Abstract
Rationale:
Following prolonged endurance events such as marathons, elevated levels of cardiospecific biomarkers are commonly reported. Although transiently raised levels are generally not considered to indicate clinical myocardial damage, comprehension of this phenomenon remains incomplete. The popularity of high-intensity interval training highlights a paucity of research measuring cardiac biomarker response to this type of exercise. This a posteriori case report discusses the elevation of cardiac troponins (cTn) associated with short interval, high-intensity exercise.
Patient concerns:
In this case report, an apparently healthy 29-year-old recreationally active female presented clinically raised cardiac troponin I (cTnI) levels (>0.04 ng/mL), after performing high-intensity cycle ergometer sprints. As creatine kinase (CK) is expressed by multiple organs (e.g., skeletal muscle, brain, and myocardium), cTnI assays were performed to determine any changes in total serum CK levels not originating from skeletal muscle damage.
Diagnosis:
A posteriori the individual's daily energy expenditure indicated chronically low-energy availability. Psychometric testing suggested that the individual scored positive for disordered eating, highly for fatigue levels, and low in mental health components.
Outcomes:
The current case report provides novel evidence of elevated cTnI occurring as a result of performing short duration, high intensity, cycle ergometer exercise in an individual with self-reported chronically depleted energy balance. A schematic to identify potentially “at risk” individuals is presented.
Lessons:
Considering this as a case report, results cannot be generalized; however, the main findings suggest that individuals who habitually restrict their calorie intake below their bodies’ daily energy requirements, may have elevated biomarkers of exercise induced myocardial stress from performing high-intensity exercise.
Keywords: cardiac biomarkers, cardiac troponin I, case report, energy deficit, high-intensity exercise
1. Introduction
The death of the Ancient Greek herald Phidippides in 490 BC is the first record of a sudden cardiac fatality apparently associated with very high volumes of physical activity. Phidippides reportedly collapsed and died immediately after bringing news to Athens of battle victory for the Greeks against the mighty Persians. His demise occurred after completing a 250-mi round trip from Marathon to Sparta in 2 days, followed by a 26.2-mi run from Marathon to Athens.
In the 21st century AD, such heroic feats of endurance and sacrifice to deliver a message are generally not necessary; however, participation in physical activity for health benefits and competitive events such as marathons, Ironman triathlons, and ultramarathons have grown in popularity.
During the past 3 decades, numerous studies have reported an increase in cardiospecific biomarkers, cardiac troponin I (cTnI), cTnT, and N-terminal probrain natriuretic peptide, in the hours following marathon running.[1] This has previously been interpreted as convincing evidence of direct myocardial damage.[2] However, due to the inherent transient nature of this increase in cardiobiomarkers, it is now generally considered to be an acute physiological phenomenon hypothesized to be linked to increased mechanical stress, raised catecholamines, pH changes,[3] and bleb formation[4]; rather than clinically meaningful cardiac damage indicating myocardial necrosis.[5] Frank myocardial damage follows a bi-phasic pattern as necrosis develops over ∼5 days[6], whereas exercise-induced raised serum levels generally fall below upper reference limits (URL) within 48 hours,[6,7] and do not demonstrate the same sustained elevation observed as a result of direct myocardial injury.[8] Nevertheless, there is substantial individual variation in the magnitude of exercise induced cTn release,[9] and the long-term implications of sustained exercise-related myocardial strain throughout a lifetime remain contentious.[10]
Exertional increase of cardiac biomarkers during endurance exercise are considered to be due to cardiac fatigue from prolonged exercise and not expected following other nonendurance based exercise modalities. This case report discusses the occurrence of clinically raised levels of cTnI in a physically active female following a bout of short duration high-intensity exercise consisting of repeated sprint performance on a cycle ergometer. Incidents such as this are unexpected, and they rarely fit with a priori study aims. It is therefore important to report these results as they represent clinically important findings.
2. History and presenting condition
2.1. Methods A
Following institutional ethical approval from the School of Science and Sport Ethics committee at The University of the West of Scotland, and participant written informed consent, a 29-year-old recreationally active female (P1) was recruited for a study investigating the effects of load application on power output and blood markers of muscle damage. At baseline, she presented as healthy and free from any ongoing disease or injury. She reported taking the oral contraceptive pill.
Repeated sprint protocols (8 × 8-s Wingate Anaerobic Test) were performed on a cycle ergometer (Lode Excalibur Sport; Lode B.V., Groningen, The Netherlands) at 2 randomly assigned trials, 7 days apart, by a cohort of similarly active females and males (Table 1 for group characteristics). Braking force was applied relative to total body mass (Trial 1), or relative to fat-free mass (Trial 2). Blood draws were taken from an antecubital vein at rest, 30 minutes, 24, and 48 hours postexercise.
Table 1.
Anthropometric characteristics of P1 compared to mean values of recreationally active females (n = 8) and males (n = 9) who participated in 8 × 8 second WAnT sprints in trial 1.
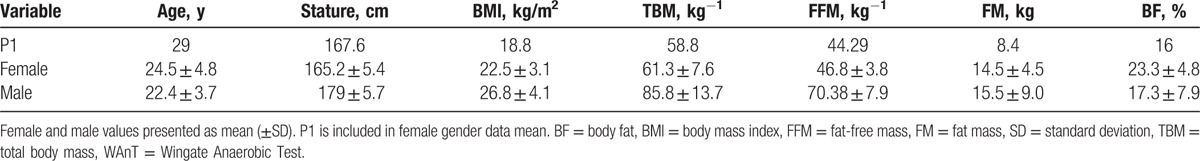
Collected blood samples were stored for ∼3 months at −80 °C, and then analyzed for the biomarkers creatine kinase (CK) (IU/L) and cTnI (ng/mL) at a local National Health Service (NHS) hospital (Architect Stat assay precision coefficient of variation (CV) ≤ 10%, NHS CV < 5%). The URL for cTn in healthy populations was defined as the 99th percentile. The observed normal value for apparently health females aged 18 to 62 years was ≤0.013 ng/mL; males aged 18 to 64 years ≤0.033 ng/mL; values ≥0.04 ng/mL were considered clinically significant for males and females (Abbot Laboratories, Chicago, IL). It was hypothesized that serum cTnI would not increase, and therefore, any change in total CK levels could be attributed to effects of the exercise on skeletal muscle metabolism.
2.2. Statistical analysis
Statistical calculations were conducted using Minitab17 statistical software (2016) (Minitab, Inc. [www.minitab.com], State College, PA). The original distribution of CK was assessed for normality using an Anderson–Darling test at the 5% significance level. The associated P value was (P = .029), indicating CK data distribution were of a nonnormal nature. The CK data were then log10() transformed to investigate if skewedness could be corrected and was further assessed for normality (P < .005). These transformed data were analyzed as part of a repeated measures analysis of variance test to compare serum CK values at each of the time points between Trials 1 and 2. The following model was considered at the 5% significance level: CK levels (log10) = Study + Time + Gender + Study × Time + Study × Gender + Gender × Time + Error. Statistical analysis was not performed on cTnI values as concentrations <0.04 ng/mL were not available from the assay analysis.
3. Results A
3.1. Creatine kinase
No significant interactions were found (P > .05) and CK serum concentrations did not change through time (P = .984); the adjusted R2 value for the model was 21.72%. The fixed variable Gender was considered a significant effect at the 5% significance level (P < .001). A Bonferroni Pairwise Comparison was conducted for the levels of the variable Gender, and a 95% confidence interval (CI) for the difference in mean CK levels between females and males calculated. Females were shown to have lower mean serum CK levels than males (95% CI −0.256 to −0.139 IU/L [transformed units]).
3.2. Cardiac troponin I
Group mean serum cTnI results were all <0.04 ng/mL at rest. P1 had normal levels of cTnI at rest (<0.04 ng/mL); however, levels of cTnI rose above the normal URL, 48-hour post-Trial 1 (0.063 ng/mL), and 24 hours (0.078 ng/mL) and 48-hour post-Trial 2 (0.155 ng/mL). Two other females each had a single incident of levels marginally above the URL (0.043 ng/mL 24-hour post-Trial 2, and 0.045 ng/mL, 48-hour post Trial 1).
3.3. Methods and results B
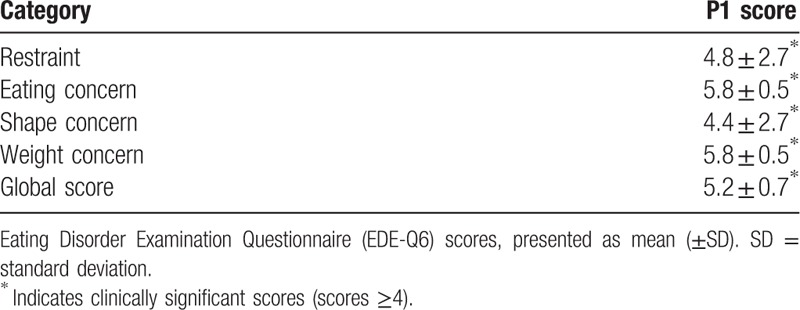
A secondary prospective study was designed. Data relating to the perception of general health and attitudes to exercise and eating were recorded. This was to assess if any potential relationship and/or interaction existed between exercise and lifestyle which could explain the presence of sustained elevated cTnI found in P1, compared to the cohort. The Exercise Motivation Inventory version 2 was used to measure attitudes and motivation to exercise.[11] Scores indicated P1 was strongly motivated to exercise for weight management and appearance reasons. Data from the Short Form Health Survey 36 version 2 (SF36v2) showed P1 had scores around average for physical health components (Physical Health Summary score 45 [USA norm 50 ± 10]), and below average scores for mental health components (Mental Health Summary score 36, [USA norm 50 ± 10]). The lowest scoring health domain for P1 was “Vitality” (score of 26 [USA norm 50 ± 10]) The “Vitality” scale measures energy levels and fatigue. Low scores in this domain indicate feelings of tiredness and being “worn out”. Although “Vitality” is very strongly associated with mental health components, it is also closely linked to physical health. P1 had clinically high scores (≥4) on the Eating Disorder Examination (28 Item) Questionnaire (EDE-Q 6.0)[12] in all categories (Table 2), and also reported regularly exercising in a compulsive driven manner to control weight. In addition, P1 informed of the cessation of her menses for >1 year.
Table 2.
EDE-Q6 scores for case report (P1).
Three-day food (Nutri-Check; Health Options LTD, Eastbourne, UK) and energy diaries (nutritional analysis tool [NAT] advanced online energy calculator, Information Systems Technology, Illinois Council on Food and Agricultural Research, University of Illinois, Champaign, IL) were used to determine energy intake and output in kilocalories (kcal/d). Information gathered was subsequently used to calculate approximate values for total daily energy expenditure (TDEE), exercise-associated thermogenesis (EAT), and nonexercise-associated thermogenesis (NEAT).[13] Basal resting metabolic rate was estimated using the equation by Cunningham.[14] TDEE, EAT, and NEAT were estimated using guidelines and equations recommended by the World Health Organization (WHO) Expert Consultation.[15]
Physical activity diaries showed that P1 reported an average TDEE of 3182 kcal/d, which included very high levels of NEAT amounting to an expenditure of ∼2347 kcal/d (∼70% of the TDEE). Structured exercise thermogenesis accounted for only 10% of the TDEE. A total energy intake of ∼2038 kcal/d was estimated from the 3-day food diaries for P1. The recommended calorie intake based on WHO energy requirement equations, calculated to individual physical activity levels (vigorous active lifestyle), gender, height, weight, and body mass index (BMI) was ∼2800 kcal/d. Therefore, P1 had a daily calorie deficit of between ∼762 (calculated from WHO recommendations) and ∼1144 kcal/d (calculated from P1 self-reported food and activity diaries). A time line of interventions and outcomes is presented in Fig. 1.
Figure 1.
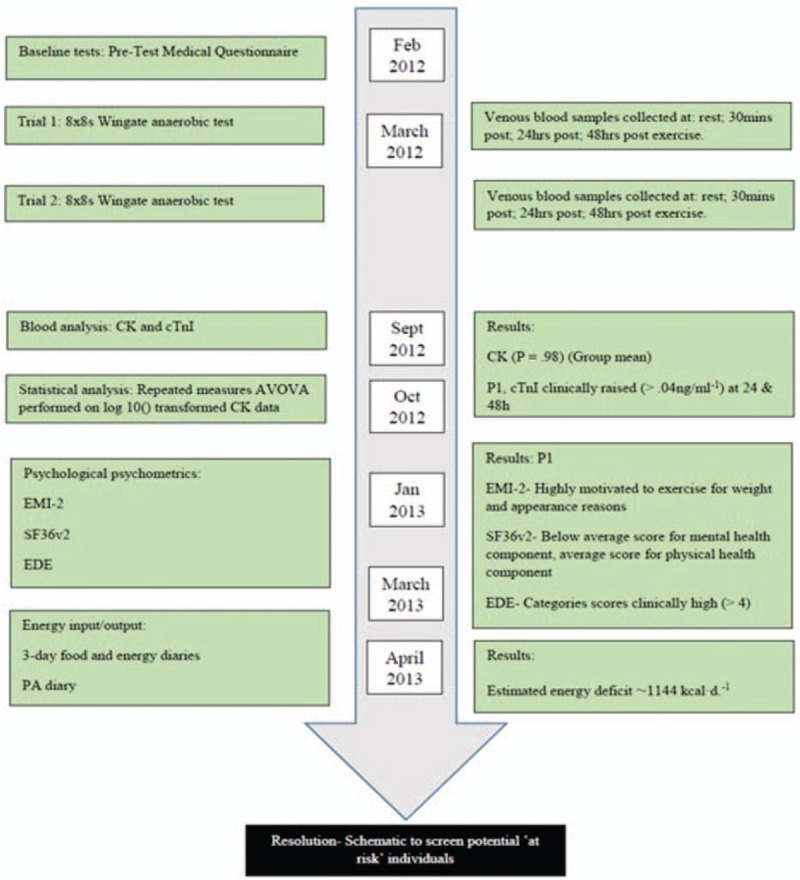
Timeline of interventions and outcomes.
4. Discussion
This case report associates the leakage of cardiac proteins from cardiomyocytes, following high-intensity short-duration exercise, with chronic energy deficit. Increased cTn serum levels, which are highly specific for cardiac damage, frequently occur in individuals to some degree, during prolonged exercise with high cardiac demand[16–18]; likely due to sheer stress, augmented catecolamine release, ischemia,[19] and changes in cardiac function.[20] In most cases, this transient increase in cardiac biomarkers is thought to be part of the integrated physiological response to exercise rather than indicative of serious myocardial injury.[21] This finding of clinically elevated levels of cTnI (≥0.04 ng/mL) in response to very short duration high-intensity exercise is novel and presents the possibility that this case is related to calorie restriction, rather than the unrelenting demands of endurance exercise.
Serum CK levels were measured to help differentiate between skeletal and cardiac damage. Results showed CK levels were not significantly different at any time point (P = .98), and there was no significant interaction between time point and study (P = .57), or study and gender (P = .42). Thus, indicating no significant level of skeletal muscle damage had occurred in response to the cycling exercise performed. Analysis showed that the statistical model was a very poor fit for the CK data (R2 = 21.72%), likely due to the nonnormal nature of the CK data, and the small sample number. This should be considered when interpreting the data. However, as cycling involves predominantly concentric contraction of skeletal muscle, it is not surprising that mean serum CK levels did not change significantly.[22] Females had lower resting levels of serum CK than males (P < .000, 95% CI −0.256 to −0.139 IU/L [transformed units]). This is commonly found[23] and is thought to be due to the greater muscle mass in males and/or generally greater levels of physical activity of males, compared to females.[24]
Clearly there are limitations in a solitary case report. Subsequently, direct cause and effect cannot be substantiated or generalized to the wider population. However, the isolated occurrence in this work also acts as a strength. Out of a cohort of 17 similar participants, P1 was the only 1 to present with clinically elevated levels of cTnI after participating in high-intensity exercise on 2 occasions. Baseline levels of cTnI were normal that is, <0.04 ng/mL for all participants before each cycle ergometer test (1 week between crossover). P1's levels had returned to normal within 5 days from the previous elevated measure at 48-hour postexercise, however, rose to clinically elevated levels on a second occasion postexercise. This provides compelling evidence that the elevation in cTnI did result from performing the exercise protocol.
If elevated cTnI levels were indeed elevated because of energy deficit, what mechanism(s) could be responsible? During strenuous exercise, augmented myocardial demand for oxygen[25] incurs a greater cardiac energy demand.[26] A chronic energy deficit, with potential glycogen loss at the myocardium, could render the myocardium vulnerable to insult from augmented energy utilization, and reduced adenosine triphosphate (ATP) regeneration, during high-intensity exercise; indeed, this is known to be fatal in the rodent model.[27] In humans, during low-to-moderate intensity exercise, myocardial glucose consumption increases, however, in high-intensity exercise, glucose utilization is observed to decline; this may be a mechanism by which glucose is spared for gluconeogenesis.[26] This hypothesized process may be related to the cardioprotective importance of the high ATP yield of glycogen. It is also worth considering other related metabolic factors which may contribute to this incidence of raised cTnI. Exercise induced magnesium (Mg2+) deficiency in otherwise healthy people can result in reduced work capacity and an increased energy demand, with potential for myocardial stress.[28] P1's dietary mineral content was not available from the analysis; however, it did contain a variety of Mg2+ rich foods. Nevertheless, inadequate calorie intake,[29] intensive exercise, and stress-induced catecholamine release[19,30] may deplete Mg2+. Consequently, a combination of restrictive calorie intake, exercise,[31] and stress[30] could potentially attenuate bioavailability of Mg2+.
Recent publications[32,33] show that factors associated with increased cardiac biomarkers after exercise are yet to be fully elucidated, and remain of clinical importance and interest. This case report and literature review would be useful to researchers and clinicians to identify when cases of extreme leanness and/or energy deficiency may contribute to elevated biomarkers after exercise. Myocardial damage is known to occur in anorexia nervosa, and cTnI can be higher at rest.[34] The case did not come into this extreme category as her BMI and body fat percentage, although low, were within the normal range; additionally, her cTnI levels were within the normal reference range at rest. However, Hoch et al[35,36] have shown that females who have symptoms of the Female Triad syndrome demonstrate increased prevalence of endothelium dysfunction. This suggests there exists an unknown number in the population who are asymptomatic, with subclinical eating disorders, and who may demonstrate this unexpected cTnI response to very short-duration exercise. As such the use of cutoff thresholds for normal healthy populations may need to be reconsidered in lean and energy deficient populations, particularly for use in long-term risk stratification. Franzini et al[37] propose subgroup numbers necessary for the calculation of cTnT should be 300 per group; whether this also applies to cTnI is not known. In addition, Apple and Jaffe[38] recommend that as females have lower physiological levels of both cTnI and T, specific assay cutoffs should be used in the determination of clinical decisions. Therefore, the increase in cTnI in P1 to ≥0.04 ng/mL could have more clinical significance in females than males. This may be important in the over or under diagnosis of both chronic and acute cardiac injury.
In this work, self-reported dietary and activity diaries showed that P1 engaged in large volumes of physical activity which resulted in a negative energy balance. Furthermore, P1 scored high in the EDE-Q6, suggesting she was at significant risk of having an eating disorder; high levels of fatigue in SF36v2 suggested low energy availability. P1 reported feeling more fatigue than usual 24 and 48 hours postexercise, however, did not develop any clinically significant issues related to the elevation of cTnI. A limitation of this case report is that dietary and activity behaviors were self-reported, therefore, calorie balance was estimated. Underestimating food intake in self-reporting diaries is extremely common, particularly in females, and those who are weight conscious.[39] It was considered that P1 may have overestimated her physical activity and/or under-reported her food intake substantially; however, it was supported by the low BMI and cessation of menses. In addition, there may be errors in the analysis of energy expenditure using the NAT online energy calculator. Nevertheless, there is other compelling evidence gathered in this work which supports an energy deficit in P1. Even if the maximum calorie intake recommended by the WHO for a “vigorous lifestyle” was assumed (2800 kcal/d) for P1, a deficit of ∼382 kcal/d would still exist. Together these findings indicate that a chronically low or negative calorie balance may increase some individuals’ susceptibility to exercise-induced myocardial stress, which in turn could be related to myocardial glycogen depletion.
The main findings of this case report are that low-energy availability may be associated with augmented cTnI release following anaerobic exercise. Whether the appearance of clinically raised levels of cTnI in this work is because of pathological mechanisms, or, a temporary and benign physiological response like that in endurance exercise, or an isolated individual response is not certain. As energy balance in this work was estimated from self-reported diaries, the actual kilocalorie deficit of the case cannot be accurately established. However, other data collected in this work support the existence of an energy deficit. Future work should determine energy balance using more accurate methods for example, accelerometers and more closely controlled dietary analysis like that in Hoch et al[35] Other biomarkers of cardiac function, for example, B-type natriuretic peptide (BNP) or N-Terminal pro-BNP and echocardiography images collected after exercise would assist in any possible diagnosis. This would be necessary to examine the interaction between high-intensity exercise and low-energy availability in young active females where calorie deficit may exist. A greater understanding would be vitally important, not only for those working with females competing in so called “lean sports”, but also for the general exercising population and matters relating to public health. If a deleterious link between energy deficit and high-intensity exercise was identified, individuals who may be “at risk” could be identified from the risk stratification model proposed in Fig. 2. Subsequently, the most appropriate intervention for the individual could be initiated. This information may also assist with clinical interpretations and judgments when conducting risk stratification of individuals presenting with raised cTn levels in the absence of other clinical evidence of acute coronary syndrome.
Figure 2.
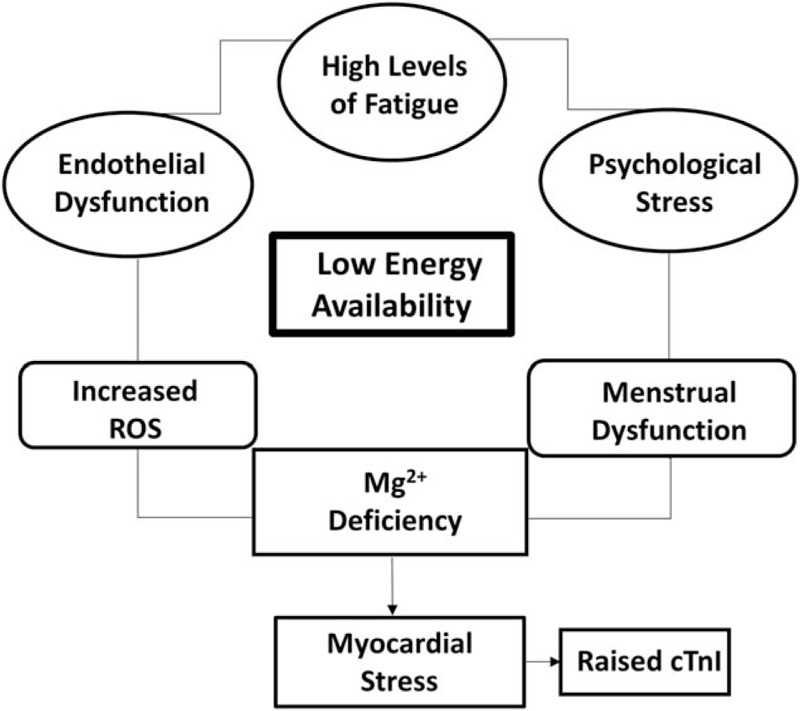
The figure shows the potential pathways which could result in augmented myocardial stress. Low-energy availability is depicted as central to this outcome.
Acknowledgments
The authors thank Johnathan Love of Strathclyde University, Glasgow, Scotland for statistical analysis consultation. Permission was received for this acknowledgment.
Footnotes
Abbreviations: ATP = adenosine triphosphate, BMI = body mass index, BNP = B-type natriuretic peptide, CI = confidence interval, CK = creatine kinase, cTn = cardiac troponin, CV = coefficient of variation, EAT = exercise associated thermogenesis, EDE-Q 6.0 = Eating Disorder Examination (28 Item) Questionnaire, Mg2+ = magnesium, NAT = nutritional analysis tool, NEAT = nonexercise associated thermogenesis, NHS = National Health Service, SD = standard deviation, SF36v2 = Short Form Health Survey 36 version 2, TDEE = total daily energy expenditure, URL = upper reference limit, WHO = World Health Organization.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Neilan TG, Januzzi JL, Lee-Lewandrowski E, et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston Marathon. Circulation 2006;114:2325–33. [DOI] [PubMed] [Google Scholar]
- [2].Rifai N, Douglas PS, O’Toole M, et al. Cardiac troponin T and I, echocardiographic [correction of electrocardiographic] wall motion analyses, and ejection fractions in athletes participating in the Hawaii Ironman Triathlon. Am J Cardiol 1999;83:1085–9. [DOI] [PubMed] [Google Scholar]
- [3].Shave R, Oxborough D. Exercise-induced cardiac injury: evidence from novel imaging techniques and highly sensitive cardiac troponin assays. Prog Cardiovasc Dis 2012;54:407–15. [DOI] [PubMed] [Google Scholar]
- [4].Hickman PE, Potter JM, Aroney C, et al. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta 2010;411:318–23. [DOI] [PubMed] [Google Scholar]
- [5].Collinson PO, Gaze DC. Biomarkers of cardiovascular damage and dysfunction—an overview. Heart Lung Circ 2007;16(13 suppl):S71–82. [DOI] [PubMed] [Google Scholar]
- [6].Le Goff C, Laurent T, Kaux JF, et al. Intense physical exercise related to the emergent generation of cardiovascular risk markers: a review. Biol Sport 2012;29:11–6. [Google Scholar]
- [7].Traiperm N, Gatterer H, Wille M, et al. Cardiac troponins in young marathon runners. Am J Cardiol 2012;110:594–8. [DOI] [PubMed] [Google Scholar]
- [8].Shave R, Baggish A, George K, et al. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol 2010;56:169–76. [DOI] [PubMed] [Google Scholar]
- [9].Legaz-Arrese A, George K, Carranza-Garcia LE, et al. The impact of exercise intensity on the release of cardiac biomarkers in marathon runners. Eur J Appl Physiol 2011;111:2961–7. [DOI] [PubMed] [Google Scholar]
- [10].O’Keefe JH, Patil HR, Lavie CJ, et al. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin Proc 2012;87:587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Markland D, Ingledew DK. The measurement of exercise motives: factorial validity and invariance across gender of a revised exercise motivations inventory. Br J Health Psychol 1997;2:361–76. [Google Scholar]
- [12].Fairburn CG, Beglin SJ. Assessment of eating disorders—interview or self-report questionnaire. Int J Eat Disord 1994;16:363–70. [PubMed] [Google Scholar]
- [13].Levine JA. Non-exercise activity thermogenesis (NEAT). Nutr Rev 2004;62(7 Pt 2):S82–97. [DOI] [PubMed] [Google Scholar]
- [14].Cunningham JJ. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am J Clin Nutr 1980;33:2372–4. [DOI] [PubMed] [Google Scholar]
- [15].FAO/WHO/UNU. Human energy requirements. Scientific background papers from the joint FAO/WHO/UNU Expert Consultation. October 17–24, 2001. Rome, Italy. Public Health Nutr 2005;8:929–1228. [DOI] [PubMed] [Google Scholar]
- [16].Agewall S, Giannitsis E, Jernberg T, et al. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J 2011;32:404–11. 2011 February 1. [DOI] [PubMed] [Google Scholar]
- [17].Regwan S, Hulten EA, Martinho S, et al. Marathon running as a cause of troponin elevation: a systematic review and meta-analysis. J Interv Cardiol 2010;23:443–550. [DOI] [PubMed] [Google Scholar]
- [18].Shave R, Ross P, Low D, et al. Cardiac troponin I is released following high-intensity short-duration exercise in healthy humans. Int J Cardiol 2010;145:337–9. [DOI] [PubMed] [Google Scholar]
- [19].Rowe WJ. Extraordinary unremitting endurance exercise and permanent injury to normal heart. Lancet 1992;340:712–4. [DOI] [PubMed] [Google Scholar]
- [20].Shave R, Dawson E, Whyte G, et al. Altered cardiac function and minimal cardiac damage during prolonged exercise. Med Sci Sports Exerc 2004;36:1098–103. [DOI] [PubMed] [Google Scholar]
- [21].Banfi B, Colombini A, Lombardi G, et al. Metabolic markers in sports medicine. Adv Clin Chem 2012;56:1–54. [DOI] [PubMed] [Google Scholar]
- [22].Driss T, Vandewalle H. The measurement of maximal (anaerobic) power output on a cycle ergometer: a critical review. Biomed Res Int 2013;2013:589361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Neal RC, Ferdinand KC, Ycas J, et al. Relationship of ethnic origin, gender, and age to blood creatine kinase levels. Am J Med 2009;122:73–8. [DOI] [PubMed] [Google Scholar]
- [24].Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 2002;81(11 suppl):S52–69. [DOI] [PubMed] [Google Scholar]
- [25].Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 2008;88:1009–86. [DOI] [PubMed] [Google Scholar]
- [26].Kemppainen J, Fujimoto T, Kalliokoski KK, et al. Myocardial and skeletal muscle glucose uptake during exercise in humans. J Physiol 2002;542(Pt 2):403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Scheuer J, Stezoski SW. Protective role of increased myocardial glycogen stores in cardiac anoxia in the rat. Circ Res 1970;27:835–49. [DOI] [PubMed] [Google Scholar]
- [28].Lukaski HC, Nielsen FH. Dietary magnesium depletion affects metabolic responses during submaximal exercise in postmenopausal women. J Nutr 2002;132:930–5. [DOI] [PubMed] [Google Scholar]
- [29].Clarkson PM, Haymes EM. Exercise and mineral status of athletes: calcium, magnesium, phosphorus, and iron. Med Sci Sports Exerc 1995;27:831–43. [PubMed] [Google Scholar]
- [30].Ryzen E, Servis KL, Rude RK. Effect of intravenous epinephrine on serum magnesium and free intracellular red blood cell magnesium concentrations measured by nuclear magnetic resonance. J Am Coll Nutr 1990;9:114–9. [DOI] [PubMed] [Google Scholar]
- [31].Lafontan M, Moro C, Berlan M, et al. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab 2008;19:130–7. [DOI] [PubMed] [Google Scholar]
- [32].Gresslien T, Agewall S. Troponin and exercise. Int J Cardiol 2016;221:609–21. [DOI] [PubMed] [Google Scholar]
- [33].Sedaghat-Hamedani F, Kayvanpour E, Frankenstein L, et al. Biomarker changes after strenuous exercise can mimic pulmonary embolism and cardiac injury—a meta-analysis of 45 studies. Clin Chem 2015;61:1246–55. [DOI] [PubMed] [Google Scholar]
- [34].Zastrow A, Wolf J, Giannitsis E, et al. Elevated myocardial enzymes and natriuretic peptides in anorexia nervosa: prototypic condition for the pathophysiology of cachexia. Cardiology 2015;111:256–9. [DOI] [PubMed] [Google Scholar]
- [35].Hoch AZ, Dempsey RL, Carrera GF, et al. Is there an association between athletic amenorrhea and endothelial cell dysfunction? Med Sci Sports Exerc 2003;35:377–83. [DOI] [PubMed] [Google Scholar]
- [36].Hoch AZ, Papanek P, Szabo A, et al. Associatio between the female athlete triad and enothelial dysfunction in dancers. Clin J Sport Med 2011;21:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Franzini M, Lorenzoni V, Masotti S, et al. The calculation of cardiac troponin T 99th percentile of the reference population is affected by age, gender, and population selection: a multicenter study in Italy. Clin Chim Acta 2015;1: 483–481. [DOI] [PubMed] [Google Scholar]
- [38].Apple FS, Jaffe AS. Men are different from women: its true for troponin too. Clin Biochem 2014;47:867–8. [DOI] [PubMed] [Google Scholar]
- [39].McClung HL, Sigrist LD, Smith TJ, et al. Monitoring energy intake: a hand-held personal digital assistant provides accuracy comparable to written records. J Am Diet Assoc 2009;109:1241–5. [DOI] [PubMed] [Google Scholar]


