Abstract
Obesity is associated with an atherogenic lipid profile. No data exists on lipoprotein particle profiles in metabolically healthy obese (MHO) individuals. Our aim is to characterize lipoprotein size, particle, and subclass concentrations in MHO women after 3 months of weight loss through dietary restriction and physical exercise.
A total of 115 nondiabetic women (aged 35–55 years) with a body mass index (BMI) of 30 to 40 kg/m2 and ≤1 of the following criteria: blood pressure ≤135/85 mm Hg, fasting plasma glucose ≤100 mg/dL, HDL-cholesterol ≤50 mg/dL, and triglycerides ≤150 mg/dL were included. After 3 months of intensive lifestyle modification (Mediterranean diet and physical exercise), they were classified according to their weight loss: <5%, ≥5% to <10%, and ≥10%. Lipoprotein size, particle, and subclass concentrations were measured using 1H NMR.
The final sample, after dropouts, comprised 104 women (age: 44.4 ± 3.7 years, BMI: 36.3 ± 4.7 kg/m2), of whom 47 (45.2%), 27 (26%), and 30 (28.8%) lost <5%, ≥5% to <10%, and ≥10% of baseline body weight, respectively. All participants experienced significant weight loss and decreases in BMI. The lipid profiles showed an increase in small, medium, and large very low density lipoprotein (VLDL) particles in all groups of study with the exception of small VLDL particles in women with ≥10% of weight loss, in which it decreased. The number of VLDL particles decreased in women who had ≥10% weight loss. On the other hand, we detected a decrease in all low density lipoprotein (cLDL) and high density lipoprotein (cHDL) concentrations.
These results indicate that intensive lifestyle modification alters lipid profiles. In particular, it decreases small LDL and HDL particle numbers and does not increase medium or large HDL particles numbers.
Keywords: lipid profile, Mediterranean diet, metabolically healthy obese, nuclear magnetic resonance spectroscopy, physical exercise
1. Introduction
Obesity is a health condition that has increased in recent years, especially in developed countries.[1–4] Body mass index (BMI) is the most widely used measure of obesity. A higher BMI has been linked to the development of various metabolic diseases and their underlying pathophysiologies, which could lead to increased mortality. However, this association is not necessarily linear; many studies suggest the existence of an “obesity paradox,” with different ranges of optimal BMIs associated with mortality.[5] Possible explanations for this inter-study heterogeneity include differences in study population characteristics, ethnicities, follow-up periods, or the use of different BMI classifications.[6–8] Metabolic health status or comorbidities could also be important confounding factors in this relationship.[9,10] Recently, subgroups of obese people with unexpected metabolic profiles that deviate from the normal BMI-metabolic disturbance relationship have generated much interest.[11,12] Although there is a lack of consensus on the defining criteria, these subgroups are usually classified by BMI and degree of insulin resistance or the number of metabolic abnormalities. Although categorized into the same BMI group, metabolic conditions and clinical outcomes may differ across different metabolic health statuses. Some obese individuals exhibit lower degrees of insulin resistance and visceral adiposity along with more favorable cardiovascular risk (CVR) profiles despite their high BMI, and therefore are classified as having a metabolically healthy obese (MHO) phenotype.[13,14]
Some authors postulate that MHO people have a healthy metabolic profile because they have lower inflammatory levels[15–18] and favorable lipid, hormonal, and immunological profiles.[19] Obesity is known to be associated with an atherogenic lipid profile (atherogenic dyslipidemia), characterized by increased levels of total cholesterol, LDL cholesterol, non-HDL cholesterol, small, dense LDL particles, triglycerides, and Lp(a); decreased levels of HDL cholesterol and apoprotein A1; presence of postprandial hyperlipidemia with accumulation of atherogenic remnants; and hepatic hyperproduction of apoprotein B. The key factor in the relationship between obesity and dyslipidemia may be the development of insulin resistance in peripheral tissues with increased hepatic flow of dietary fatty acids, intravascular lipolysis, and resistance of adipose tissue to the antilipolytic effect of insulin.[20]
Currently, clinical laboratories are faced with the problem of standardizing current methods for correctly quantifying low density lipoproteins bound to cholesterol (cLDL).[21] This, along with variability of some lipid components among individuals, has led to an incorrect interpretation of lipid profile results, which is fundamental in an MHO diagnosis.
Conventional lipoprotein quantification methods used in clinical laboratories only allow for the quantification of concentrations.[22] Therefore, it is necessary to find new techniques that allow for a much more precise estimate of CVR. This requires the ability to distinguish between different classes and subclasses of lipoproteins through the use of nuclear magnetic resonance spectroscopy (1H NMR). NMR has been shown to be a more accurate technique across different population types. To date, no studies have been performed using NMR in an MHO population. Using this technique, we can more accurately estimate CVR by calculating the number of particles of the aforementioned classes of lipoproteins.[23–29] Recently, the concentration of lipid particles (very low density lipoprotein [VLDL], intermediate density lipoprotein [IDL], LDL, and HDL) bound to cholesterol and the concentration of lipoproteins (VLDL, IDL, LDL, and HDL) bound to triglycerides have been quantified using this technique,[30,31] as well as numbers of VLDL, LDL, and HDL particles. Measuring lipid profiles using this technique represents a great advance in estimating CVR because it is able to distinguish between different particle sizes and densities[32,33] according to their physicochemical characteristics,[34] allowing for a more precise, accurate determination of the degree of penetration of these particles in the vascular endothelium.[35]
These particles have different sizes and are able to be classified into different subclasses: small, medium, and large.[31] The particle number and lipoprotein size are very important aspects in assessing CVR.[32–34] Lipid metabolism can be altered through physical exercise and diet. Although the role of VLDL in the lipid profile of MHO subjects is not clear, the role of LDL particle size is indeed clear. As LDL particles decrease in size and increase in density, so their degree of atherogenicity increases.[31–33]
The size of HDL is very important because bigger particles can transport more cholesterol to the liver whereas small HDL particles are less functional and, due to their size, do not capture cholesterol.
MHO subjects have a less atherogenic lipid profile, with lower triglyceride and oxidized LDL values and higher HDL cholesterol levels, with no significant differences in total cholesterol, LDL cholesterol, or fatty acid levels.[36,37] However, metabolically abnormal, normal weight subjects have a more atherogenic lipid profile, with higher levels of oxidized LDL and LDL particles of a smaller diameter.[38]
Low density lipoproteins (LDL) that are small are highly atherogenic, favoring the formation of atheromatous plaques,[32,35] which ultimately lead to cardiovascular disease (CVD). Whether weight loss improves the lipid profiles of MHO subjects is also a topic of discussion. Therefore, our objective is to analyze the changes in lipid profiles using 1H NMR in a population of MHO women without atherogenic dyslipidemia detected in routine baseline analysis after 3 months of an intensive lifestyle intervention.
2. Material and methods
An open, single-blind study was conducted. A population of 115 MHO women was recruited from 4 primary health care centers in the Málaga district of the Andalusian Health Service (SAS). Study participants were recruited by their family physicians between June 2013 and April 2014.
MHO participants met 0 or 1 of the following criteria: blood pressure ≥135/85 mm Hg (or use of blood pressure lowering agents), fasting plasma glucose ≥100 mg/dL, HDL cholesterol ≤50 mg/dL, and triglycerides ≥150 mg/dL (or the use of lipid-lowering therapies).
Inclusion criteria were women aged 35 to 55 years with a BMI between 30 and 40 kg/m2.
Exclusion criteria were a diagnosis of diabetes, glucose intolerance as detected on a 2-hour, 75-g oral glucose tolerance test (OGTT); pregnancy or becoming pregnant during the study; CVD; severe disease (advanced organ failure, dementia, and cancer); immobility; alcohol or drug abuse; and severe psychiatric illness and weight loss ≥5 kg in the last 6 months due to an unknown cause.
After signing written informed consent forms, they underwent a clinical interview conducted by an internal medicine specialist, a nurse and a nutritionist, who were trained for the study.
The study is based on implementing a hypocaloric Mediterranean diet and exercise program for all study participants. The Mediterranean diet was based on a hypocaloric diet with extra virgin olive oil and nuts. For this hypocaloric diet, energy intake was reduced by about 600 kcal (or approximately 30% less than estimated energy needs). Fat comprised 35% to 40% (8%–10% saturated fatty acids), carbohydrates comprised 40% to 45% (low glycemic index), and protein comprised 20% of total daily calorie intake. Participants were encouraged and assessed on how to gradually increase their level of physical activity to reach at least 45 minutes a day after 3 months of intervention. Physical activity and a specific exercise protocol was measured through the use of pedometers. Sedentarism was evaluated using Rapid Assessment of Physical Activity (RAPA) questionnaires.[39]
Adherence to the Mediterranean diet was measured as described by Trichopoulou et al.[40] In this study, a participant was considered to have dropped out when they did not adhere to the visit schedule. Dietary and physical intervention involved weekly individual visits with a nutritionist over the 3-month period. After 3 months of intervention, the participants were divided into 3 groups according to weight loss with respect to their initial weight: group 1, with weight loss <5%; group 2, with weight loss between ≥5% and <10%; and group 3, with weight loss ≥10%.
Body weight was measured in kilograms and height was measured in meters. BMI was calculated as weight/height2 (kg/m2). Waist circumference was measured using an inelastic measuring tape with millimeter marks (Gulick II, Country Technology, Inc.) at the midpoint between the iliac crest and the costal margin, parallel to the ground and upon exhalation. Blood pressure was determined as the mean of 3 measurements after 5 minutes of rest between each. All parameters were measured by the medical staff at the Regional University Hospital of Málaga.
Peripheral blood samples were obtained from all participants after completing a fast of at least 12 hours. Fasting was necessary so as to ascertain that the lipid profile was not influenced by food intake and to accurately assess the results of the lipid profile. Samples from patients were immediately processed after reception and frozen following procedures in force. These samples were managed and provided by IBIMA's Hospital Biobank of Málaga, which belongs to the Andalusian Public Health System Biobank, part of the Spanish National Biobank Network (project PT13/0010/0006).
The conventional biochemical parameters were measured at the Clinical Analysis Laboratory at the Regional University Hospital of Málaga using routine methods. The conventional lipid profile included total cholesterol, high density lipoproteins bound to cholesterol (cHDL), cLDL, and triglycerides. cHDL was analyzed using direct measurement by homogeneous methods and total cholesterol and triglycerides by enzymatic methods. The cLDL was calculated using the Friedewald equation. Glucose was measured using the hexokinase method. All these parameters were analyzed using commercial equipment (Advia 2400, Siemens, Germany) and following the manufacturer's recommendations.
The advanced lipid profile of lipoproteins was measured by the Biosfer Teslab company (Tarragona, Spain) using the Liposcale Test.[23] The Liposcale test is a novel method for characterizing lipoprotein particles based on 2D Diffusion-Ordered 1H NMR spectroscopy (DOSY). DOSY allows for measurement of the diffusion coefficients and for direct calculation of lipoprotein sizes through the Stokes–Einstein equation. It is should be noted that a direct measurement of lipoprotein sizes is of particular importance, as they are used to compute lipoprotein particle numbers by dividing the spatial volume of the total lipid molecules by the mean volume (ie, size) of the lipoprotein particles. This test determined concentrations of VLDL, IDL, LDL, and HDL bound to cholesterol as well as concentrations of VLDL, IDL, LDL, and HDL bound to triglycerides. The number of VLDL, LDL, and HDL particles and the different sizes of these lipoproteins were calculated and classified as either small, medium, or large particles.
All patients participating in the study gave informed consent, and protocols were approved by institutional ethical committees (Comité Coordinador de Ética de la Investigación Biomédica de Andalucía).
2.1. Statistical analysis
For statistical analysis, the Simple Interactive Statistical Analysis (SISA) program was used to calculate sample size. It was based on other studies that have demonstrated metabolic benefits after weight loss (≥5% of body weight) in MHO subjects.[41,42] We assumed a 95% confidence interval (α level of 5%). With an 80% test power and a dropout rate of 5%, we included115 MHO women.
Variables measured using a conventional lipid profile and a Liposcale lipid profile were analyzed. A Deming regression line was run with MATLAB R2015b software (8.6.0.267246).
Weight loss at 3 months was analyzed using SPSS statistical software for Windows, version 20.0 (IBM Corporation, INC. Somers, NY). Quantitative variables were analyzed as the mean ± standard deviation (SD), and qualitative variables were expressed as percentages. To analyze the quantitative variables, we used a paired t test whereas for qualitative variables, we used the Chi-square test and Mantel–Haenszel test.
3. Results
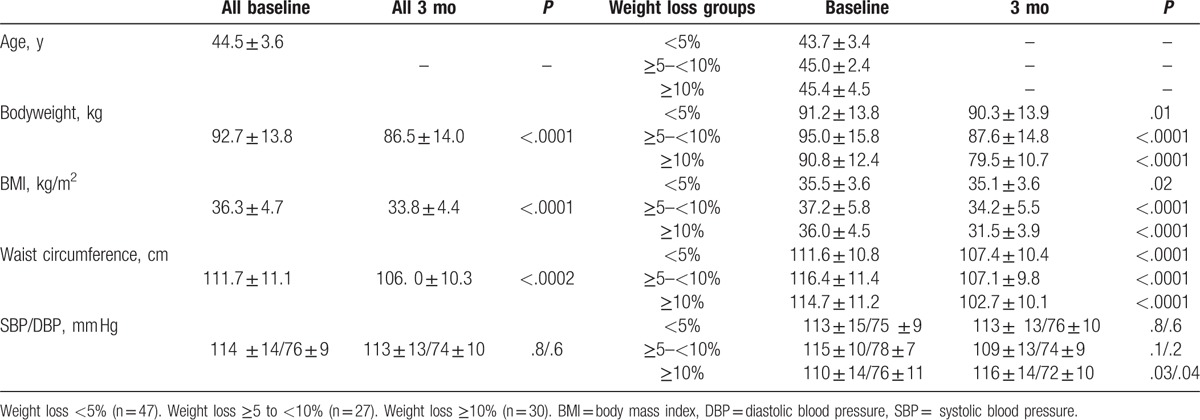
A total of 115 MHO participants with a mean age of 44.5 ± 3.6 years and a mean BMI of 36.3 ± 4.7 kg/m2 began the hypocaloric dietary intervention and physical exercise program. At baseline, the participant population had an abdominal circumference of 111.7 ± 11.1 cm and systolic and diastolic blood pressure of 114 ± 14/76 ± 9 mm Hg, respectively.
After 3 months and 11 (9.6%) dropouts, 104 women completed the program. Of these, 47 (45.2%), 27 (26.0%), and 30 (28.8%) lost <5%, ≥5% to <10%, and ≥10% of baseline body weight, respectively. Table 1 shows the anthropometric variables studied according to the percentage of weight loss, baseline, and 3 months after the intervention.
Table 1.
Anthropometric characteristics at baseline and after 3 mo of dietary intervention, according to percentage of weight loss.
“Conventional Lipid Profile.”
The conventional lipid profile is detailed in Table 2. The authors observed that total cholesterol decreased after 3 months of dietary restriction and physical exercise (194.6 ± 28.2 vs 181.1 ± 32.8 mg/dL, P < .0001). This decrease was observed in all weight loss groups.
Table 2.
Lipid profile at baseline and after 3 mo of dietary intervention, according to percentage of weight loss (conventional measurement).

After 3 months, cLDL also decreased significantly in groups with weight loss of <5% and >10%. Nevertheless, MHO subjects that lost ≥5 to <10% of their weight had significantly increased cLDL levels. On the other hand, cHDL levels also changed in all groups after following the hypocaloric diet. MHO subjects that lost <5% of their weight had significantly increased cHDL levels and the group that lost ≥10% of their weight had significantly decreased cHDL levels. Significant changes in triglyceride levels were not observed.
3.1. Advanced lipid profile by nuclear magnetic resonance spectroscopy (1H NMR)
The different classes and sizes of lipoproteins analyzed by 1H NMR spectroscopy are detailed in Table 3. They constitute the advanced lipid profile.
Table 3.
Characterization of lipid profile by nuclear magnetic resonance spectroscopy (1H RMN) at baseline and after 3 mo of dietary intervention, according to percentage of weight loss.
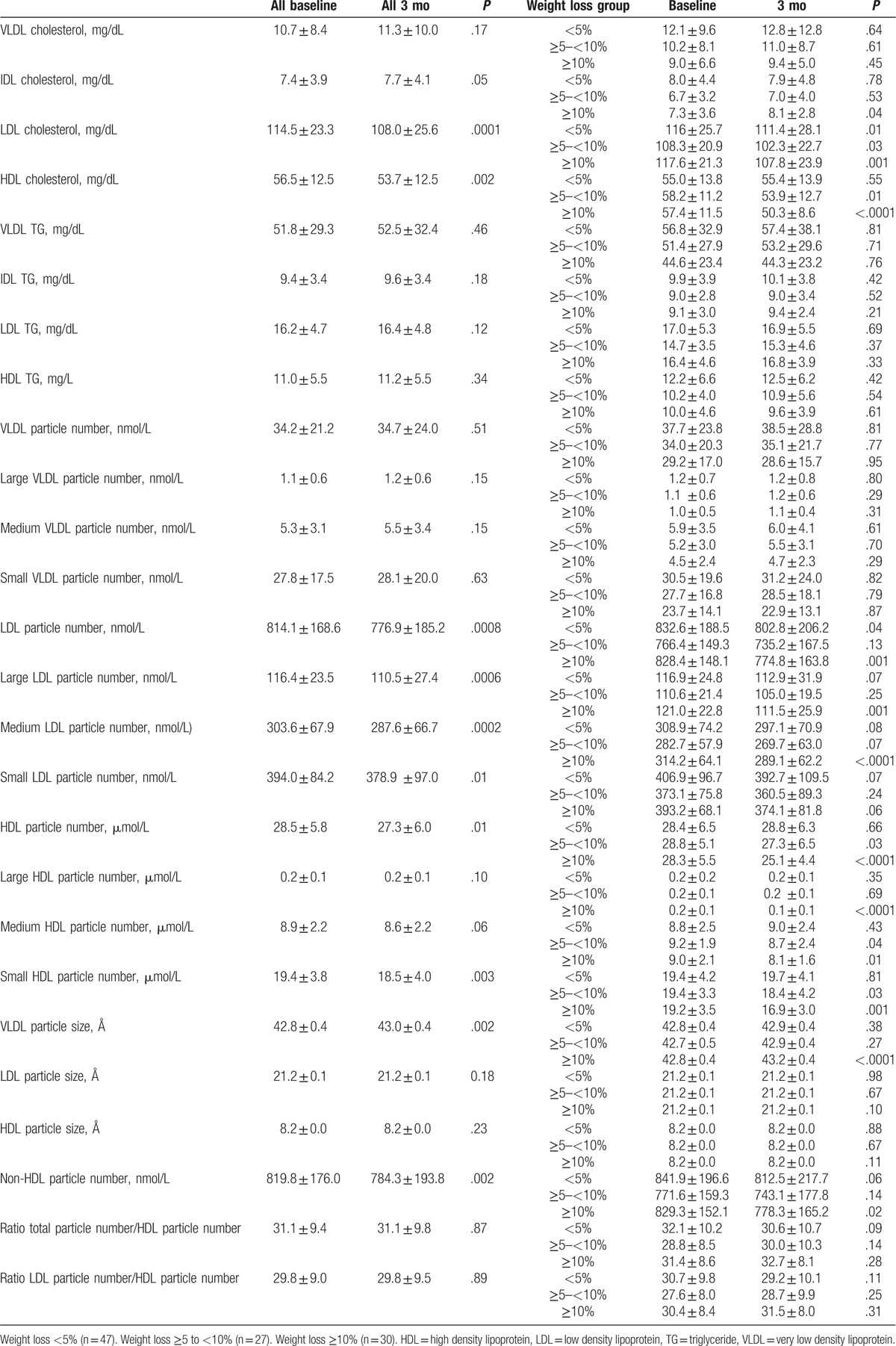
Plasma levels of LDL particles bound to cholesterol decreased significantly in all 3 study groups regardless of the percentage of body weight lost. In contrast, for LDL particles bound to triglycerides, there was no significant decrease in LDL particles in any of the 3 groups. In addition, there was a significant reduction in the number of LDL particles in 2 of the study groups (<5% and ≥10%). In contrast, when we analyzed the different sizes of LDL particles, though the small, dense particles were the most abundant in the 3 study groups and were reduced after the dietary and physical exercise intervention, they did not decrease significantly. However, there was a significant overall decline in LDL particles in all population before versus after the intervention. In contrast, when participants lost more than 10% of their body weight, concentrations of both medium and large particles did show a significant decrease (314.2 ± 64.1 vs 289.1 ± 62.2 nmol/L, P < .0001, 121.0 ± 22.8 vs 111.5 ± 25.9 nmol/L, P = .001, respectively).
Despite 3 months of following a hypocaloric diet based on the Mediterranean diet and physical exercise, levels of VLDL particles did not change significantly in the different groups. Cholesterol-bound IDL particle levels only increased in MHO women with weight loss >10% (7.3 ± 3.6 vs 8.1 ± 2.8 mg/dL, P = .04).
Plasma HDL-cholesterol levels decreased significantly when participants lost more than 5% of their body weight. This was not observed in HDL bound to triglycerides. On the other hand, the number of HDL particles decreased after the intervention in the 2 groups that lost more than 5% of their body weight, as previously observed in other studies on conventional methods of lipid measurement. When analyzing the different sizes of HDL particles, we observed that the small particles are the most abundant in the population of MHO women. Participants who reduced their body weight by more than 5% showed a significant decrease in small and medium particles. Participants who lost more than 10% of their body weight also showed a significant decrease in large particles after the intervention.
Therefore, as we have shown, the only metabolically significant changes in MHO women were observed in the levels of cIDL and cLDL. However, cHDL levels decreased in those women who lost more than 5% of their body weight. Likewise, levels of all LDL and HDL particles (small, medium, and large) decreased in all study groups.
4. Discussion
The study of the 1H NMR lipid profile makes an important contribution to our understanding of the molecular mechanisms underlying obesity. Although the lipid profiles remain within normal values, our results show that the lipid profiles of MHO subjects improved with the weight loss that resulted from the intervention with Mediterranean diet and physical exercise.
We demonstrated that MHO subjects display a range of favorable lipoprotein phenotypes, in accordance with the results of Phillips and Perry. When compared to non-MHO people who are obese, we observed lower concentrations of small LDL and HDL particles, medium and large VLDL particles, and greater numbers of LDL and HDL particles in all weight loss groups.
Significant differences in VLDL were not observed, though levels increased the following intervention. This could perhaps be due to increased hepatic cholesterol synthesis as a compensatory mechanism. However, levels of IDL particles increased in MHO women with weight loss >10%. IDL are unstable, transitional particles that are most likely changing between other subclasses of lipoproteins. The increase in IDL can also be related to a redistribution of body fat.
Associations between lipoprotein profiles and MH phenotypes were generally not dependent on the definition of MHO.[43] Despite the inclusion of lipid profiles in most MH definitions, limited data exist regarding lipoprotein particle profiles in MHO phenotypes. Conventional methods of lipid profiling have been analyzed and all particle subclasses have been researched.[44–47] In comparing our work to these authors’ work, we found that our participants had lower concentrations of small LDL particles but a similar LDL particle size, independently of the percentage of weight lost. Moreover, also reported that the larger LDL particle values in MHO women are still within normal parameters.
The use of 1H NMR in our study allowed us to examine both the number and size of each of the main lipoprotein subclasses. The size of LDL particles has an important influence on CVR, especially regarding small LDL particles, which are the most atherogenic. However, after a 3-month intervention, a significant decrease was not observed in the groups studied. Nevertheless, the medium and large particles were decreased in the group that had the greatest weight loss (≥10%).
Our results are consistent findings of Phillips and Perry,[43] which report lower numbers of small LDL particles and higher concentrations of large LDL particles among the MHO subjects.
The concentration of VLDL affects the concentration of the remaining lipoproteins. We report the same concentration of large VLDL and higher concentrations of medium VLDL particles in all weight loss groups as well as less small concentrations of small VLDL particles in the group with the greatest weight loss, in contrast with the results obtained by Phillips and Perry.
HDL concentrations in the MHO population require more in-depth analysis. Paradoxically, HDL concentrations, which should increase due to diet and physical exercise, decreased in all participants and decreased to a greater extent in those who lost the greatest percentage of their body weight (≥10%). In this study, low concentrations of small HDL were observed in all groups studied, but principally in women with weight loss ≥5%, results corroborated by Phillips and Perry. Small HDL particles are the most atherogenic and have a positive correlation with CVD, whereas large HDL particles have an inverse correlation.[29,47–50] Small HDL particles may be less protective against LDL oxidation or may be indicative of overproduction of VLDL.[29] These findings are consistent across all MHO definitions, regardless of BMI status and independent of a range of confounding factors, including markers of liver fat and function.
On the other hand, from a dietary point of view, one of the possible causes of this increase in HDL levels after an intensive diet may be that increasing fiber intake not only reduces the intestinal absorption of cholesterol and saturated fats, but also of unsaturated fats. This would lower the concentration of HDL cholesterol in the blood.
It has been described that physical exercise has a paradoxical effect in MHO individuals, causing a decrease in HDL concentrations.[41–53] Our results support this finding. However, 1H NMR shows that although HDL levels decrease, the overall atherogenic profile improves as there is a greater reduction in small and medium HDL particles.
5. Conclusion
Adequate analysis of the lipid profile using novel techniques such as 1H NMR is necessary for the correct characterization of the MHO population since both the number and sizes of the different lipoprotein particles better estimate CVR after a short-term, intensive intervention with a hypocaloric Mediterranean diet, and physical exercise. The results of our study can only be identified through 1H-NMR, hence the importance of including this technique in normal clinical practice.
Our study demonstrates that weight loss in MHO women slightly improves their lipid profile, but within normal ranges. We do not know what this may imply in the long-term, since weight regain in this phenotype could alter their “healthy” metabolic profile.
More long-term studies are needed to see if there is a change in the lipid profile of this MHO population after weight loss.
Acknowledgments
We thank Tatiana Diaz-Cordoba and Inmaculada Martin-Martin for their excellent laboratory assistance in the Malaga Hospital-IBIMA Biobank and Claire Alexandra Conrad for her help with the final English-language version. This work was supported by grants from the Instituto de Salud Carlos III, cofinanced by the Fondo Europeo de Desarrollo Regional-FEDER (PI12/01373 and “Centros de Investigación En Red" [CIBER, CB06/03/0018]). M Rosa Bernal-Lopez was supported by “Miguel Servet Type I" program (CP15/00028) from the ISCIII-Madrid (Spain), cofinanced by the Fondo Europeo de Desarrollo Regional-FEDER.
Footnotes
Abbreviations: 1H NMR = nuclear magnetic resonance spectroscopy, cHDL = high density lipoproteins bound to cholesterol, cLDL = low density lipoproteins bound to cholesterol, CVD = cardiovascular disease, CVR = cardiovascular risk, IDL = intermediate density lipoprotein, MHO = metabolically healthy obese, VLDL = very low density lipoprotein.
ER-G and MRB-L contributed equally to this work.
Funding/support: This work was supported by grants from the Instituto de Salud Carlos III, cofinanced by the Fondo Europeo de Desarrollo Regional-FEDER (PI12/01373 and “Centros de Investigación En Red” (CIBER, CB06/03/0018)), and M Rosa Bernal-Lopez was supported by “Miguel Servet Type I” program (CP15/00028) from the ISCIII-Madrid (Spain), cofinanced by the Fondo Europeo de Desarrollo Regional-FEDER.
The authors have no conflicts of interest to disclose.
References
- [1].Bandera EV, Maskarinec G, Romieu I, et al. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Adv Nutr 2015;6:803–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xu W, Zhang H, Paillard-Borg S, et al. Prevalence of overweight and obesity among Chinese adults: role of adiposity indicators and age. Obes Facts 2016;9:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee JH, Han KD, Jung HM, et al. Association between obesity, abdominal obesity, and adiposity and the prevalence of atopic dermatitis in young Korean adults: the Korea National Health and Nutrition Examination Survey 2008–2010. Allergy Asthma Immunol Res 2016;8:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci 2006;331:166–74. [DOI] [PubMed] [Google Scholar]
- [5].Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 2011;364:719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010;363:2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhao W, Katzmarzyk PT, Horswell R, et al. Body mass index and the risk of all-cause mortality among patients with type 2 diabetes mellitus. Circulation 2014;130:2143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Di Angelantonio E, Kaptoge S, et al. Emerging Risk Factors Collaboration. Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sung KC, Ryu S, Cheong ES, et al. All-cause and cardiovascular mortality among Koreans: effects of obesity and metabolic health. Am J Prev Med 2015;49:62–71. [DOI] [PubMed] [Google Scholar]
- [11].Ruderman N, Chisholm D, Pi-Sunyer X, et al. The metabolically obese, normal-weight individual revisited. Diabetes 1998;47:699–713. [DOI] [PubMed] [Google Scholar]
- [12].Karelis AD, St-Pierre DH, Conus F, et al. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 2004;89:2569–75. [DOI] [PubMed] [Google Scholar]
- [13].Karelis AD, Faraj M, Bastard JP, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 2005;90:4145–50. [DOI] [PubMed] [Google Scholar]
- [14].Seo MH, Rhee EJ. Metabolic and cardiovascular implications of a metabolically healthy obesity phenotype. Endocrinol Metab 2014;29:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Karelis AD, Faraj M, Bastard J-P, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 2005;90:4145–50. [DOI] [PubMed] [Google Scholar]
- [16].Shin M-J, Hyun YJ, Kim OY, et al. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes 2006;30:1529–34. [DOI] [PubMed] [Google Scholar]
- [17].Koster A, Stenholm S, Alley DE, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010;18:2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marques-Vidal P, Velho S, Waterworth D, et al. The association between inflammatory biomarkers and metabolically healthy obesity depends of the definition used. Eur J Clin Nutr 2012;66:426–35. [DOI] [PubMed] [Google Scholar]
- [19].Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes 2011;35:971–81. [DOI] [PubMed] [Google Scholar]
- [20].Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013;5:1218–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Korzun WJ, Nilsson G, Bachmann LM, et al. Difference in bias approach for commutability assessment: application to frozen pools of human serum measured by 8 direct methods for HDL and LDL cholesterol. Clin Chem 2015;61:1107–13. [DOI] [PubMed] [Google Scholar]
- [22].Méndez González J, Martín Campos J, Ordóñez Llanos J. El laboratorioclínico y lasdislipemias. Endocrinol Nutr 2008;55:89–96. [DOI] [PubMed] [Google Scholar]
- [23].Mallol R, Amigó N, Rodríguez MA, et al. Liposcale: a novel advanced lipoprotein test based on 2D diffusion-ordered 1H NMR spectroscopy. J Lipid Res 2015;56:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Blake GJ, Otvos JD, Rifai N, et al. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation 2002;106:1930–7. [DOI] [PubMed] [Google Scholar]
- [25].El Harchaoui K, van der Steeg WA, Stroes ESG, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol 2007;49:547–53. [DOI] [PubMed] [Google Scholar]
- [26].Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 2006;26:847–70. [DOI] [PubMed] [Google Scholar]
- [27].Mora S, Szklo M, Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007;192:211–7. [DOI] [PubMed] [Google Scholar]
- [28].Otvos JD, Mora S, Shalaurova I, et al. Clinical implications of discordance between LDL cholesterol and LDL particle number. J Clin Lipidol 2011;5:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol 2002;90:89–94. [DOI] [PubMed] [Google Scholar]
- [30].Otvos JD. NMR measurement of LDL particles and its clinical advantages. J Clin Ligand Assay 2004;27:94–6. [Google Scholar]
- [31].Mallol R, Rodríguez MA, Heras M, et al. Particle size measurement of lipoprotein fractions using diffusion-ordered NMR spectroscopy. Anal Bioanal Chem 2012;402:2407–15. [DOI] [PubMed] [Google Scholar]
- [32].Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol 2010;21:305–11. [DOI] [PubMed] [Google Scholar]
- [33].Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 2002;43:1363–79. [DOI] [PubMed] [Google Scholar]
- [34].Williams PT, Zhao X-Q, Marcovina SM, et al. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis 2014;233:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 2008;31:811–22. [DOI] [PubMed] [Google Scholar]
- [36].Pajunen P, Kotronen A, Korpi-Hyövälti E, et al. Metabolically healthy and unhealthy obesity phenotypes in the general population: the FIN-D2D Survey. BMC Public Health 2011;11:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bo S, Musso G, Gambino R, et al. Prognostic implications for insulin-sensitive and insulin-resistant normal-weight and obese individuals from a population-based cohort. Am J Clin Nutr 2012;96:962. [DOI] [PubMed] [Google Scholar]
- [38].Lambert E, Sari CI, Dawood T, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension 2010;56:351. [DOI] [PubMed] [Google Scholar]
- [39].Topolski TD, LoGerfo J, Patrick DL, et al. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- [40].Trichopoulou A, Costacou T, Bamia C, et al. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608. [DOI] [PubMed] [Google Scholar]
- [41].Arsenault BJ, Côté M, Cartier A, et al. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis 2009;207:530–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care 2010;33:1957–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Phillips CM, Perry IJ. Lipoprotein particle subclass profiles among metabolically healthy and unhealthy obese and non-obese adults: does size matter? Atherosclerosis 2015;242:399–406. [DOI] [PubMed] [Google Scholar]
- [44].Iacobellis G, Ribaudo MC, Zappaterreno A, et al. Small, dense low-density lipoprotein and C-reactive protein in obese subjects with and without other criteria for the metabolic syndrome. J Clin Lipidol 2007;1:599–604. [DOI] [PubMed] [Google Scholar]
- [45].Kim M, Paik JK, Kang R, et al. Increased oxidative stress in normal-weight postmenopausal women with metabolic syndrome compared with metabolically healthy overweight/obese individuals. Metab Clin Exp 2013;62:554–60. [DOI] [PubMed] [Google Scholar]
- [46].Kim S, Lee H, Lee DC, et al. Predominance of small dense LDL differentiates metabolically unhealthy from metabolically healthy overweight adults in Korea. Metab Clin Exp 2014;63:415–21. [DOI] [PubMed] [Google Scholar]
- [47].Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol 2015;6:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kuller L, Arnold A, Tracy R, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol 2002;22:1175–80. [DOI] [PubMed] [Google Scholar]
- [49].Mora S, Otvos JD, Rifai N, et al. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].El Harchaoui K, Arsenault BJ, Franssen R, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med 2009;150:84–93. [DOI] [PubMed] [Google Scholar]
- [51].Huang XL, Pan JH, Chen D, et al. Efficacy of lifestyle interventions in patients with type 2 diabetes: a systematic review and meta-analysis. Eur J Intern Med 2016;27:37–47. [DOI] [PubMed] [Google Scholar]
- [52].Maillard F, Rousset S, Pereira B, et al. High-intensity interval training reduces abdominal fat mass in postmenopausal women with type 2 diabetes. Diabetes Metab 2016;42:433–41. [DOI] [PubMed] [Google Scholar]
- [53].Al-Eisa ES, Alghadir AH, Gabr SA. Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin Interv Aging 2016;11:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


