Abstract
Our past research has focused on identifying an effective carrier composed of histidine and lysine for delivery of nucleic acid into cells. For this purpose, we developed histidine-lysine-rich (HK) polymers with specific sequences and branching. We have found that branched HK polymers in complex with Raf-1 siRNA markedly decreased Raf-1 mRNA and induced apoptosis in cell lines in vitro. The primary focus of the present study was to determine an effective carrier to deliver siRNA systemically to tumor xenografts. After comparing HK:Raf-1 polyplexes for their in vivo efficacy, we investigated in greater detail whether one of these polymers, H3K(+H)4b, in complex with Raf-1 siRNA, inhibited the growth of MDA-MB-435 xenografts. H3K(+H)4b is a four-branched HK peptide whose predominant repeating sequence within the terminal arm is -HHHK-. After the first tail vein injection in a mouse model, there was a statistically significant reduction in tumor size between the H3K(+H)4b:Raf-1 siRNA treated and the control groups (P<0.01). By the third injection, there was nearly a 50% reduction in the Raf-1 siRNA-treated group compared to the control siRNA-treated or untreated group. Consistent with a significant effect of the HK:Raf-1 polyplex on the tumor, there were marked histological changes, increased apoptosis, and fewer vessels in the Raf-1 siRNA-treated group. Raf-1 protein within the tumor was significantly decreased after treatment with the HK:Raf-1 siRNA polyplex compared to the control treatment groups. Despite the striking effect on the tumor by the HK Raf-1 siRNA, there was little evidence of toxicity in normal tissues with this therapy. By harnessing the ability to modify the amino acid sequence and branching of HK polymers, we expect continued development of HK polymers as in vivo carriers of siRNA.
Keywords: Histidine, lysine, peptides, Raf-1, tumor xenograft
Introduction
The ability to down-regulate target genes by using double-stranded RNA interference (RNAi) has revolutionized basic science research on signal transduction and gene function 1. RNAi also has tremendous therapeutic potential for treating diseases such as cancer in which an oncogene or angiogenic growth factor is overexpressed. Despite the promise of siRNA therapy, it shares the classic delivery problem of antisense and gene therapies: nucleic acids are highly negatively charged and cannot easily be transported through the blood stream to their cytosolic targets. A host of carriers for siRNA are now being tried in several animal disease models including cholesterol conjugates 2, liposomes (e.g., stable nucleic acid lipid particles), atelocollagen 3, cyclodextrin 4–6, polyethylenimine 7,8, and fusion protamine-antibody carriers 9.
We have focused on developing histidine-lysine (HK) polymers as carriers of nucleic acids including siRNA. To this end, we developed a series of such polymers with specific sequences and branching 10,11. Whereas the histidine component is essential for buffering and lysing endosomes, thereby releasing the nucleic acid in vitro, the lysine component is important in binding the HK polymer with the negatively charged phosphates of the nucleic acids. Even though HK polymers can deliver both siRNA and DNA, the requirements for successful delivery of siRNA usually differ from those for delivering DNA. For example, we have found that H2K4b with a predominant repeating pattern of -HHK- was an effective carrier of plasmids, but was ineffective as a carrier of siRNA in vitro. For delivery of siRNA to SVRbag4 cells expressing β-galactosidase, the most effective polymer was the eight-branched peptide, H3K8b, although this polymer was not particularly effective as a carrier of plasmids 12. The predominant pattern in H3K8b within the terminal branch is the repeating amino acid sequence of -HHHK- (e.g., H3K8b). In addition to being an effective carrier of siRNA into SVRbag4 cells, H3K8b proved effective in several cell lines as an in vitro carrier of Raf-1 siRNA and resulted in reduced Raf-1, decreased cell number, and induced apoptosis in several cell lines 13. Selection of the optimal HK polymer for nucleic acid delivery appears to depend on various patterns within the HK polymer that have a role in binding and release of different forms of nucleic acids. Although the eight-branched polymer, H3K8b, was an effective carrier of siRNA in vitro, the four-branched HK polymer, H3K4b, was significantly more effective carrier of siRNA when injected intratumorally 13. HK carriers with fewer than four branches were less effective as carriers of nucleic acids (DNA or RNA) unless combined with another carrier (e.g., liposomes) 14–16. Thus, together with different amino acid patterns, the degree of branching and transport medium may be important in determining the most effective HK carrier of siRNA.
To determine whether one or more of several HK siRNA polyplexes can effectively reduce tumor growth, Raf-1 was targeted. We and other laboratories have found that targeting Raf-1 through a variety of therapeutic strategies can reduce the growth of tumor xenografts 13,17–20. Raf-1 also has a central role in tumor angiogenesis. In endothelial cells, oncogenic Ras or its activated substrate, Raf-1, is associated with increased levels of vascular endothelial growth factor (VEGF), which is the primary growth factor responsible for inducing tumor angiogenesis 21. Furthermore, because it is differentially phosphorylated at separate sites by VEGF or bFGF signal transduction pathways, Raf-1 protects endothelial cells from apoptosis 22. Finally, Raf-1 has also been shown to increase cancer metastases 23. Thus, Raf-1 is an attractive therapeutic target for siRNA since its down-regulation affects multiple pathways, including tumor angiogenesis, tumor cell growth, and formation of metastases.
Our current goal is to develop an effective HK carrier for systemic delivery of Raf-1 siRNA to inhibit the growth of tumors in vivo. Consequently, several systemically injected HK peptides, differing in their branching and their terminal amino acid patterns, were compared for their ability to reduce the growth of tumor xenografts. We found that systemically delivered HK polymers in complex with Raf-1 siRNA can effectively reduce MDA-MB-435 xenografts in a mouse model.
MATERIALS AND METHODS
Cell line
MDA-MB-435 cells, a human malignant cell line, and SVRbag4, a mouse transformed endothelial cell line, were obtained from the American Type Culture Collection and maintained in Dulbecco’s Minimal Essential Medium (DMEM) containing 10% fetal calf serum (FCS) and 20 mM glutamine. Primary human umbilical vein endothelial cells (HUVEC, Clonetics) were grown in EGM-2 Bullet Kit medium (Clonetics).
Polymers
The biopolymer core facility at the University of Maryland synthesized HK polymers on a Ranin Voyager synthesizer (PTI) as previously described. Three polymers (H3K8b, H3K(+H)4b), H3K(+G)4b) were used as siRNA carriers in this study. H3K8b has 8 terminal branches and a molecular weight of 22 922; H3K(+H)4b and H3K(+G)4b have 4 terminal branches and molecular weights of 10 191 and 10 539, respectively. See Table 1 for structure of the three HK polymers. Nomenclature of HK polymers: 1) for H3K4b, the dominant repeating sequence in its terminal branch is -HHHK-, thus “H3K” is part of the name; the “4b” refers to the number of terminal branches; 2) for H3K(+H)4b, four-branched analog of H3K4b in which 1 extra histidine is inserted in the terminal branch of H3K4b; 3) for H3K(+G)4b, four-branched analog of H3K4b in which 2 glycines are interspersed within the dominant pattern of the terminal branch; 4) for H3K8b, an eight-branched HK polymer with a dominant terminal sequence of –HHHK-.
Table 1.
Sequence and Size of HK Polyplexes
| Polymer | Structure | Terminal Sequence | Molecular Weight | Size (nm)_ |
|---|---|---|---|---|
| H3K(+H)4b |

|
R=KHHHKHHHKHHHHKHHHK | 10 191 | 230±129 |
| H3K(+G)4b | R=KHHHKHHHKGHHHKHHHKG | 10 035 | 274±128 | |
| H3K8b |
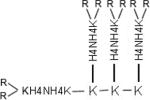
|
R=HHHKHHHKHHHKHHHK | 22 908 | 137±56 |
SiRNA
The siRNA sequences were screened for lack of homology to other genes within the mouse and human transcriptome to minimize cross-hybridization and inadvertent knockdown of unrelated genes by off-target effects.
Raf-1 siRNA
The Raf-1 siRNA duplex is as follows: Sense, 5′-UGU CCA CAU GGU CAG CAC C-dTdT-3′; Antisense, 5′ GGU GCU GAC CAU GUG GAC AdTdT-3′
Control siRNA
Sense, 5′-CAG UUG CGC AGC CUG AAU GdTdT-3′; Antisense, 5′-CAU UCA GGC UGC GCA ACU GdTdT-3′; (no target, Qiagen)
Fluorescent labeled siRNA
Alexa Fluor 555 Allstars siRNA (no target, Qiagen)
β-galactosidase siRNA
Sense 5′-CAG UUG CGC AGC CUG AAU G dTdT-3′; Antisense, 5′-CAU UCA GGC UGC GCA ACU GdTdT-3′.
In vitro siRNA Transfection
Two cell lines, MDA-MB-435 and HUVEC, were examined for the ability of HK in complex with Raf-1 siRNA to inhibit cell growth and to induce apoptosis. The SVRbag4 cell line, which expresses β-galactosidase, was also examined for the ability of the HK polymers in complex with β-gal siRNA to reduce expression of the intracellular marker. In brief, 3 × 104 cells were plated into a 24-well plate containing 500 μl of DMEM and 10% serum. After 24 h, when the cells were 40% confluent, transfection polyplexes were added to the media. To prepare polyplexes of HK:siRNA, siRNA (2 μg) in 50 μl of OptiMEM was briefly mixed well with HK (16 μg) and maintained at room temperature for 30 min. This polyplex was then added dropwise to the cells in 0.5 ml of DMEM/10% serum; 24–48 h after transfection, cell number or β-galactosidase activity was determined as described previously 12,13. Data are represented as percent of untreated control.
Measurement of particle size of HK siRNA polyplexes
H3K8b, H3K(+H)4b and H3K(+G)4b, (300 μg) in complex with siRNA (75 μg) (4:1 wt:wt, polymer: siRNA) were prepared similarly to the transfection polyplexes described previously. After the polyplexes were prepared in D5W (5% Dextrose; 375 μl) for 30 min, water (625 μl) was added. Particle size was determined by measurement of light scattering at a 90-degree angle on an N4 Submicron Particle Size Analyzer (Beckman Coulter, Hialeah, FL), and is reported as the average size obtained from a Unimodal analysis carried out using the software provided by the instrument manufacturer. Each data point represents the mean ± S.D. of three measurements.
Preparation of HK:siRNA polyplexes for in vivo experiments
To the siRNA (50 μg in 125 μl of D5W), HK (100, 200, 300, or 400 μg in 125 μl D5W) was added quickly and mixed briefly with a Vortex mixer. After the polyplexes were maintained for 30 min at room temperature, 250 μl of each were injected into the tail vein of each mouse.
In vivo xenograft experiments
Female adult nude mice (4–6 weeks) were obtained from NCI Frederick. MDA-MB-435 cells (7×105 cells/injection) were injected bilaterally into the mammary fat pads of each mouse. Ten days later, when the subcutaneous tumors were established, the mice were separated into groups of four mice to determine efficacy of the HK in complex Raf-1 siRNA. Depending on the experiment, the mice were given two or seven intravenous injections of polymer-siRNA complex, each separated by 4 days (50 μg of siRNA/tumor/injection and various amounts of HK). Polyplexes were administered every three days based on down-regulation of the target gene and maximum tumor response and recovery from therapy. Tumor size was assessed with caliper measurements of the tumors in two dimensions before each injection and 3 days after the last injection; the volume was calculated by formula 1/2 × length×width2. Animal care and treatment were conducted in accordance with established guidelines and protocols approved by the University of Maryland Baltimore Institutional Animal Care and Use Committee.
Tumor localization and distribution
H3K(+H)4b in complex with fluorescently-labeled (AlexaFluor 555) siRNA was used to validate siRNA delivery. After implanted MDA-MB-435 tumor xenografts reached 50 mm3, H3K(+H)4b in complex with AlexaFluor-555 siRNA was administered by i.v. injections; mice were euthanized 6 h later. Frozen sections were prepared from several organs (lungs, liver, kidney) and tumor xenografts. Images of these tissues were obtained with a Diaphot-TMD fluorescence microscope fitted with a Z-motor and deconvolved with 3-D Volocity Restoration software.
Immunohistochemical detection of Raf-1, Ki67, and CD31
Tumors were fixed with 4% formalin overnight, after which tumor sections (5 μm) were deparaffinated with xylene and hydrated with ethanol. Antigens were retrieved in 10 mM citrate, pH 6.0, by boiling for 20 min, then cooling to room temperature. Endogenous peroxidase activity was blocked by 3% H2O2 in 100% methanol for 10 min, and the sections were then incubated with 3% goat serum for 30 min at room temperature. Rabbit anti-human polyclonal antibody (Raf-1, diluted 1:100, Genscript, Ab-259; Ki67, 1:200 dilution, Chemicon; CD31, 1:100 dilution, Cell Signal) was added to the tumor sections for 1 h at room temperature and the secondary horseradish peroxidase-labeled antibody was applied to the sections for 30 min at room temperature (Histoscan HRP universal rabbit kit; Biomeda, cat. No.06-602). The chromagen diaminobenzene (DAB) was incubated with the tumor sections for 10 min to permit color development, and the tumor sections were dehydrated and mounted with glass coverslips. A brown color indicated positive staining.
In vivo apoptosis as determined by TUNEL assay
The TUNEL (terminal deoxynucleotidyl transferase) assay was done according to the manufacturer’s instructions in the FragEL™ DNA Fragmentation Detection Kit (Calbiochem). The tumors were first fixed in 4% formalin, embedded in paraffin, and tissue sections were then deparaffinated. After the sections were treated with proteinase K (20 mg/ml) for 20 min at room temperature, endogenous peroxidase was inhibited with 3% H2O2. The TUNEL reaction mixture (deoxynucleotidyl transferase) was incubated with the tumor section for 90 min at 37°C in a humidified chamber. The labeling reaction was then stopped and conjugated buffer was added to the sections for 30 min at room temperature. After the DAB substrate was applied to the tumor sections for 10 min, the slides were mounted with glass coverslips and examined by light microscopy.
Apoptotic and proliferation Index
To determine the apoptotic and proliferation indices, four randomly chosen fields (100×) of stained slides were examined and the total numbers of cells and positively stained cells in each field were then counted. The percentages of apoptotic and proliferating cells were calculated by the following formula: (number of positively stained cells/total tumor of cells in a field) × 100.
Statistical analysis
When multiple groups were compared, results were analyzed using one-way analysis of variance with multiple comparisons (Bonferroni’s t-test); when only two groups were compared, results were analyzed using a two-tailed Student’s t-test. Values of P < 0.05 were considered significant.
Results
In vitro comparison of HK polymers as carriers of siRNA
We had previously found that H3K8b was an effective carrier of siRNA in vitro. Thus, in SVRbag4 cells expressing β-galactosidase, the H3K8b siRNA polyplex reduced β-gal by nearly 75% compared to untreated cells. In a similar study (Fig 1A), we compared the efficacy of H3K8b with two other polymers, H3K(+H)4b and H3K(G)4b, for their ability to inhibit β-gal in SVRbag4 cells. As shown in Fig. 1, the H3K8b siRNA polyplex was markedly more effective than H3K(+H)4b, reducing β-gal by 71% (P<0.001), compared with 43% for the latter polyplex (P<0.01); H3K(+G)4b, a polymer which contained glycine in its terminal branches, was the least effective.
Figure 1.
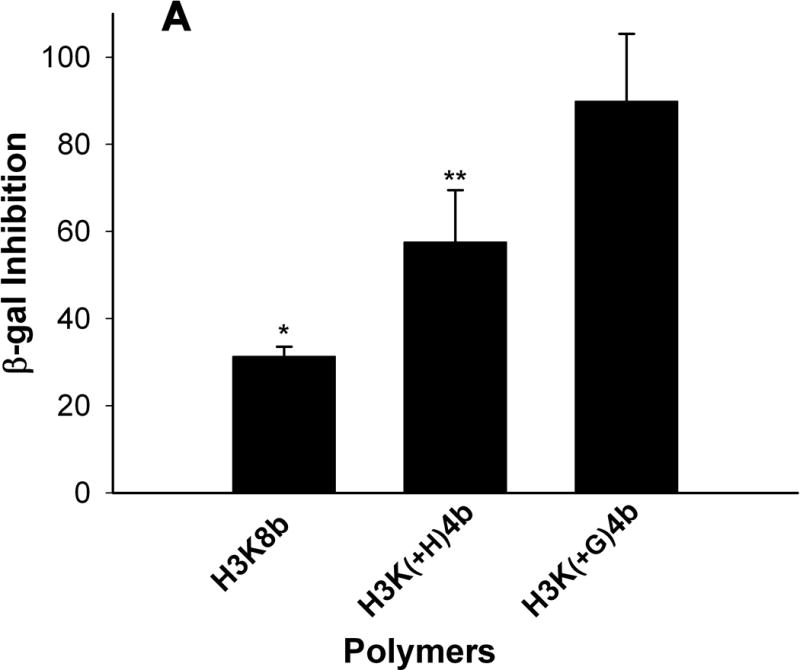
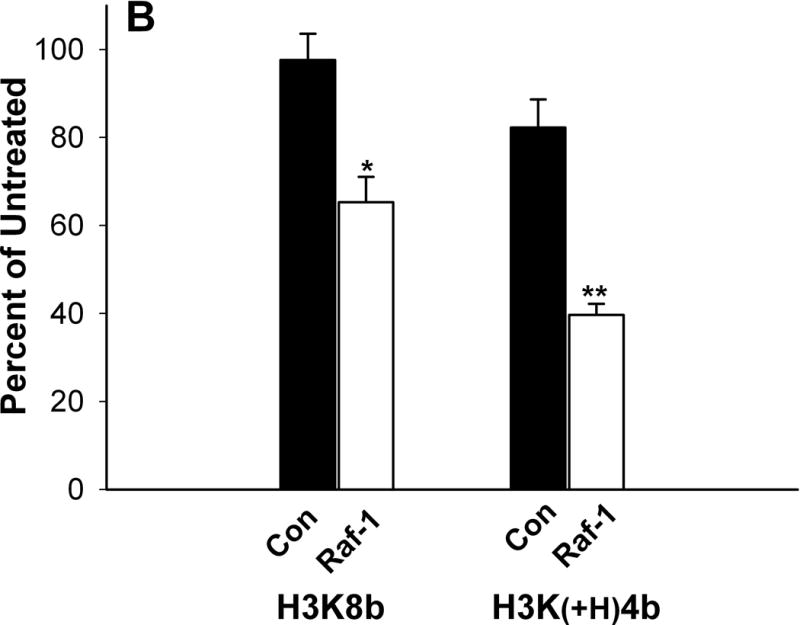
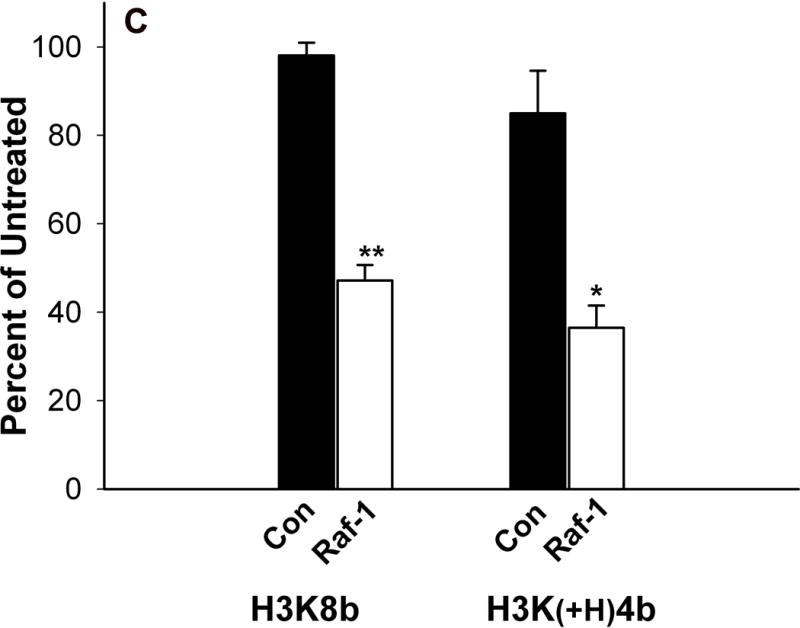
Comparison of HK polymers as carriers of siRNA in cell lines. Cells (5 × 104 cells per well; A, SVRbag4; B, MDA-MB-435; C, HUVEC) were plated into 24-well plates. After 24 h, when the cells were 50% confluent, 2 μg of siRNA in complex with an HK polymer was prepared and added to the cells as described under “Method and Materials.” A) Three carriers (H3K8B, H3K(+H)4B, H3K(+G)4B) of β-gal siRNA were tested for their ability to inhibit β-galactosidase expression in SVRbag4 cells. *, P<0.001, untreated vs. H3K8b; **, P<0.01, untreated vs. H3K(+H)4b (Multiple Comparisons versus Control Group (Bonferroni t-test). B, C) Forty-eight hours after transfection with HK: Raf-1 siRNA, MDA-MB-435 (B) or HUVEC (C) cells were treated with trypsin-EDTA and counted in a Coulter apparatus. *, P<0.001; **, P=0.002, Raf-1 siRNA vs. Control siRNA in MDA-MB-435 cells or HUVEC cells (Student t-test). H3K(+H)4b in complex with the control siRNA showed an 11% and 18% reduction in HUVEC and MDA-MB-435 cells, respectively, compared to untreated cells. Results represent percent of untreated controls and are expressed as the mean ± S.D. of three independent experiments.
Surprisingly, in two cell lines, H3K8b was not the optimal carrier of siRNA. In complex with Raf-1 siRNA, H3K(+H)4b was more effective in reducing the number of MDA-MB-435 cells compared to H3K8b (Fig. 1B). H3K(+H)4b:Raf-1siRNA polyplex reduced cell numbers by about 60% while the H3K8b polyplex reduced the cell number by 35%. In HUVEC, H3K8b: and H3K(+H)4b:Raf-1 siRNA polyplexes were comparable in reducing cell number by 53% and 64%, respectively (Fig. 1C). We also examined the toxicity of these polymers. Compared to the H3K8b polyplex, H3K(+H)4b in complex with the control siRNA had moderately increased toxicity (Fig. 1B, 1C). Whereas the H3K8b control siRNA polyplex exhibited little to no toxicity compared to untreated cells, the H3K(+H)4b control siRNA polyplex showed 11% and 18% reduction in number of HUVEC and MDA-MB-435 cells, respectively.
In a previous study, we had found that plasmids delivered by HK polyplexes were markedly affected by conditions used for their preparation; in particular, salt conditions that altered the HK polyplex from spherical to needle-like morphology were associated with higher transfection activity 24. In contrast, the efficacy of the three HK-siRNA polyplexes used in this study was not affected by salt conditions during their formation. We found no evidence of needle-like morphology of HK siRNA polyplexes despite their formation in medium of different salt concentrations; indeed, HK siRNA polyplexes had a spherical morphology when they were formed in different media. Not surprisingly, regardless of whether the HK-siRNA polyplex was formed in water, isotonic salt, or sequential water and isotonic salt conditions, reduction of its mRNA targets was similar (data not shown).
H3K(+H)4b and H3K8b are comparable carriers of Raf-1 siRNA in vivo
We then screened the three HK polymers to determine which was the most effective carrier of Raf-1 siRNA in reducing the growth of tumor xenografts. After the tumors reached 20 mm3, we injected HK polymers (200 μg) in complex Raf-1 siRNA (50 μg) into the tail vein of a mouse tumor xenograft model. After the second injection, we assessed the antitumor efficacy of each of these HK polyplexes. H3K(+H)4b and H3K8b Raf-1 siRNA polyplexes reduced the growth of tumors to a comparable degree (Fig. 2A) (P<0.02, untreated vs. H3K(+H)4b, H3K8b). Similar to the situation in vitro, H3K(+G)4b was an ineffective carrier of Raf-1 siRNA in vivo (Fig. 2A). The size of the polyplexes could not explain differences in their antitumor efficacy, because the H3K(+H) K4b (230.2 ± 129 nm) and H3K(+G)K4b (274.3 ±128) polyplexes were comparable in size, while H3K8b polyplexes were smaller (137±56 nm) (Table I).
Figure 2.


Selection and optimization of HK polymer for systemic delivery of siRNA. A) In vivo screening of HK polymers to determine effective carrier of Raf-1 siRNA. Ten days after the injection of MDA-MB-435 cells into the mammary fat pad, mice with visible tumors were separated into treatment groups (untreated, H3K(+G)4b/Raf-1 siRNA, H3K(+H)4b/Raf-1 siRNA, H3K8b/Raf-1 siRNA). Each group had 4 mice with 8 tumors and tumor size and treatment efficacy were evaluated after the second injection. Results of tumor size per treatment group 3 days after the second injection are expressed as the mean ± S.D. *, P<0.02: H3K(+H)4b, H3K8b vs untreated. B) Determination of the optimal ratio of H3K(+H)4b/siRNA polyplex that inhibits tumor growth. After MDA-MB-435 xenografts became visible, different ratios of H3K(+H)4b to Raf-1 siRNA were administered to determine the optimal ratio (2.0, 4.0, 6.0, 8.0: 1). After two injections, tumor size was assessed in the treatment and untreated groups. P<0.05, untreated vs. 4:1 ratio, one-way analysis of variance with multiple comparisons versus untreated group (Bonferroni t-test)
Systemically delivered H3K(+H)4b:Raf-1 siRNA markedly reduce tumor growth
Because the four-branched H3K(+H)4b was markedly easier to synthesize than is the eight-branched H3K8b peptide, we selected this polymer to study in greater detail. We first examined the optimal ratio of H3K(+H)4b to siRNA. We examined four ratios (w/w; 2:1, 4:1, 6:1, 8:1) and found that the 4:1 ratio was the most effective in reducing tumor growth (Fig. 2B) (P<0.05, untreated vs 4:1 ratio). To examine uptake by the tumor and normal tissues of the polyplex, the HK peptide in complex with Alexafluor-labeled siRNA was injected into tumor-bearing mice. Six hours later, frozen sections of the tumor and tissues were prepared. Intratumoral accumulation of fluorescently-labeled H3K(+H)4b/polyplex was evident (Fig. 3) and there was widespread deposition in other tissues of these polyplexes with the greatest accumulation in the kidneys.
Figure 3.

Distribution of HK siRNA polyplex in tissues after systemic injection. H3K4b in complex with fluorescently-labeled (AlexaFluor 555) siRNA was used to validate siRNA delivery. After implanted MDA-MB-435 tumor xenografts reached 50 mm3, H3K4b in complex with AlexaFluor-555 siRNA was administered by i.v. injection; mice were euthanized 6 h later. Frozen sections were prepared from several organs (lungs, liver, kidney) and tumor xenografts. Images of these tissues were obtained with a Diaphot-TMD fluorescence microscope fitted with a Z-motor and deconvolved with 3-D Volocity Restoration software. A, lung; B, tumor; C, liver; D, kidney.
With the optimal HK:siRNA ratio, we evaluated in greater detail by continuing for 7 injections the H3K(+H)4b :Raf-1 siRNA polyplex in reducing tumor xenografts. After the tumors became visible, H3K(+H)4b in complex with Raf-1 siRNA (50 μg) was injected every 4 days for seven injections. After the first injection, there was a 40% reduction in tumor size between the HK/Raf-1 polyplex and the untreated control group (Fig. 4, P<0.01, untreated, control siRNA vs. Raf-1 siRNA). After the third injection, there was nearly a 50% difference between the control and Raf-1 siRNA treatment groups (Fig. 4, P<0.01, untreated, control siRNA vs. Raf-1 siRNA). This difference in tumor size between the control and Raf-1 treatment groups was maintained through the last (seventh) injection.
Figure 4.

Systemic injection of H3K(+H)4b: Raf-1 siRNA polyplex into athymic nude mice bearing MDA-MB-435 tumors. Tumor volume (mm3) was measured in the groups (untreated-close circles, control siRNA-close inverted triangles, or Raf-1 siRNA-open circles) before to each treatment injection time. After the tumors became visible, H3K(+H)4b (200 μg) in complex with siRNA (50 μg) were injected every 4 days for seven injections. By the second injection, there was a significant difference between the control siRNA and Raf-1 siRNA groups. *, P<0.01. One-way analysis of variance with multiple comparisons versus control siRNA or untreated group (Bonferroni’s t-test).
H3K(+H)4b:Raf-1 siRNA polyplex induces marked histological and immununochemical changes within the tumor
Histology and immunological staining studies showed marked antitumor effects in the H3K(+H)4b:Raf-1 siRNA treatment group (Fig. 5). Three days after the last treatment, mice were euthanized, and tumors and tissues were removed and examined histologically. Tumors from mice treated with H3K(+H)4b-Raf-1 siRNA polyplex showed marked cell death within the tumor compared to control siRNA polyplex or the untreated groups. Furthermore, in viable areas of the tumor, there was marked reduction of Raf-1, suggesting that therapeutic Raf-1 siRNA reduced its desired target. Because Raf-1 has a significant role in cellular mitosis, tumor angiogenesis, and preventing apoptosis, we examined whether the Raf-1 siRNA polyplex affected these pathways in the tumor. As evidenced by CD31 immunohistochemistry, the number of tumor vessels was decreased. Moreover, decreased proliferation and increased apoptosis were observed in the Raf-1 siRNA-treated group (Fig. 6). Proliferation and apoptotic indices of tumors validated the impression from the immunohistochemical images (Fig. 6). While the proliferation marker, Ki67, was decreased by nearly 50% within tumor sections, apoptosis increased by nearly 700% (P<0.001, untreated, control siRNA vs. Raf-1 siRNA). We also examined the histology of a variety of tissues (heart, lungs, liver, and kidneys; Fig. 7); there was no toxicity observed despite seven injections of the H3K(+H)4b siRNA polyplexes.
Figure 5.
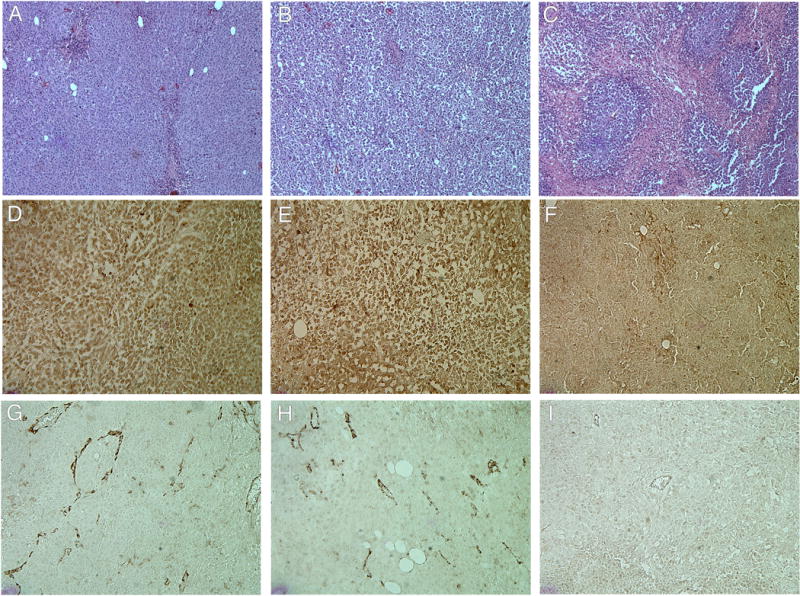
Histologic and immunohistochemical analysis of CD31 and Raf-1 content of tumor xenografts. Hematoxylin and eosin staining of tumors excised from mice after various treatments (A, untreated; B, control siRNA; C, Raf-1 siRNA). Immunohistochemical staining detection of Raf-1 expression (D, untreated; E, control siRNA; F, Raf-1 siRNA). A diaminobenzidine substrate was used to visualize antibody binding to Raf-1 and is shown as the dark brown precipitate. Immunohistochemical staining detection of CD31 to determine vessel content within treatment groups (G, untreated; H, control siRNA; I, Raf-1 siRNA). For further details, see “Materials and Methods.”
Figure 6.
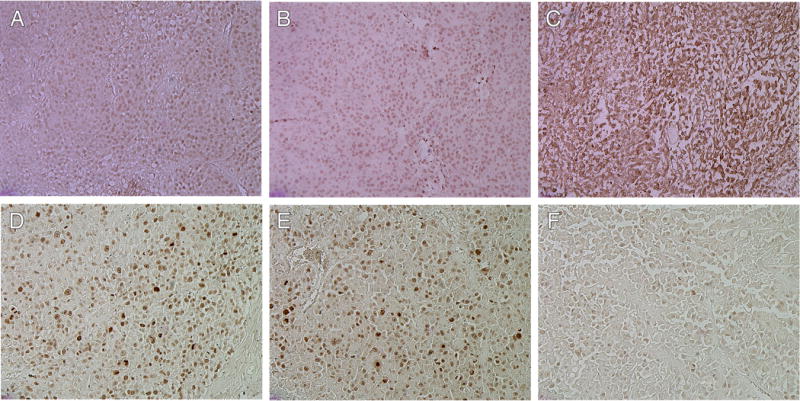

Proliferation and apoptosis of tumors. Apoptosis of tumor induced by Raf-1 siRNA (C), control siRNA (B), or untreated (A) as determined by TUNEL assay. Detection of Ki67 as an indicator of proliferation of tumor cells within various treatment groups (D, untreated; E, control siRNA, F, Raf-1 siRNA) × 100 magnification. Proliferation and apoptotic indices were calculated by counting positive cells in four random fields at ×100 magnification from untreated (black), control siRNA-treated (red), and Raf-1 siRNA-treated (green) mice (G) (P<0.001, untreated, Control siRNA vs. Raf-1 siRNA, One-way analysis of variance with multiple comparisons versus control siRNA and untreated groups (Bonferroni’s t-test)).
Figure 7.
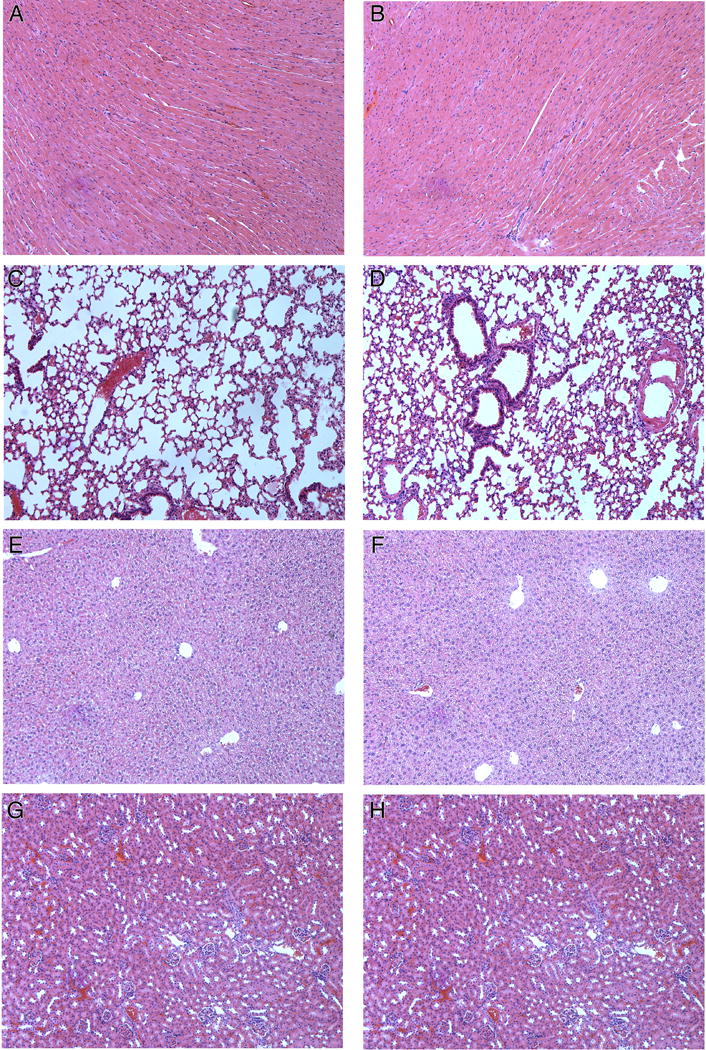
Hematoxylin and Eosin staining of several normal tissues (A,B, heart; C,D, lung; E,F, liver; G,H, kidney) of untreated and Raf-1 siRNA treatment groups. There was no toxicity in the control (A,C,E,G) or Raf-1 siRNA (B,D,F,H) treatment groups.
Discussion
Prior to studying the effects of amino acid patterns of HK on siRNA delivery, we examined conditions that might increase the efficacy of such delivery systems, because we had previously found that changes in the medium during the preparation of the HK:plasmid polyplexes were associated with a needle-like morphology and a marked increase in gene expression 24. Unlike plasmids delivered by HK peptides, however, perturbations in the salt conditions during the HK polyplex formation failed to affect morphology or efficacy of siRNA polyplexes (data not shown). This likely indicates significant differences between the binding of plasmids and siRNA to HK peptides.
As a result, it perhaps is not surprising that there were differences in the ability of various HK peptides to transport siRNA and plasmids in vivo 12. We considered a number of sequence and branching patterns of the HK polymer that might improve transport of siRNA. Although the H2K4b polymer with a repeating pattern of -HHK- was effective as a carrier of plasmids in vitro and in vivo 11,25, it was not an effective carrier of siRNA under either condition (data not shown). Thus, in this study we focused on HK polymers with a repeating pattern of -HHHK-, which we had previously shown to be effective for transporting siRNA in vitro. Of the polymers tested for siRNA delivery in a screening assay to silence β-gal expression in vitro, H3K8b was the most effective 12. This is a highly branched polymer with a histidine-rich domain and terminal branches with a repeating pattern of –HHHK-. Initially, H3K8b was synthesized because its histidine-rich domain was expected to buffer the acidic endosomal compartment and thereby enable nucleic acid to escape from endosomes. This strategy was only partially successful in that the polymer augmented siRNA delivery but not plasmid delivery. In our tumor xenograft model, H3K8b was an effective carrier in vivo and did not exhibit significant toxicity. For several reasons, however, we sought other polymers that might be effective carriers of siRNA. Compared to H3K(+H)4b, H3K8b is expensive, more challenging to synthesize, and more difficult to modify by pegylation at specific sites. We therefore screened a number of polymers that had only four branches, but with the repeating patterns of -HHHK-.
In general, four-branched polymers with this pattern were effective carriers of siRNA in vivo except for H3K(+G)4b. Because glycine has the smallest side group and may increase the flexibility of the terminal branch, we tested the effect of adding glycine to the -HHHK- pattern. Insertion of glycines within the terminal branches of H3K(+G)4b decreased the efficacy of siRNA delivery significantly both in vitro and in vivo compared to the H3K(+H)4b carrier. Perhaps, the mechanism for the difference in these two peptides for their ability to carry siRNA into cells is due to the reduced α-helical structure of H3K(+G)4b. Indeed, preliminary data with circular dichroism spectroscopy established that H3K(+G)4b has less α-helical structure in a hydrophobic environment compared to its H3K(+H)4b counterpart. The differences in the structure between these two polymers may affect interaction and release of siRNA. We are continuing our biophysical and structural studies to understand why H3K(+H)4b and H3K(+G)4b differ as carriers of siRNA.
Although HK siRNA polyplexes appear to have significant tumor-inhibitory activity with low toxicity in vivo, developing therapies such as this one often fail because toxicity reduces the therapeutic index window. To identify polymers with increased efficacy with lower toxicity, one strategy is to examine a variety of HK polymers with different amino acid sequences. For instance, preliminary studies suggest that H3K(+H)4b and a close analog H3K4b in complex with Raf-1 siRNA have comparable antitumor activity when given intravenously. Nevertheless, there were differences in toxicities between these two HK polymers in vitro even though their amino acid sequences differ by only a single histidine in each branch; the H3K(+H)4b polyplex is moderately more toxic in vitro compared to the H3K4b siRNA polyplex (data not shown). Although H3K(+H)4b siRNA showed no toxicity in vivo at dosages used in this study, the in vitro findings raise the possibility that there may be differences in toxicity among polymers and their polyplexes in vivo at higher dosages. A complementary strategy that our lab is developing to improve HK carriers with reduced toxicity is to increase the specificity and delivery of HK siRNA polyplexes to the tumor target. Although toxicity of HK siRNA polyplexes appears minimal, their wide distribution in a variety of tissues suggests that modification of HK with PEG and with a ligand may increase the specificity, increase antitumor efficacy, and decrease potential toxicity of the polyplexes. At least for luciferase-expressing plasmids, modification of HK peptides with PEG and a tumor-selective ligand has improved the efficacy and specificity of these polyplexes (data not shown). Other laboratories have reported similar findings 7,26–28. There are some distinguishing characteristics in pegylating the HK polymer compared to other polymers. By incorporating cysteines within the polymer, the precise location of pegylation is known and the number of PEGs/molecule is the same. In contrast, many polymers such as polyethylenimine are randomly pegylated and an average number of PEGs/molecule is obtained.
In summary, we screened several HK polymer candidates for their ability to carry Raf-1 siRNA systemically to tumor xenografts in a mouse model. Although the eight-branched polymer was an effective carrier, the easier to synthesize and less expensive four-branched carrier was equally effective. In future studies, further modification of the four–branched HK peptide either through its amino acid sequence and/or by addition of PEG/ligand is required to improve its ability to carry siRNA systemically.
Acknowledgments
We are grateful to Dr. Pamela Talalay for her careful reading and useful comments concerning the manuscript. We thank Drs. Nicholas Ambulos and Pat Campbell of the Maryland Biopolymer lab for synthesizing the peptides in this study. This study was supported by the National Cancer Institute CA70394.
References
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz C, Hadwiger P, John M, Vornlocher HP, Unverzagt C. Steroid and lipid conjugates of siRNAs to enhance cellular uptake and gene silencing in liver cells. Bioorg Med Chem Lett. 2004;14:4975–4977. doi: 10.1016/j.bmcl.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S, Muramatsu T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res. 2004;64:3365–3370. doi: 10.1158/0008-5472.CAN-03-2682. [DOI] [PubMed] [Google Scholar]
- 4.Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- 5.Heidel JD, Yu Z, Liu JY, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci U S A. 2007;104:5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug Chem. 2007;18:456–468. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffelers RM, Ansari A, Xu J, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li BJ, Tang Q, Cheng D, et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med. 2005;11:944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song E, Zhu P, Lee SK, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 10.Leng Q, Goldgeier L, Zhu J, Cambell P, Ambulos N, Mixson AJ. Histidine-lysine peptides as carriers of nucleic acids. Drug News Perspect. 2007;20:77–86. doi: 10.1358/dnp.2007.20.2.1083026. [DOI] [PubMed] [Google Scholar]
- 11.Leng Q, Scaria P, Ioffe OB, Woodle M, Mixson AJ. A branched histidine/lysine peptide, H2K4b, in complex with plasmids encoding antitumor proteins inhibits tumor xenografts. J Gene Med. 2006;8:1407–1415. doi: 10.1002/jgm.982. [DOI] [PubMed] [Google Scholar]
- 12.Leng Q, Scaria P, Zhu J, Ambulos N, Campbell P, Mixson AJ. Highly branched HK peptides are effective carriers of siRNA. J Gene Med. 2005;7:977–986. doi: 10.1002/jgm.748. [DOI] [PubMed] [Google Scholar]
- 13.Leng Q, Mixson AJ. Small interfering RNA targeting Raf-1 inhibits tumor growth in vitro and in vivo. Cancer Gene Ther. 2005;12:682–690. doi: 10.1038/sj.cgt.7700831. [DOI] [PubMed] [Google Scholar]
- 14.Chen QR, Zhang L, Luther PW, Mixson AJ. Optimal transfection with the HK polymer depends on its degree of branching and the pH of endocytic vesicles. Nucleic Acids Res. 2002;30:1338–1345. doi: 10.1093/nar/30.6.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen QR, Zhang L, Stass SA, Mixson AJ. Co-polymer of histidine and lysine markedly enhances transfection of liposomes. Gene Ther. 2000;7:698–704. doi: 10.1038/sj.gt.3301294. [DOI] [PubMed] [Google Scholar]
- 16.Yu W, Pirollo KF, Yu B, et al. Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide. Nucleic Acids Res. 2004;32:e48. doi: 10.1093/nar/gnh049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cioca DP, Aoki Y, Kiyosawa K. RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther. 2003;10:125–133. doi: 10.1038/sj.cgt.7700544. [DOI] [PubMed] [Google Scholar]
- 18.Hood J, Granger HJ. Protein kinase G mediates vascular endothelial growth factor-induced Raf-1 activation and proliferation in human endothelial cells. J Biol Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- 19.Rudin CM, Marshall JL, Huang CH, et al. Delivery of a liposomal c-raf-1 antisense oligonucleotide by weekly bolus dosing in patients with advanced solid tumors: a phase I study. Clin Cancer Res. 2004;10:7244–7251. doi: 10.1158/1078-0432.CCR-04-0642. [DOI] [PubMed] [Google Scholar]
- 20.Pei J, Zhang C, Gokhale PC, et al. Combination with liposome-entrapped, ends-modified raf antisense oligonucleotide (LErafAON) improves the anti-tumor efficacies of cisplatin, epirubicin, mitoxantrone, docetaxel and gemcitabine. Anticancer Drugs. 2004;15:243–253. doi: 10.1097/00001813-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Grugel S, Finkenzeller G, Weindel K, Barleon B, Marme D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995;270:25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- 22.Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol. 2003;162:933–943. doi: 10.1083/jcb.200304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatib AM, Fallavollita L, Wancewicz EV, Monia BP, Brodt P. Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res. 2002;62:5393–5398. [PubMed] [Google Scholar]
- 24.Leng Q, Kahn J, Zhu J, Scaria P, Mixson J. Needle-like morphology of H2K4b polyplexes associated with increases in transfection in vitro. Cancer Ther. 2007;5B:193–202. [PMC free article] [PubMed] [Google Scholar]
- 25.Chen QR, Zhang L, Stass SA, Mixson AJ. Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res. 2001;29:1334–1340. doi: 10.1093/nar/29.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hood JD, Bednarski M, Frausto R, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 27.Bellocq NC, Pun SH, Jensen GS, Davis ME. Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug Chem. 2003;14:1122–1132. doi: 10.1021/bc034125f. [DOI] [PubMed] [Google Scholar]
- 28.Kunath K, Merdan T, Hegener O, Haberlein H, Kissel T. Integrin targeting using RGD-PEI conjugates for in vitro gene transfer. J Gene Med. 2003;5:588–599. doi: 10.1002/jgm.382. [DOI] [PubMed] [Google Scholar]


