Abstract
Objectives
To compare staging correctness between contrast-enhanced FDG PET/ceCT and 64-slice multi–detector-row CT (ceCT64) for initial staging and response evaluation at the end of treatment (EOT) in patients with Hodgkin lymphoma, diffuse large B cell lymphoma (DLBCL), and follicular lymphoma.
Methods
This prospective study compared initial staging and response evaluation at EOT. One hundred eighty-one patients were randomly assigned to either ceCT64 or FDG PET/ceCT. A nuclear medicine physician and a radiologist read FDG PET/ceCT scans independently and achieved post hoc consensus, whereas another independent radiologist interpreted ceCT64 separately. The reference standard included all clinical information, all tests, and follow-up. Ethics committees of the participating centers approved the study, and all participants provided written consent.
Results
Ninety-one patients were randomized to ceCT64 and 90 to FDG PET/ceCT; 72 had Hodgkin lymphoma, 72 had DLBCL, and 37 had follicular lymphoma. There was excellent correlation between the reference standard and initial staging for both FDG PET/ceCT (κ = 0.96) and ceCT64 (κ = 0.84), although evaluation of the response at EOT was excellent only for FDG PET/ceCT (κ = 0.91).
Conclusions
Our study demonstrated satisfactory agreement between FDG PET/ceCT (κ = 0.96) and ceCT64 (κ = 0.84) in initial staging compared with the reference standard (P = 0.16). Response evaluation at EOT with FDG PET/ceCT (κ = 0.91) was superior compared with ceCT64 (κ = 0.307) (P < 0.001).
Key Words: CT, FDG PET/CT, Hodgkin lymphoma, lymphoma, non-Hodgkin lymphoma
Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) are malignant clonal neoplastic processes characterized by the proliferation of cells originating from lymphoid tissues, lymphocytes, and their precursors and derivatives. They jointly comprise 5% to 6% of all malignant tumors and are the fifth leading cause of cancer-related mortality in the United States.1 The 2016 revision of the classification of lymphomas by the World Health Organization divides HL into two groups: nodular lymphocyte predominant HL and classic HL with nodular sclerosis, lymphocyte-rich classic, mixed cellularity classic, and lymphocyte-depleted classic HL. Non-Hodgkin lymphoma is divided into two categories: precursor lymphoid neoplasms and mature cell neoplasms.2,3 The most frequent subtypes of NHL are diffuse large B cell lymphoma (DLBCL) and follicular lymphoma (FL) comprising 30% and 22%, respectively. Together, HL, DLBCL, and FL constitute approximately 80% of adult lymphomas.4
18F-FDG PET demonstrates glucose metabolic differences that may occur before the appearance of structural changes.5,6 Hodgkin lymphoma, DLBCL, and FL are FDG avid.7–9 FDG PET/CT modifies lymphoma stage in between 14% and 59% of patients when compared with CT or FDG PET without CT.10–18 There are few high-evidence-level prospective studies validating the use of PET/CT in the treatment of lymphoma. Moreover, PET/CT is relatively expensive and may present pitfalls and artifacts.19 Although there are reports comparing PET/CT to 4–detector-row CT16–20 or CT without documentation of detector number,19 there are no validation studies comparing PET/ceCT with 64-detector ceCT for lymphoma staging.
Although there are histological and immunochemistry differences, published data suggest similar FDG uptake for HL, DLBCL, and FL.9 In a prospective study, we demonstrated FDG avidity in more than 90% of patients with DLBCL and 80% with FL.16
FDG PET/CT lymphoma staging and response assessment recommendations were presented at the fourth International Workshop on PET in Lymphoma, and a working paper was presented at the 12th International Congress of Malignant Lymphoma. The Lugano classification includes FDG PET/CT for end-of-treatment (EOT) evaluation in HL and a consensus on the use of FDG PET/CT in staging and monitoring response.21–24
The aim of this prospective study was to determine the agreement between FDG PET/ceCT and ceCT64 in the initial staging and during EOT evaluation in patients with HL, DLBCL, and FL. Therapeutic response was characterized based on FDG uptake as complete response (CR), partial response (PR), or progressive disease (PD). Outcomes were correlated with pretherapy and posttherapy ceCT64 and FDG PET/ceCT.
MATERIALS AND METHODS
Study Design
This is a prospective, multicenter, randomized, comparative study conducted at the Autonomous University of Madrid, University Hospital La Princesa, University Hospital Clínico San Carlos, University Hospital Fundación Jiménez Díaz, University Hospital La Paz, and San Pedro Hospital.
Patients
Between January 2012 and May 2015, a total of 181 consecutive patients with biopsy-proven untreated lymphoma were included. Patients were randomized either to ceCT64 or FDG PET/ceCT for initial staging and response assessment at the EOT. The study was approved by the ethics committee of each participating hospital and conducted according to the Declarations of Helsinki and Tokyo. Written informed consent was obtained from all participants. Participants were recruited from 5 hematology departments in Spain. Each hospital evaluated clinical data and performed confirmatory biopsies, and the pathology department of each center classified lymphoma subtypes based on the 2016 World Health Organization classification.2,3 All participating centers had similar equipment.
Independent of the branch assigned, FDG PET/ceCT was always performed at EOT21 for all patients with HL. Therefore, patients with HL were asked to sign informed consent specifying both tests would be done.
Inclusion criteria were as follows: (a) biopsy-pathology–proven diagnosis of HL, DLBCL, or FL not treated previously for lymphoma and without previous imaging tests for initial staging; (b) signed informed consent; and (c) age older than 18 years.
Exclusion criteria were as follows: (a) renal failure with a creatinine clearance below 30 mL/min; (b) human immunodeficiency virus infection; (c) known inflammatory or granulomatous disease (tuberculosis, sarcoidosis); (d) a life expectancy of less than 3 months or poor general health (a Zubrod performance scale status >3); (e) known allergies to CT contrast agents; (f) a positive pregnancy test; and (g) suspicion or confirmation of alcohol or drug abuse.
Reference or Criterion Standard
As other authors have done previously,12,18,20,25 we defined the reference or criterion standard from a combination of factors including clinical history, physical examination, laboratory evaluation including β2-microglobulin and lactate dehydrogenase, bone marrow biopsy, endoscopy for gastrointestinal symptoms, lumbar puncture for central nervous system signs, and biopsies of other suspicious areas. Based on all available information, each patient was clinically staged based on the Ann Arbor staging system that included modifications introduced in the 11th International Conference on Malignant Lymphoma in Lugano in 2014.21
18F-FDG PET/ceCT
PET/ceCT studies were performed using 2 similar Siemens Biograph PET/CT systems (Siemens, Erlangen, Germany). Of the 90 patients randomized to the PET/ceCT branch, 80 patients (80/90 [89.5%]) were scanned in a PET/CT system with a 6-detector row CT (Biograph 6 TruePoint; Siemens) with a theoretical 3D PET spatial resolution of 3 to 4 mm. It includes 4 rows of detectors with LSO crystals with topogram CT dose modulation. The remaining 10 patients (10/90 [10.5%]) were scanned with a 16-detector-row CT (Biograph 16; Siemens), with no differences in the other technical factors. The FDG PET/ceCT procedure was similar for all the patients in all centers.
Patient Preparation and Study Protocol
Participants avoided strenuous exercise for 24 hours and fasted for 6 hours before FDG administration. Blood glucose level was measured with an upper threshold of 200 mg/dL. 18F-FDG dosage was 5 MBq/kg using an automatic injector (Medrad Intego 200; Bayer, Leverkusen, Germany). All patients had an uptake period of 45 to 60 minutes in a quiet room. The 18F-FDG PET/ceCT procedure was performed following the European Association of Nuclear Medicine (EANM) guidelines,26,27 administering 130 mL of iodinated contrast with a delay of 45 seconds and a speed of 2.5 mL/s. An arterial-phase thoracic CT (110 kV and 60 mAs, pitch of 1.2, and 2.5-mm thickness) was performed with deep inspiration, followed by whole-body portal-phase CT (110 kV and 95 mAs, 0.5 second tube rotation, pitch of 6, and a thickness of 5 mm) in a craniocaudal direction from the top of the skull to thighs. PET was acquired from caudal to cranial with 3-minute beds and 20% overrun. Iterative reconstruction was applied.
Interpretation and Image Analysis
Studies were analyzed by a nuclear medicine physician and a radiologist, all with experience in the field using the same workstation (Syngo™ software system; Siemens Medical Imaging). Both readings were done independently with post hoc consensus. Discrepancies were resolved by discussion. PET/ceCT was interpreted as an integrated examination.
As previously described,21–24 semiquantitative 18F-FDG uptake was compared between potential lesions and areas of physiological uptake. Criteria for classifying imaging findings as positive or negative were as follows:
Visual analysis: Pathological 18F-FDG uptake was considered when there was increased regional 18F-FDG uptake where lymphoma was clinically suspected. If there were no increased uptakes of 18F-FDG in any region where lymphoma was clinically suspected, PET was categorized as negative for lymphoma.
Semiquantitative analysis: SUVmax was measured and compared with the physiological uptake (measured as SUVmax) in 2 regions: the mediastinal blood pool (MBP) and the liver. A 5-point scale was applied, where 0 was the absence of pathological uptake, 1 when the SUVmax was lower than the MBP, 2 when the SUVmax was higher than the MBP but lower than the liver, 3 when SUVmax was higher than the liver without duplicating the SUVmax, and 4 when the SUVmax was more than double the liver uptake.
In addition, to monitor the response to therapy, PERCIST (version 1.0) criteria were applied following a previous work done with PERCIST criteria lymphomas by Tadashi et al.28 These criteria assess response to therapy as the percentage of change measured in SULpeak in the most active lesion (target lesion) between the pretherapy and posttherapy FDG PET/ceCT studies.
ceCT64 Interpretation and Image Analysis
Contrast-enhanced CT was performed using GE and Siemens 64 slice multi-detector row scanners (ceCT64). Oral (750 cm3 of Gastrografin) and intravenous iodinated contrast (2 mL/kg of iodinated contrast administered at a rate of 3 mL/s) were administered. Arterial-phase thoracic CT was performed in deep inspiration with 35-second delay (120 kVp, 370 mAs, pitch of 1.35, and single collimation width 1.2 mm), followed by portal-phase abdominal CT from the top of the liver to the thighs with 65- to 70-second delay (120 kVp, 500 mAs, pitch of 1.35, single collimation width 1.2 mm). Finally, scanning from the base of the skull to supraclavicular region with 90 seconds of delay (120 kVp, 380 mAs, pitch of 0.9, single collimation width 0.6 mm) was performed.
Lymph nodes with a short-axis diameter of more than 10 mm were considered positive, except for inguinal lymph nodes, which were considered positive if more than 15 mm.6 Extranodal disease positivity was considered with increased organ size, abnormal contrast enhancement, soft tissue nodules or masses, and bone changes. An independent radiologist with broad experience in CT interpreted the ceCT64 examinations. A second radiologist performed consensus reading. RECIST (Response Evaluation Criteria In Solid Tumors) criteria one-dimensional measures were used to monitor treatment results.29 To apply RECIST criteria, target lesions were defined at baseline and compared with follow-up.
Statistical Analysis
Qualitative variables were presented by frequency distribution, and quantitative variables were presented as mean and standard deviation (SD). A validity analysis was performed. Agreement between each diagnostic technique with the criterion standard was also calculated (kappa coefficient [κ] and 95% confidence intervals [CIs]). All statistical tests were considered bilateral, and significant results were considered when P < 0.05 was achieved.
The statistical program used was IBM SPSS Statistics version 22.00 (IBM, New York, NY).
RESULTS
Between January 2012 and May 2015, 181 patients were enrolled and randomly assigned to either the FDG PET/ceCT group (90 patients) or ceCT64 group (91 patients); there were 91 men and 90 women. Mean ages were 52 and 51 years, respectively, with a period of patient inclusion of 2.5 years and a follow-up that was prolonged until the EOT, death, or the end of the study with a mean follow-up of 16 months.
Table 1 demonstrates that there were no clinical differences between the 2 randomized groups. The flowchart for participant selection is presented in Figure 1.
TABLE 1.
Characteristics of the Participants at Baseline

FIGURE 1.

Flowchart for participant selection.
Table 2 summarizes comparative results for initial staging and the final response to treatment for FDG PET/ceCT compared with ceCT64.
TABLE 2.
Results for Initial Staging and Final Response With ceCT64 and FDG PET/ceCT Compared With the Reference Standard

Initial Staging With ceCT64
Sixty-four-slice multi–detector row CT was concordant with the reference standard for staging in 90.2% of cases (82/91) with κ = 0.84 (P < 0.001), as presented in Table 2. Based on the reference standard, the following stages were identified: stage I (n = 7), stage II (n = 23), stage III (n = 17), and stage IV (n = 6). Stages I and III were correctly evaluated, with sensitivity between 85% and 100% and specificity between 92% and 100%, as presented in Table 2. Two patients were overstaged: (a) one with lymphoma in the central nervous system (stage IV) when he would have been considered stage IE applying the Lugano classification and (b) a patient with apparent bone disease on CT who was also considered stage IV, in whom lymphomatous bone disease was later excluded by biopsy, reducing her to stage II DLBCL (Fig. 2). One patient presented with neurological symptoms associated with leptomeningeal involvement on MRI and with stage I disease only on CT. Six patients were considered a stage III, although they were later confirmed as stage IV because of positive extranodal involvement confirmed in the bone marrow biopsy (5 with DLBCL, 1 with HL).
FIGURE 2.

A 48-year-old woman diagnosed with DLBCL in whom ceCT64 was false positive in the bone and therefore was overstaged. Initial staging with ceCT64 showed a lymphadenopathy in the right hilum (A) and multiple bone lytic lesions in the pelvis (B). At the EOT, ceCT64 demonstrated complete resolution of the lymphadenopathy in the right hilum (C) and persistent multiple bone lytic lesions in the pelvis with no significant changes (D). Lymphomatous involvement of the bone was suspected based on the ceCT64 images, but the biopsy was negative. The pathology study (E) revealed a fibrous lesion composed of extracellular matrix and fibroblastic cells; no lymphoid cells were present (hematoxylin-eosin stain, original magnification ×200). Immunohistochemistry confirmed the absence of lymphoma cells. After lymphomatous bone disease was later excluded by biopsy, the initial stage IV by ceCT64 due to bone disease was corrected to stage II.
Initial Staging With FDG PET/ceCT
Contrast-enhanced FDG PET/CT was concordant with the reference standard for staging in 97.8% of cases (88/90) with κ = 0.96 (P < 0.001), and sensitivity and specificity for each stage were 98% to 100% and 98% to 100%, respectively, as presented in Table 2. Two patients were erroneously staged. One was considered stage III but ultimately confirmed as stage IV because of bone marrow infiltration, and another case was a bone lesion in the femur that was categorized as stage IV being finally confirmed as stage II. Interestingly, all of the 45 patients classified as stage IV had extranodal or bone marrow involvement.
Comparison of the Initial Staging With Both Tests
There were no significant differences in the κ index between both tests (P = 0.16), and both were considered to be excellent. Follow-up was done at EOT or death or withdrawal. The mean follow-up time after treatment was 16.2 ± 9.5 months with a median follow-up of 14 months (range, 1–36 months).
All patients were evaluated at EOT with the same diagnostic modality as in the initial staging, except for HL in which a PET/ceCT was performed independently of the initial test, following the current clinical guidelines.
Response at the EOT With ceCT
There was concordance with the reference standard in 78% of cases (71/91). Most of the discordances were in patients with PR (n = 13 [14.3%]), which were confirmed as CRs, and sensitivity and specificity were low. The most frequent reason for the overestimation of response was the detection of residual lymphadenopathy of pathological size lacking malignant infiltration (n = 8). Regarding the extranodal lesions, the most frequent reason for incorrectly detecting lymphomatous infiltration in the lung was the detection of nonspecific inflammation. One patient had lung infarction diagnosed with CT angiography as a pulmonary embolism. Another discrepancy was due to discovery of a tonsillar mass with cervical lymphadenopathy initially interpreted as disease progression, finally determined to be a primary squamous carcinoma of the tonsil with complete lymphoma remission. Figure 3 shows a patient studied with ceCT64 for initial staging and EOT evaluation.
FIGURE 3.
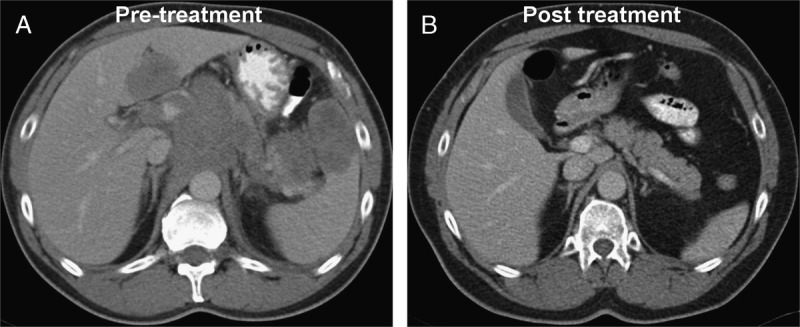
A 54-year-old man diagnosed with DLBCL stage IVB in October 2012. Initial staging (A) with ceCT64 demonstrated supradiaphragmatic and infradiaphragmatic lymphadenopathies, splenic and hepatic infiltration, and an abdominal bulky disease (9 cm). At the EOT in June 2013, ceCT64 demonstrated a CR (B). In April 2016, 34 months after the completion of treatment, the patient remains with a CR.
Response at the EOT With FDG PET/ceCT
There was concordance with the reference standard in 97.8% of the cases (88/90) with κ = 0.91 (P < 0.001), corresponding with a CR in 83.3% (n = 75), a PR in 4.4% (n = 4), and a PD in 10% (n = 9), with good sensitivity and specificity for response assessment as presented in Table 2. There were only 2 discordant findings in cases of partial clinical response that FDG PET/ceCT reported as CR. One corresponded to a splenomegaly, and the other to bone marrow infiltration as demonstrated by abnormal peripheral blood. Figure 4 shows a patient studied with FDG PET/CT for initial staging and EOT evaluation.
FIGURE 4.

A 61-year-old man diagnosed with DLBCL in April 2013. Initial staging with FDG PET/ceCT (A–C) evidenced stage IV supradiaphragmatic and infradiaphragmatic lymphadenopathies, multiples organs with lymphomatous infiltration (gastric, pancreatic, splenic, and renal), and multiple bone lesions, and soft tissue, intratracheal, muscular, and cutaneous infiltration, the largest FDG accumulation being located in the right shoulder with an SUVmax of 23.7. At the EOT in November 2013, FDG PET/ceCT demonstrated a CR (D–F).
All HL patients who had been assigned to the CT branch (n = 37) were evaluated at the EOT with CT and also with FDG PET/ceCT. The concordance was perfect; 34 (91.9%) reached a CR, and 3 (8.1%) a PR.
Most of the patients in both localized stages and advanced stages achieved CR. Fourteen patients showed progression of the disease. One patient had appearance of a new tumor, and another patient demonstrated PD in the orbit. Twelve patients died in the period ranging from the inclusion in the study to the end of the follow-up. The causes of withdrawal were progression in 9 patients, treatment toxicity in two, and sepsis in one patient. All patients withdrawn from this study were older than 65 years at the time of diagnosis.
DISCUSSION
In this study, we prospectively compared FDG PET/ceCT and ceCT64 in the initial staging and response assessment at the EOT in 181 patients with lymphoma. Our findings confirm that both imaging tests provide accurate staging. It is important to note that, similar to the findings of Fuertes et al,30 the percentage of correct staging reached 89.5% with CT and 97.8% with FDG PET/ceCT. The difference between both tests was not significant, but because of sample size limitations [(1 − β) was 0.73], no firm conclusion can be recommended regarding initial imaging for the 3 lymphoma types considered here. On the other hand, FDG PET/ceCT was clearly superior to ceCT64 for EOT evaluation (P < 0.05), with (1 − β) = 0.85.
Sixty-four-slice multi-detector row CT correctly identified all patients with stage III lymphoma. One patient classified as stage IV because of suspected bone and bone marrow infiltration was determined to be a false-positive finding, with no lymphomatous infiltration. One patient with central nervous system involvement was classified as stage IV, although it would now be classified as stage IE, as at the time of participant recruitment the new Ann Arbor (Lugano 2014) classification had not been published yet.21 Six patients with confirmed stage IV were incorrectly downstaged. Downstaging was most frequently due to insensitivity of CT for the detection of bone marrow infiltration. Our CT results have improved with respect to previous studies,16–18 although we confirm what has been described in the literature regarding the limitations of CT in the detection of bone marrow infiltration.
Prospective studies comparing PET/CT versus CT, including the one published previously by our group that compared the diagnostic accuracy of 2 PET/CT options with both PET and CT in the initial staging of HL and NHL,17 concurred with our results indicating the limitation of CT due to false-negative findings. However, previous studies did not indicate the numbers of CT detectors,13 except that our previous study was done with a 4-detector row CT.
Staging accuracy with PET/ceCT was excellent, with a higher proportion of patients correctly staged in locations with extranodal disease and bone marrow infiltration where CT was less accurate.
FDG PET/ceCT correctly staged all of the patients with stages I, III, and IV.
Our results coincide with the published literature regarding the superiority of FDG PET/ceCT compared with CT in the detection of extranodal disease. It is reasonable that bone marrow biopsy can be excluded from the diagnostic workup when FDG PET/ceCT demonstrates focal marrow disease.31,32 Other studies report a low sensitivity with a high specificity of FDG PET/CT in the detection of bone marrow involvement.33
A recent study in 87 patients with DLBCL demonstrated that most cervical lymph nodes with FDG uptake that appeared after treatment were ultimately benign. However, FDG uptake appeared earlier in the malignant group.34
Our results agree with previously reports20 except in the initial staging of HL, DLBCL, and FL, where we noted no significant differences between ceCT64 and FDG PET/ceCT. Thus, although perhaps not as effective as FDG PET/ceCT, ceCT64 can be used for initial staging of lymphoma.
The results of our study are excellent for FDG PET/ceCT compared with ceCT64, particularly at EOT. Sixty-four-slice multi–detector row CT overestimated residual lymphadenopathy in the absence of malignancy (n = 8). Nonspecific pulmonary inflammation was a frequent confound. One patient developed a new pulmonary opacity associated with a lung infarction confirmed by CT angiography. Another discrepancy was due to the appearance of a tonsillar mass with cervical lymphadenopathy interpreted as disease progression, but biopsy confirmed a primary squamous carcinoma of the tonsil with a complete remission of the lymphoma.
All HL patients who had been assigned to the ceCT branch (n = 37) and FDG PET/ceCT (n = 35) were evaluated at the EOT with CT and also with FDG PET PET/ceCT. Concordance with the reference standard was perfect. This corresponds with the results of previous publications.20,21
Strengths of the Study
We present a prospective study comparing the efficacy of ceCT64 with FDG PET/ceCT in the initial staging and monitoring of response in HL and NHL. Our results demonstrate the usefulness of ceCT64 for the initial staging in HL, DLBCL, and FL, because of its accessibility and lower cost. However, FDG PET/ceCT provides the most accurate results in the evaluation at EOT, especially in HL.
Limitations of the Study
Study limitations include modest sample size that was underpowered to confirm differences in initial staging and imperfection of the criterion or reference standard based on all available clinical data rather than histology.
CONCLUSIONS
Our study demonstrates that both ceCT64 and FDG PET/ceCT provide accurate initial staging when compared with the reference standard. However, for monitoring EOT response, FDG PET/ceCT is superior to ceCT64 (P < 0.001).
ACKNOWLEDGMENTS
The authors thank the members of the multicenter and multidisciplinary research team for their participation in this study. Participating centers and researchers include (a) M. Josefa Nájera and M. Mar Hermosilla (hematology), M. Puy Garrastachu (nuclear medicine), and Leticia Salazar (radiology) from the Hospital San Pedro and Centre for Biomedical Research of La Rioja (CIBIR), Logroño, La Rioja; (b) Joaquín Díaz-Mediavilla (hematology) from the University Hospital San Carlos, Madrid; and (c) Raquel Jover (nuclear medicine) from the University Hospital Rey Juan Carlos, Madrid.
Footnotes
Conflicts of interest and sources of funding: This work was financially supported by the Spanish Health Ministry through the Fondo de Investigaciones Sanitarias (F.I.S.) of the Instituto de Salud Carlos III, Madrid (file no. 11/01800) granted in 2011. The final report was presented in 2016.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed Lyon, France: IARC; 2008. [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hicks RJ, Mac Manus MP, Seymour JF. Initial staging of lymphoma with positron emission tomography and computed tomography. Semin Nucl Med. 2005;35:165–175. [DOI] [PubMed] [Google Scholar]
- 5.Kwee TC, Kwee RM, Nievelstein RA. Imaging in staging of malignant lymphoma: a systematic review. Blood. 2008;111:504–516. [DOI] [PubMed] [Google Scholar]
- 6.Keil S, Behrendt FF, Stanzel S, et al. RECIST and WHO criteria evaluation of cervical, thoracic and abdominal lymph nodes in patients with malignant lymphoma: manual versus semi-automated measurement on standard MDCT slices. Rofo. 2009;181:888–895. [DOI] [PubMed] [Google Scholar]
- 7.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507. [DOI] [PubMed] [Google Scholar]
- 8.Barrington SF, O’Doherty MJ. Limitations of PET for imaging lymphoma. Eur J Nucl Med Mol Imaging. 2003;30:S117–S127. [DOI] [PubMed] [Google Scholar]
- 9.Elstrom R, Guang L, Baker G, et al. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood. 2003;101:3875–3876. [DOI] [PubMed] [Google Scholar]
- 10.Bar-Shalom R. Normal and abnormal patterns of FDG-PET/CT in lymphoma. Radiol Clin N Am. 2007;45:677–688. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer NG, Hany TF, Taverna C, et al. Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging—do we need contrast enhanced CT? Radiology. 2004;232:823–829. [DOI] [PubMed] [Google Scholar]
- 12.Tatsumi M, Cohade C, Nakamoto Y, et al. Direct comparison of FDG PET and CT findings in patients with lymphoma: initial experience. Radiology. 2005;237:1038–1045. [DOI] [PubMed] [Google Scholar]
- 13.La Fougère C, Hundt W, Bröckel N, et al. Value of PET/CT versus PET and CT performed as separate investigations in patients with Hodgkin’s disease and non-Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging. 2006;33:1417–1425. [DOI] [PubMed] [Google Scholar]
- 14.Allen-Auerbach M, Quon A, Weber WA, et al. Comparison between 2-deoxy-2[18F]fluoro-d-glucose positron emission tomography and positron emission tomography/computed tomography hardware fusion for staging of patients with lymphoma. Mol Imaging Biol. 2004;6:411–416. [DOI] [PubMed] [Google Scholar]
- 15.Raanani P, Shasha Y, Perry C, et al. Is CT scan still necessary for staging in Hodgkin and non-Hodgkin lymphoma patients in the PET/CT era? Ann Oncol. 2006;17:117–122. [DOI] [PubMed] [Google Scholar]
- 16.Hernández-Maraver D, Hernández-Navarro F, Gómez-León N, et al. Positron emission tomography/computed tomography: diagnostic accuracy in lymphoma. Br J Haematol. 2006;135:293–307. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Vigil B, Gómez-León N, Pinilla I, et al. PET/CT in lymphoma: prospective study of enhanced full-dose PET/CT versus unenhanced low-dose PET/CT. J Nucl Med. 2006;47:1643–1648. [PubMed] [Google Scholar]
- 18.Pinilla I, Rodríguez-Vigil B, Gómez-León N. Integrated FDG PET/CT: utility and applications in clinical oncology. Clin Med Oncol. 2008;2:181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elstrom RL, Leonard JP, Coleman M, et al. Combined PET and low-dose, noncontrast CT scanning obviates the need for additional diagnostic contrast-enhanced CT scans in patients undergoing staging or restaging for lymphoma. Ann Oncol. 2008;19:1770–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinilla I, Gómez-León N, Del Campo-Del Val L, et al. Diagnostic value of CT, PET and combined PET/CT performed with low-dose unenhanced CT and full dose enhanced CT in the initial staging of lymphoma. Q J Nucl Med Mol Imaging. 2011;55:567–575. [PubMed] [Google Scholar]
- 21.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallamini A, Barrington SF, Biggi A, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica. 2014;99:1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallanca F, Alongi P, Incerti E, et al. Diagnostic accuracy of FDG PET/CT for clinical evaluation at the end of treatment of HL and NHL: a comparison of the Deauville Criteria (DC) and the International Harmonization Project Criteria (IHPC). Eur J Nucl Med Mol Imaging. 2016;43:1837–1848. [DOI] [PubMed] [Google Scholar]
- 25.Tatsumi M, Kitayama H, Sugahara H, et al. Whole-body hybrid PET with 18F-FDG in the staging of non-Hodgkin’s lymphoma. J Nucl Med. 2001;42:601–608. [PubMed] [Google Scholar]
- 26.Boellaard R, O’Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadashi W, Mitsuaki T, Kayako I, et al. Response evaluation with total lesion glycolysis in patients with malignant lymphoma: comparison to SULpeak in PERCIST on 18F-FDG PET/CT study. J Nucl Med. 2012;53(Suppl 1):1361; abstract no. 136. [Google Scholar]
- 29.van Persijn van Meerten EL, Gelderblom H, Bloem JL. RECIST revised: implications for the radiologist. A review article on the modified RECIST guideline. Eur Radiol. 2010;20:1456–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuertes S, Setoain X, López-Guillermo A, et al. The value of positron emission tomography/computed tomography (PET/CT) in the staging of diffuse large B-cell lymphoma. Med Clin (Barc). 2007;129:688–693. [DOI] [PubMed] [Google Scholar]
- 31.Pakos EE, Fotopoulos AD, Ioannidis JP. 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med. 2005;46:958–963. [PubMed] [Google Scholar]
- 32.Gheysens O, Thielemans S, Morscio J, et al. Detection of bone marrow involvement in newly diagnosed post-transplant lymphoproliferative disorder: (18)F-fluorodeoxyglucose positron emission tomography/computed tomography versus bone marrow biopsy. Leuk Lymphoma. 2016;57:2382–2388. [DOI] [PubMed] [Google Scholar]
- 33.Adams HJ, Kwee TC, Fijnheer R, et al. Direct comparison of visual and quantitative bone marrow FDG-PET/CT findings with bone marrow biopsy results in diffuse large B-cell lymphoma: does bone marrow FDG-PET/CT live up to its promise? Acta Radiol. 2015;56:1230–1235. [DOI] [PubMed] [Google Scholar]
- 34.An YS, Yoon JK, Lee SJ, et al. Clinical significance of post-treatment 18F-fluorodeoxyglucose uptake in cervical lymph nodes in patients with diffuse large B-cell lymphoma. Eur Radiol. 2016;26:4632–4639. [DOI] [PubMed] [Google Scholar]


