 to 3.54) | 0.853 | - | - |
| Surgical characteristics | |||||||
| Urgency of surgeryc | |||||||
| Elective | 195/270 (72.2) | 8447/9425 (89.6) | <0.001 | 1 (Reference) | 1 (Reference) | ||
| Urgency | 52/270 (19.3) | 764/9425 (8.1) | 2.95 (2.15 to 4.04) | <0.001 | 2.56 (1.64 to 3.99) | <0.001 | |
| Emergency | 23/270 (8.5) | 214/9425 (2.3) | 4.66 (2.96 to 7.32) | <0.001 | 5.83 (2.74 to 12.37) | <0.001 | |
| Type of incision | |||||||
| Peripheral | 111/270 (41.1) | 5369/9427 (57.0) | <0.001 | 1 (Reference) | 1 (Reference) | ||
| Abdominal | 159/270 (58.9) | 4058/9427 (43.0) | 1.89 (1.48 to 2.42) | <0.001 | 1.18 (0.73 to 1.89) | 0.501 | |
| Type of surgery | |||||||
| Nonlaparoscopic | 230/270 (85.2) | 7595/9427 (80.6) | 0.057 | 1 (Reference) | 1 (Reference) | ||
| Laparoscopic | 40/270 (14.8) | 1832/9427 (19.4) | 0.72 (0.51 to 1.01) | 0.059 | 0.49 (0.27 to 0.86) | 0.014 | |
| Duration of surgery (min)d | 115 [63 to 200] | 71 [40 to 121] | <0.001 | 1.00 (1.00 to 1.00) | <0.001 | 1.00 (0.99 to 1.01) | 0.644 |
| Duration of anaethesia (min)e | 155 [63 to 200] | 100 [65 to 160] | <0.001 | 1.00 (1.00 to 1.00) | <0.001 | 1.00 (0.99 to 1.01) | 0.328 |
| Intraoperative characteristics | |||||||
| Tube type | |||||||
| Endotracheal | 246/270 (91.1) | 7684/9421 (81.6) | 1 (Reference) | 1 (Reference) | |||
| Nasotracheal | 4/270 (1.5) | 122/9421 (1.3) | 1.02 (0.37 to 2.79) | 0.963 | 1.65 (0.44 to 6.21) | 0.458 | |
| Supra-glottic | 16/270 (5.9) | 1523/9421 (16.2) | 0.33 (0.20 to 0.55) | <0.001 | 0.57 (0.29 to 1.12) | 0.103 | |
| Other | 4/270 (1.5) | 92/9421 (1.0) | 1.36 (0.49 to 3.72) | 0.552 | 0.81 (0.11 to 6.21) | 0.843 | |
| Ventilation mode | |||||||
| Volume controlled | 181/267 (67.8) | 6518/9288 (70.2) | 1 (Reference) | 1 (Reference) | |||
| Pressure controlled | 55/267 (20.6) | 1498/9288 (16.1) | 0.237 | 1.32 (0.97 to 1.80) | 0.075 | 1.07 (0.71 to 1.61) | 0.740 |
| Assisted | 15/267 (5.6) | 607/9288 (6.5) | 0.89 (0.52 to 1.52) | 0.668 | 1.50 (0.67 to 3.34) | 0.322 | |
| Otherf | 16/267 (6.0) | 665/9288 (7.2 | 0.87 (0.52 to 1.45) | 0.587 | 1.54 (0.76 to 3.11) | 0.226 | |
| SpO2 (%) | 99 [98 to 100] | 99 [98 to 100] | 0.489 | 0.94 (0.86 to 1.02) | 0.135 | 0.84 (0.75 to 0.94) | 0.002 |
| EtCO2 (kPa) | 4.4 [4.0 to 4.8] | 4.5 [4.1 to 4.9] | 0.001 | 0.96 (0.94 to 0.99) | 0.005 | 1.03 (0.98 to 1.08) | 0.238 |
| Transfusion of PRBC | 39/270 (14.4) | 282/9427 (3.0) | <0.001 | 5.47 (3.82 to 7.84) | <0.001 | 1.82 (1.08 to 3.05) | 0.024 |
Data are presented as median [LQ to UQ] or n/N (%). Driving pressure = plateau pressure − PEEP.
ASA, American Society of Anaesthesiology; ARISCAT, Assess Respiratory Risk in Surgical Patients in Catalonia risk score; bpm, breaths per minute; CI, confidence interval; COPD, chronic obstructive pulmonary disease; EtCO2, expiratory carbon dioxide; FiO2, inspired fraction of oxygen; LQ, lower quartile; OR, odds ratio; PBW, predicted body weight; PEEP, positive end-expiratory pressure; PPC, composite endpoint of postoperative pulmonary complications; severe PPCs, same composite as total PPCs, without unplanned supplemental O2; PRBC, packed red blood cells; OR, odds ratio; SpO2, peripheral oxygen saturation; UQ, upper quartile.
aPlateau pressure and driving pressure was only reported and calculable in 60% of all patients; Peak pressure, plateau pressure, and driving pressure had high co-linearity, therefore only peak pressure was entered into the model, as plateau pressure had missing values.
bNeuromuscular disease affecting the respiratory system.
cUrgency of surgery: elective, surgery that is scheduled in advance because it does not involve a medical emergency; urgent, surgery required within <48 h; emergency, nonelective surgery performed when the patient's life or well being is in direct jeopardy.
dDuration of surgery is the time between skin incision and closure of the incision.
eDuration of anaesthesia is the time between start induction and extubation or discharge from operation room if mechanical ventilation remained.
fOther (e.g. high frequency oscillatory ventilation, jet ventilation, synchronised intermittent mandatory ventilation).
Post-hoc analyses
To provide more insight into the effects of stratification using the three original ARISCAT risk groups, we analysed the data according to the original boundaries (eTables 6, 7, 8Fig. 5, and eFigures 1, 2). The incidence of PPCs, mortality rates, and duration of stay in hospital significantly increased from the lowest to the highest risk group (eTable 8).
Fig. 5.
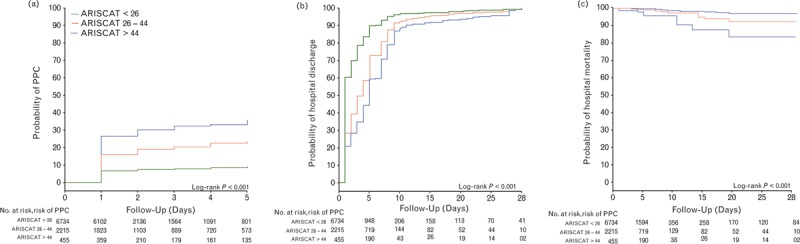
Outcome in patients at low, moderate, and high risk of PPCs: (a) probability of development of PPCs; (b) probability of hospital discharge; and (c) probability of in-hospital mortality. PPC, postoperative pulmonary complications.
Discussion
This prospective observational study with centres from 30 different countries shows that a substantial proportion of patients undergoing invasive ventilation are at risk of development of PPCs. These patients receive higher VT and higher PEEP levels compared with patients at low risk, but the differences are small. Only a minority of patients receive intraoperative ventilation with VT less than 8 ml kg−1 PBW and a PEEP level more than 5 cmH2O. The incidence of PPCs is high, and higher in patients at increased risk. Patients at risk of PPCs have longer lengths of hospital stay and increased in-hospital mortality.
To our knowledge, the LAS VEGAS study is the largest prospective investigation describing intraoperative ventilation strategies and the incidence of intraoperative events and PPCs to date. The study is also the first to show the incidence of surgical patients at increased risk of PPCs using the ARISCAT score on a truly international basis.17 The international character of this study represents practice in many countries. The 1-week prospective design of LAS VEGAS avoided the effects of changes over time as the data were collected within a short period. The findings of LAS VEGAS could help to guide hypotheses for future trials of intraoperative ventilation and the data could be employed to support sample size calculation for such a study of PPCs. It should be stressed that the design of the current study excludes the possibility of determining cause–effect relationships and does not allow the defining of any pathophysiological associations with the outcome measures.
ARISCAT is an internally and externally validated score for risk stratification that uses seven easy to obtain objective factors. The incidence of patients at increased risk of PPCs in LAS VEGAS was comparable to that in the original ARISCAT studies.17,18 In addition, the proportion of patients at moderate and at high risk of PPCs who developed one or more PPCs was similar to the original studies.17,18
LAS VEGAS extends our knowledge of the practice of intraoperative ventilation, as it is the first study to explore the use of intraoperative ventilator settings, not only in patients at increased risk of PPCs, but also in patients at low risk. Our results show remarkably little difference in ventilation practice between these two patient categories and indicate that protective ventilation (i.e. using a combination of low VT and higher PEEP levels) is not in widespread use. Notably, recent observational studies in critically ill patients with or without ARDS also show strikingly similar distributions of VT-size.6,7
The current results also confirm previous reports of ventilation practice in the surgical setting: previous audits and retrospective studies of intraoperative ventilation showed that surgical patients continue to receive ventilation with high VT.25–27 These studies, however, did not compare ventilation practice in different risk groups. The results from LAS VEGAS suggest that VT has reduced when compared with earlier studies, at least in the participating centres. This finding is in line with recent findings in a prospective study in patients with high ASA scores.28 Interestingly, VT was not a predictor for the development PPCs, a finding similar to the results of a previous observational study.29
Our results suggest that there are two preferences with regard to the PEEP level during intraoperative ventilation as the most frequently chosen levels were 0 and 5 cmH2O. PEEP levels more than 5 cmH2O were rarely used, even in patients at increased risk of PPCs. The frequent use of PEEP levels equal to or lower than 5 cmH2O is comparable to reports from previous studies.25–28 In the LAS VEGAS study no association between PEEP levels and the occurrence of PPCs was found, similar to a recent randomised controlled trial.30 In contrast to our results, three recent trials advocate the use of higher PEEP levels for protective ventilation.9–11 The absence of a protective effect of higher PEEP levels in our study could be explained by a possible parabola-shaped association between PEEP and the development of PPCs, as found in a previous analysis.29 In that study of electronic patient records, PEEP levels less than 5 cmH2O and more than 5 cmH2O were associated with higher risk of PPCs, suggesting that a PEEP level of 5 cmH2O would be most protective.29 Second, we did not examine specific operative procedures whereas a recent study suggest that PEEP can have different effects on PPCs, depending on the procedure performed.31
Our study also indicates that high peak pressures (>20 cmH2O) and high driving pressures (>12 cmH2O) are used in many patients and, in particular, in patients at increased risk of PPCs. Patients who developed PPCs were ventilated with higher peak pressures, plateau pressures, and driving pressures than patients who did not develop PPCs. This mirrors two recent investigations showing an association between increasing plateau pressures29 and the development of PPCs and between intraoperative changes in the driving pressure level and occurrence of PPCs.32 Of interest, in LAS VEGAS higher peak pressures seem to be associated with increased risk of PPCs, when corrected for known risk factors.20–22 We chose to limit the multivariable model to peak pressures, as there was high co-linearity between peak pressure, plateau pressure, and driving pressure. Intraoperative SpO2 was also associated with development of PPCs. This finding must be interpreted with caution, as SpO2 levels may have several confounders, even though the model was corrected for preoperative SpO2, smoking, chronic obstructive pulmonary disease, intraoperative PEEP and FiO2. Nevertheless, it could be that patients who develop hypoxaemia during surgery are at increased risk of PPCs, suggesting that these patients may benefit from more intensive postoperative monitoring. Although the difference between the groups in the peak pressures applied are small, it still could be of potential importance, as the analysis suggests that for every increase of 1 cmH2O in peak pressure there is a 3% increase in the odds ratio for the development of PPCs. Because of the large number of surgical procedures in which a patient requires intraoperative ventilation (approximately 234 million per year16), even a small rise in the incidence of PPCs translates into a large number of patients at risk.
Even though clinicians were not informed about the subsequent use of ARISCAT score, we cannot exclude the fact that patients expected to be at risk of developing PPCs, for example sicker patients with underlying lung disease or obese patients, received or required adjusted ventilator settings, while also developing PPCs more often.
The composite endpoint of PPCs included ‘unplanned supplementary oxygen’, a definition used in previous clinical studies,17,18 and in a recent publication on standards for definitions and the use of outcome measures for research into clinical effectiveness in perioperative medicine.33 As unplanned supplementary oxygen could have confounders, we excluded this from the composite endpoint for ‘severe PPCs’ in one post-hoc analysis. This analysis showed similar results. Of note, a recently published study showed that patients requiring prolonged postoperative oxygen had increased hospital lengths of stay.28
In line with the abovementioned study,28 the results of the current study confirm that patients who develop PPCs have longer duration of hospital admission and increased in-hospital mortality. Previously, the PERISCOPE study showed that worsening patient outcomes are associated with a rise in the number of PPCs.18 Furthermore, the probability of patient survival decreases sharply with increasing severity of postoperative respiratory failure.34 These results were confirmed by two recent large individual data meta-analyses, showing an association between development of PPCs and longer lengths of hospital stay, and increased mortality.14,15 Even though this study is not designed to evaluate the relation between occurrence of PPCs and outcome, our results mirror these earlier studies.14,15,18,34
Supraglottic devices were used markedly fewer times in patients at increased risk of PPCs compared with patients at low risk, which could have an effect on applied ventilation strategies and outcome. However, when entering supraglottic devices into the multivariable model, the variable did not have an association with development of PPCs.
The findings of this study suggest that more attention should be given to protection of the lung from the potentially harmful effects of intraoperative ventilation. First, it seems that protective ventilation is not always used during surgery. Second, although the results of this study confirm previous findings that high inspiratory and high driving pressures levels during intraoperative ventilation could play major roles in the development of PPCs,29,32 uncertainty regarding how to prevent such high pressures remains. There is a need for feasibility studies testing interventions aimed at low pressures, and for randomised controlled trials that test whether such strategies do have the potential to prevent PPCs in surgical patients.35 Currently, several randomised controlled trials are being conducted to investigate the effect of protective ventilation in specific patient populations, such as the PROBESE trial in obese patients (Clinicaltrials.gov identifier: NCT02148692, https://clinicaltrials.gov/ct2/show/NCT02148692?term=NCT02148692&rank=1), the PROTHOR trial in patients undergoing one-lung ventilation (Clinicaltrials.gov identifier: NCT02963025, https://clinicaltrials.gov/ct2/show/NCT02963025?term=NCT02963025&rank=1), and the AVATaR trial in patients undergoing robotic surgery (Clinicaltrials.gov identifier: NCT02989415, https://clinicaltrials.gov/ct2/show/NCT02989415?term=NCT02989415&rank=1).
The LAS VEGAS study has several limitations. First, the willingness of participating centres to join the study may have resulted in a selection bias towards those centres with an interest in protective ventilation, meaning that they may already use low VT during general anaesthesia for surgery. Second, any prospective observational study can interfere with daily practice, making anaesthetists more likely to use those ventilation settings that are considered to be lung-protective. Third, due to the selection of centres participating in this study, the results may not be representative of ventilation management in the different countries. Fourth, there was no restriction on the number of centres per country and, with the number of centres per country ranging from 1 to 19, this could have biased the results. Fifth, it is possible that there was a quality difference in data reporting between the centres despite extensive data cleaning. Sixth, the design of the study only allowed the recording of data collected as part of standard care and thus, we were required to restrict our collection of postoperative pulmonary complications to those that could be captured easily in all patients, without ordering extra laboratory or radiographic examinations. Last, although the large number of PPC events permitted a high number of variables in our analysis,36 the large exploratory multivariate model has its potential limitations and should not be used for cause–effect determination.
Conclusion
The incidence of patients at risk of PPCs is high. A large proportion of patients receive high VT and low PEEP levels, seemingly independent of the risk of PPCs. Patients at increased risk more frequently develop PPCs, have longer lengths of hospital stay and increased in-hospital mortality. These findings suggest that more attention could be given to the use of lung-protective modes during intraoperative mechanical ventilation in patients at risk of PPCs.
Supplementary Material
Acknowledgements relating to this article
Assistance with study: we are indebted to all participating research nurses, nurse anaesthetists, surgeons, other physicians, and our patients. We particularly wish to acknowledge Brigitte Leva, Sandrine Damster, and Benoit Plichon from the Clinical Trial Network of the European Society of Anaesthesiology for their expertise and professional help in coordinating the study and cleaning the data for the LAS VEGAS study. Without any of the aforementioned, the LAS VEGAS study would not have been possible. All members of the Steering Committee contributed to the design and conduct of the LAS VEGAS study.
The members of the LAS VEGAS Steering Committee and Collaborators can be found in the Supplementary Digital Content pp. 2 to 10.
In addition to the LAS VEGAS Study Investigators (Supplementary Digital Content:), the following individuals helped to prepare the manuscript: Sabrine N.T. Hemmes (Academic Medical Center, Amsterdam, the Netherlands); Ary Serpa Neto (Hospital Israelita Albert Einstein; Sao Paulo, Brazil; Faculdade de Medicina do ABC; Sao Paulo, Brazil); Jan M. Binnekade (Academic Medical Center, Amsterdam, the Netherlands); Jaume Canet (Hospital Universitari Germans Trias I. Pujol, Barcelona, Spain); Goran Hedenstierna (University Hospital Uppsala, Uppsala, Sweden); Samir Jaber (Saint Eloi University Hospital, Montpellier, France); Michael Hiesmayr (Medical University Vienna, Vienna, Austria); Markus W. Hollmann (Academic Medical Center, Amsterdam, the Netherlands); Gary H. Mills (Sheffield Teaching Hospitals, Sheffield, UK); Marcos F. Vidal Melo (Massachusetts General Hospital, Boston, USA); Rupert Pearse (Queen Mary University of London, London, UK); Christian Putensen (University Hospital Bonn, Bonn, Germany); Werner Schmid (Medical University Vienna, Vienna, Austria); Paolo Severgnini (University of Insubria, Varese, Italy); Hermann Wrigge (University of Leipzig, Leipzig, Germany); Marcelo Gama de Abreu (University Hospital Dresden, Dresden, Germany); Paolo Pelosi (IRCCS AOU San Martino IST Hospital, University of Genoa, Genoa, Italy); and Marcus J. Schultz (Academic Medical Center, Amsterdam, the Netherlands).
Sabrine Hemmes and Ary Serpa Neto had complete access to all the data in the study and performed data analysis, with support from Marcelo Gama de Abreu, Paolo Pelosi, and Marcus Schultz. Sabrine Hemmes, Ary Serpa Neto, Marcelo Gama de Abreu, Paolo Pelosi, and Marcus Schultz made the final decision to submit the report for publication. All contributed equally to the study.
Financial support and sponsorship: the LAS VEGAS study was endorsed and partly funded by a research grant from the European Society of Anaesthesiology through their Clinical Trial Network.
Conflicts of interest: none.
Presentation: the study was presented in part at Euroanaesthesia 2014 and 2015.
The LAS VEGAS (Local ASsessment of VEntilatory management during General Anaesthesia for Surgery) investigators are listed in the Supplementary Digital Content.
Published online 14 June 2017
Contributor Information
Collaborators: The LAS VEGAS investigators
References
- 1.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013; 369:2126–2136. [DOI] [PubMed] [Google Scholar]
- 2.Putensen C, Theuerkauf N, Zinserling J, et al. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med 2009; 151:566–576. [DOI] [PubMed] [Google Scholar]
- 3.Serpa Neto A, Nagtzaam L, Schultz MJ. Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta-analysis. Curr Opin Crit Care 2014; 20:25–32. [DOI] [PubMed] [Google Scholar]
- 4.Serpa Neto A, Simonis FD, Barbas CS, et al. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive Care Med 2014; 40:950–957. [DOI] [PubMed] [Google Scholar]
- 5.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010; 303:865–873. [DOI] [PubMed] [Google Scholar]
- 6.Bellani G, Laffey JG, Pham T, et al. for LUNG SAFE Investigators and the ESICM Trials Group. The LUNG SAFE study: a presentation of the prevalence of ARDS according to the Berlin Definition. Crit Care 2016; 20:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serpa Neto A, Barbas CS, Simonis FD, et al. Epidemiology, practice of ventilation and outcome for patients at risk of ARDS in intensive care units in 16 countries. Lancet Resp Med 2016; 4:882–893. [DOI] [PubMed] [Google Scholar]
- 8.Schultz MJ, Haitsma JJ, Slutsky AS, et al. What tidal volumes should be used in patients without acute lung injury? Anesthesiology 2007; 106:1226–1231. [DOI] [PubMed] [Google Scholar]
- 9.Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013; 118:1307–1321. [DOI] [PubMed] [Google Scholar]
- 10.Futier E, Constantin JM, Paugam-Burtz C, et al. for IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013; 369:428–437. [DOI] [PubMed] [Google Scholar]
- 11.Ge Y, Yuan L, Jiang X, et al. Effect of lung protection mechanical ventilation on respiratory function in the elderly undergoing spinal fusion. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2013; 38:81–85. [DOI] [PubMed] [Google Scholar]
- 12.Güldner A, Kiss T, Serpa Neto A, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 2015; 123:692–713. [DOI] [PubMed] [Google Scholar]
- 13.Hedenstierna G, Edmark L. Mechanisms of atelectasis in the perioperative period. Best Pract Res Clin Anaesthesiol 2010; 24:157–169. [DOI] [PubMed] [Google Scholar]
- 14.Serpa Neto A, Hemmes SN, Barbas CS, et al. for PROVE Network Investigators. Protective ventilation with lower tidal volumes and high PEEP versus conventional ventilation with high tidal volume and low PEEP in patients under general anesthesia for surgery: a systematic review and individual patient data meta-analysis. Anesthesiology 2015; 123:66–78. [DOI] [PubMed] [Google Scholar]
- 15.Serpa Neto A, Hemmes SN, Barbas CS, et al. for PROVE Network Investigators. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med 2014; 2:1007–1015. [DOI] [PubMed] [Google Scholar]
- 16.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–144. [DOI] [PubMed] [Google Scholar]
- 17.Canet J, Gallart L, Gomar C, et al. for the ARISCAT Group. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010; 113:1338–1350. [DOI] [PubMed] [Google Scholar]
- 18.Mazo V, Sabate S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014; 121:219–231. [DOI] [PubMed] [Google Scholar]
- 19.International Conference on Harmonisation (ICH). International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use: ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. J Postgrad Med 2001; 47:199–203. [PubMed] [Google Scholar]
- 20.Arozullah AM, Daley J, Henderson WG, et al. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg 2000; 232:242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arozullah AM, Khuri SF, Henderson WG, et al. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 2001; 135:847–857. [DOI] [PubMed] [Google Scholar]
- 22.Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:581–595. [DOI] [PubMed] [Google Scholar]
- 23.Ranieri VM, Rubenfeld GD, Thompson BT, et al. for the ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 24.Hemmes SN, de Abreu MG, Pelosi P, et al. ESA Clinical Trials Network 2012: LAS VEGAS – Local Assessment of Ventilatory Management during General Anaesthesia for Surgery and its effects on Postoperative Pulmonary Complications: a prospective, observational, international, multicentre cohort study. Eur J Anaesthesiol 2013; 30:205–207. [DOI] [PubMed] [Google Scholar]
- 25.Karalapillai D, Weinberg L, Galtieri J, et al. Current ventilation practice during general anaesthesia: a prospective audit in Melbourne, Australia. BMC Anesthesiol 2014; 14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bender SP, Paganelli WC, Gerety LP, et al. Intraoperative lung-protective ventilation trends and practice patterns: a report from the multicenter perioperative outcomes group. Anesth Analg 2015; 121:1231–1239. [DOI] [PubMed] [Google Scholar]
- 27.Wanderer JP, Ehrenfeld JM, Epstein RH, et al. Temporal trends and current practice patterns for intraoperative ventilation at U.S. academic medical centers: a retrospective study. BMC Anesthesiol 2015; 15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg 2017; 152:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladha K, Vidal Melo M, McLean D, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015; 351:3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.PROVE Network Investigators. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014; 384:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jong M, Ladha K, Melo M, et al. Differential effects of intraoperative positive end-expiratory pressure (PEEP) on respiratory outcome in major abdominal surgery versus craniotomy. Ann Surg 2016; 264:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serpa Neto A, Hemmes SN, Barbas CS, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 2016; 4:272–280. [DOI] [PubMed] [Google Scholar]
- 33.Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions. Eur J Anaesthesiol 2014; 31:1–18. [DOI] [PubMed] [Google Scholar]
- 34.Canet J, Sabaté S, Mazo V, et al. for the PERISCOPE group. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: a prospective, observational study. Eur J Anaesthesiol 2015; 32:458–470. [DOI] [PubMed] [Google Scholar]
- 35.Ferrando C, Soro M, Canet J, et al. Rationale and study design for an individualized perioperative open lung ventilatory strategy (iPROVE): study protocol for a randomized controlled trial. Trials 2015; 16:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol 2001; 54:979–985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


