Supplemental Digital Content is available in the text
Keywords: antineoplastic agents, Asia, castration-resistant, disease-free survival, MDV 3100, prostatic neoplasms
Abstract
Background:
Enzalutamide is an androgen receptor (AR) inhibitor that acts on different steps in the AR signaling pathway. In PREVAIL, an international, phase III, double-blind, placebo-controlled trial, enzalutamide significantly reduced the risk of radiographic progression by 81% (hazard ratio [HR], 0.19; P < .0001) and reduced the risk of death by 29% (HR, 0.71; P < .0001) compared with placebo in chemotherapy-naïve men with metastatic castration-resistant prostate cancer.
Methods:
To evaluate treatment effects, safety, and pharmacokinetics of enzalutamide in East Asian patients from the PREVAIL trial, we performed a post hoc analysis of the Japanese, Korean, and Singaporean patients. PREVAIL enrolled patients with asymptomatic or mildly symptomatic chemotherapy-naïve metastatic castration-resistant prostate cancer who had progressed on androgen deprivation therapy. During the study, patients received enzalutamide (160 mg/d) or placebo (1:1) until death or discontinuation because of radiographic progression or skeletal-related event and initiation of subsequent therapy. Centrally assessed radiographic progression-free survival (rPFS) and overall survival (OS) were coprimary endpoints. The secondary endpoints of the PREVAIL trial were investigator-assessed rPFS, time to initiation of chemotherapy, time to prostate-specific antigen (PSA) progression, and PSA response (≥50% decline).
Results:
Of 1717 patients, 148 patients were enrolled at sites in East Asia (enzalutamide 73, placebo 75). Treatment effect of enzalutamide versus placebo was consistent with that for the overall population as indicated by the HRs (95% confidence interval) of 0.38 (0.10–1.44) for centrally assessed rPFS, 0.59 (0.29–1.23) for OS, 0.33 (0.19–0.60) for time to chemotherapy, and 0.32 (0.20–0.50) for time to PSA progression. In East Asian patients, PSA responses were observed in 68.5% and 14.7% of enzalutamide- and placebo-treated patients, respectively. The enzalutamide plasma concentration ratio (East Asian:non-Asian patients) was 1.12 (90% confidence interval, 1.05–1.20) at 13 weeks. Treatment-related adverse events grade ≥ 3 occurred in 1.4% and 2.7% of enzalutamide- and placebo-treated East Asian patients, respectively.
Conclusions:
Treatment effects and safety of enzalutamide in East Asian patients were generally consistent with those observed in the overall study population from PREVAIL.
ClinicalTrials.gov number:
1. Introduction
Prostate cancer is less common in Asian countries than in Western countries,[1] but incidence appears to be increasing and may be associated with demographic aging, changes in diet, and increased utilization of prostate-specific antigen (PSA) screening.[1–4] Epidemiologic data across the 3 East Asian countries (Japan, Korea, and Singapore) that contributed patients for this analysis are similar when differences in the size of the populations are accounted for.[1,5] Prostate cancer is the 6th-leading cause of male cancer mortality in Japan and Singapore and the 7th-leading cause of male cancer mortality in Korea.[2] There are an estimated 64,900 new cases of prostate cancer[6] and 11,600 deaths from prostate cancer[7] annually in Japan. In Korea, there are approximately 9000 new prostate cancer cases and 1500 deaths annually,[8] and in Singapore, there are about 1200 new diagnoses of prostate cancer and about 170 deaths from prostate cancer annually.[2] In addition, compared with those in Western countries, patients in Asian countries often present with more advanced disease.[1,9,10]
Androgen suppression with androgen-deprivation therapy or castration is the primary first-line treatment for metastatic prostate cancer. Although most patients initially respond to androgen-deprivation therapy, the disease will eventually progress to castration-resistant prostate cancer (CRPC) in nearly all patients.[11–14] Preclinical evidence has demonstrated that overexpression of the androgen receptor (AR) is sufficient to confer resistance to androgen deprivation in prostate cancer cell lines.[15,16] Until recently, docetaxel has been the only option for men with metastatic castration-resistant prostate cancer (mCRPC)[17]; however, progression is inevitable.[18] In Japan, Korea, and Singapore, there are currently limited options for noncytotoxic agents to treat mCRPC.[19] Thus, there is a need for additional therapies in these regions that may have higher proportions of patients presenting with metastatic disease.[1,9,10]
In PREVAIL, an international, phase III, randomized trial, the AR signaling inhibitor enzalutamide (ENZA) (Medivation, Inc., San Francisco, CA [Medivation was acquired by Pfizer Inc in September 2016]) significantly reduced the risk of radiographic progression by 81% (hazard ratio [HR], 0.186; P < .001), reduced the risk of death by 29% (HR, 0.706; P < .001), and reduced the risk of skeletal-related events (SREs) by 28% (HR, 0.720; P < .001) compared with placebo in chemotherapy-naïve men with mCRPC.[20,21] The most common adverse events (AEs) with ENZA treatment in PREVAIL were fatigue, back pain, constipation, and arthralgia. ENZA is now approved for use in Japan and Korea in men with CRPC regardless of the patient's prior chemotherapy exposure. In Singapore, it is approved for use in men with mCRPC postchemotherapy.
In this post hoc analysis of the PREVAIL trial, we evaluated the treatment effects, safety, and pharmacokinetic exposure of ENZA versus placebo in patients treated in Japan, Korea, and Singapore.
2. Methods
The full methodology, including patient eligibility and full endpoint definitions, of the international, randomized, double-blind, placebo-controlled phase III PREVAIL trial (NCT01212991) has been previously reported.[20] PREVAIL was approved by the independent review board at each participating site and was conducted according to provisions of the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
2.1. Patients
Patients were eligible for enrollment if they had a histologically or cytologically confirmed adenocarcinoma of the prostate that was castration resistant, had not received cytotoxic chemotherapy or abiraterone acetate, had an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1 (no symptoms or ambulatory but restricted in strenuous activity), and were asymptomatic or mildly symptomatic per the Brief Pain Inventory Short Form question 3 (i.e., pain score 0–3).[22] Patients with conditions that could lower the seizure threshold (i.e., brain metastases, history of seizure, concurrent medications) or those with a history of chemotherapy were excluded.
2.2. Study design
Patients were enrolled from September 2010 to September 2012 at 207 sites globally, of which 21 were in Japan, 7 in Korea, and 2 in Singapore. Enrollment occurred in Japan between November 2011 and May 2012, in Korea between November 2011 and September 2012, and in Singapore between October 2011 and April 2012. Patients were randomized 1:1 to receive either 160 mg oral ENZA or placebo once daily (Fig. 1). Randomization was stratified according to the study site. Treatment was discontinued for any of the following reasons: occurrence of unacceptable side effects, confirmed radiographic progression, or confirmed SRE and the initiation of cytotoxic therapy or investigational agent for prostate cancer.
Figure 1.
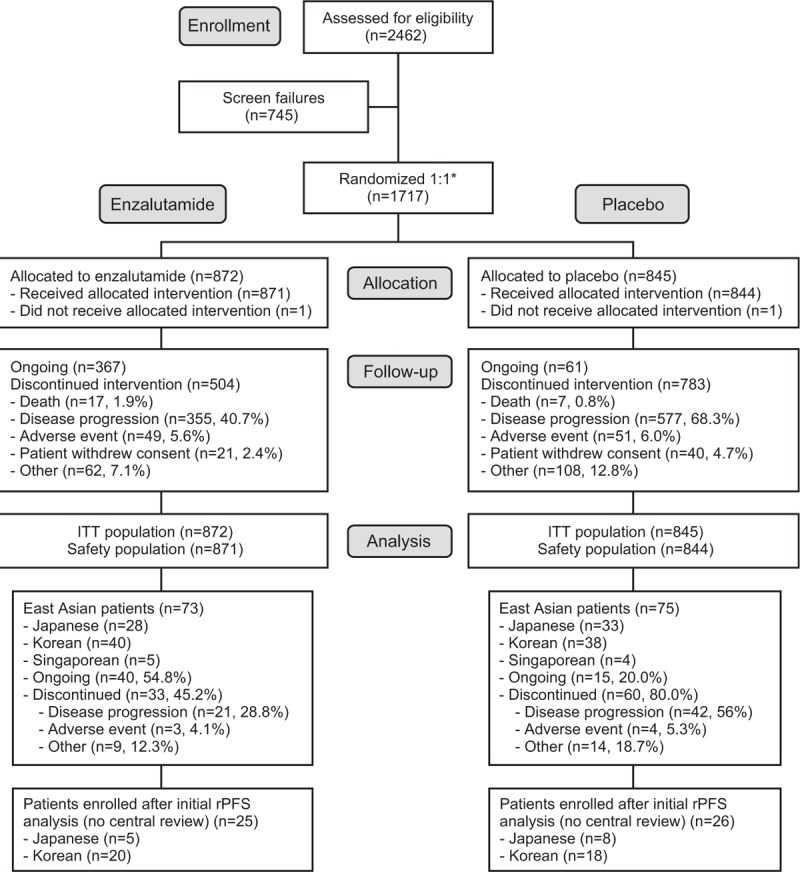
PREVAIL patient disposition (CONSORT diagram). ∗Randomization was stratified by study site. ITT = intent-to-treat, rPFS = radiographic progression-free survival.
2.3. Endpoints
The coprimary endpoints were radiographic progression-free survival (rPFS) determined by independent central review and overall survival (OS). A prespecified, interim analysis of rPFS occurred on May 6, 2012, upon the occurrence of 439 rPFS events. Secondary endpoints included rPFS by investigator review, time to initiation of chemotherapy, time to PSA progression, PSA response, and time to SRE. Predose plasma concentrations of ENZA and N-desmethyl ENZA, its active metabolite, were measured at weeks 5, 13, and 25.
Radiographic disease progression was evaluated using the Prostate Cancer Clinical Trials Working Group[23] guidelines for bone disease and Response Evaluation Criteria in Solid Tumors version 1.1 for soft-tissue disease.[24] Radiographic disease progression included confirmed new bone lesions and new soft-tissue lesions. PSA response was defined as a decline in PSA ≥50% from baseline to the lowest postbaseline PSA level as determined by the local laboratory (confirmed by a second assessment conducted ≥3 weeks later). An SRE was defined as radiation therapy or surgery to bone for prostate cancer, pathological bone fracture, spinal cord compression, or change of antineoplastic therapy to treat bone pain.
2.4. Statistical analysis
In this post hoc analysis, baseline characteristics and treatment effects were evaluated in the intent-to-treat population, defined as all randomly assigned patients, whereas safety data were analyzed using descriptive statistics in all randomized patients who received at least 1 dose of study drug. Baseline characteristics and safety data were summarized descriptively for each treatment group. Estimates of the medians and 95% confidence intervals (CIs) were determined using the Kaplan–Meier method. The HR relative to placebo, with <1.00 favoring ENZA, was determined using an unstratified Cox regression model with treatment as the only covariate. The mean minimum concentration of ENZA and the sum of ENZA plus N-desmethyl ENZA at weeks 5, 13, and 25 were adjusted for weight using log-linear regression and summarized descriptively.
3. Results
3.1. Patients
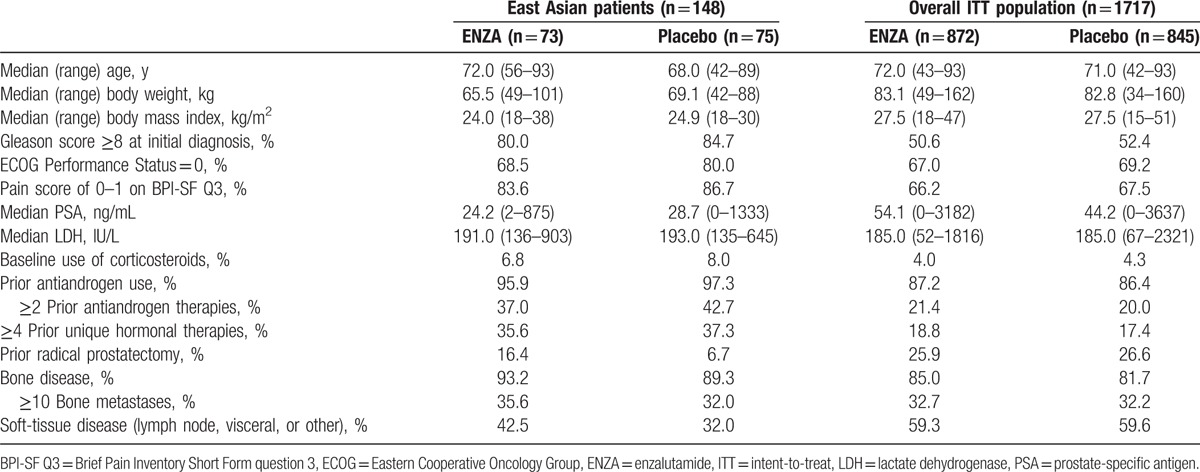
Of a total of 1717 patients, 148 East Asian patients were enrolled at sites in Japan, Korea, and Singapore (ENZA, n = 73; placebo, n = 75) (Fig. 1). Among East Asian patients, baseline demographic characteristics were well balanced between the ENZA and placebo treatment arms, except that fewer ENZA-treated patients had an ECOG Performance Status of 0 (68.5% vs. 80.0%) and more had radical prostatectomy (16.4% vs. 6.7%) and soft-tissue metastases (42.5% vs. 32.0%) (Table 1). Differences in baseline disease characteristics were observed between East Asian patients and the overall study population, including lower median body weight, lower median body mass index, greater proportion of patients with ECOG Performance Status of 0, and greater proportion of patients with low baseline pain scores in East Asian patients. Compared with the overall study population, East Asian patients may have had a greater disease burden as indicated by a greater proportion of patients with a Gleason score ≥8, bone disease, baseline corticosteroid use, and prior antiandrogen therapy. Notably, 40% of East Asian patients and 21% of patients in the overall population received at least 2 prior antiandrogen therapies. The median PSA and incidence of soft-tissue disease at baseline were lower in East Asian patients than in the overall study population.
Table 1.
Baseline patient and disease characteristics.
Per the PREVAIL protocol, the interim analysis of centrally assessed rPFS in the overall PREVAIL population was to occur when 439 rPFS events had occurred; this event happened on May 6, 2012. This cutoff date occurred during the enrollment period in East Asia; therefore, rPFS was not evaluated by central review for 51 East Asian patients (Fig. 1). For East Asian patients, results for the investigator-assessed rPFS and OS are based on a data cutoff of September 16, 2013, which corresponds to the occurrence of 540 deaths in the overall study population as specified by the protocol. At the September 16, 2013, data cutoff, enrollment at all East Asian sites was complete and 55% of East Asian patients in the ENZA arm and 20% of East Asian patients in the placebo arm were still receiving study drug (Fig. 1). After 784 deaths in the overall study population (data cutoff, June 1, 2014), an updated exploratory analysis of OS was performed for the overall PREVAIL population, and the results for East Asian patients are presented here.
3.2. Treatment effects
Relative to placebo, ENZA reduced the risk of centrally assessed radiographic progression or death by 62% (HR, 0.38; 95% CI, 0.10–1.44) in East Asian patients and by 81% (HR, 0.19; 95% CI, 0.15–0.23) in the overall population (Fig. 2A). Median rPFS by central assessment was not reached among ENZA- or placebo-treated East Asian patients. In East Asian patients, treatment effects were consistent between centrally assessed and investigator-assessed radiographic progression (HR, 0.33; 95% CI, 0.19–0.56) (Fig. 2B). Median rPFS by investigator assessment for East Asian patients was not reached in ENZA-treated patients, compared with 8 months in placebo-treated patients.
Figure 2.
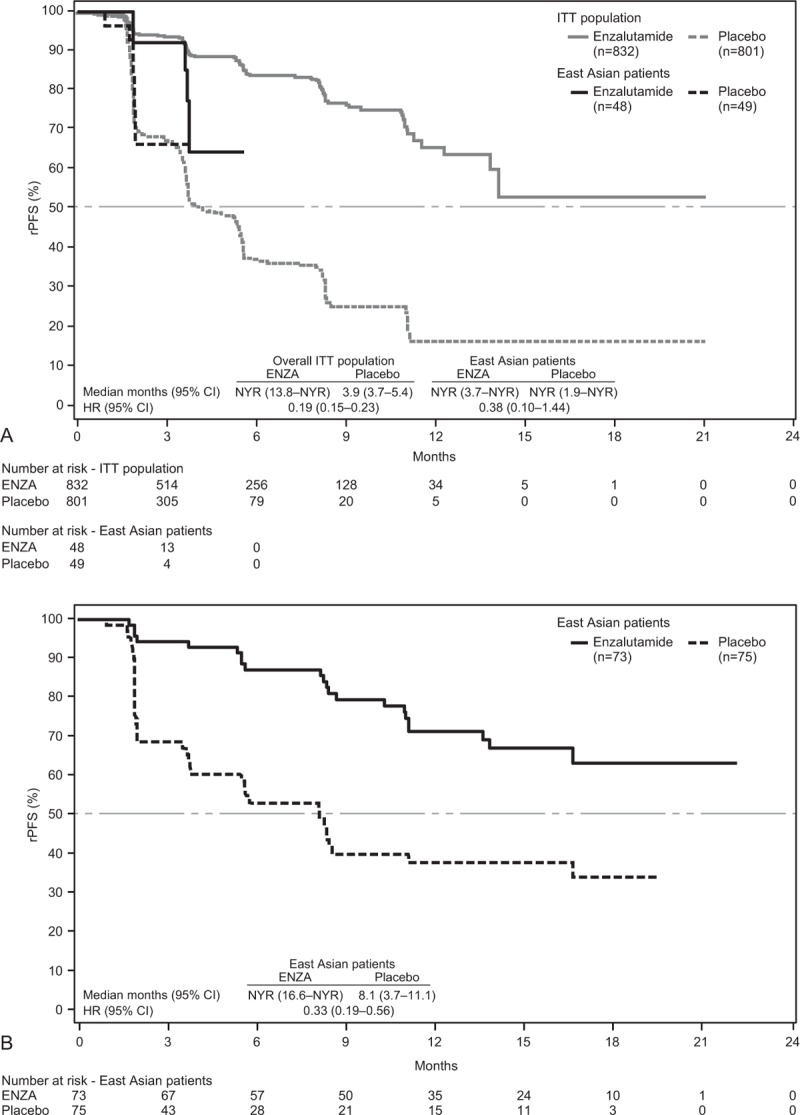
(A) Kaplan–Meier estimates of centrally assessed rPFS for East Asian patients and the overall study population (data cutoff, May 6, 2012). (B) Kaplan–Meier estimates of investigator-assessed rPFS for East Asian patients only (data cutoff, September 16, 2013). Dashed horizontal lines indicate median. Hazard ratios are based on unstratified Cox regression models with treatment as the only covariate, with values <1.00 favoring ENZA. CI = confidence interval, ENZA = enzalutamide, HR = hazard ratio, ITT = intent-to-treat, NYR = not yet reached, rPFS = radiographic progression-free survival.
Treatment with ENZA reduced the risk of death by 41% (HR, 0.59; 95% CI, 0.29–1.23) in East Asian patients and by 29% (HR, 0.71; 95% CI, 0.60–0.84) in the overall study population (Fig. 3A). In an updated OS analysis that included an additional 9 months of patient follow-up, ENZA reduced the risk of death among East Asian patients by 37% (HR, 0.63; 95% CI, 0.36–1.10) relative to placebo (Fig. 3B).
Figure 3.
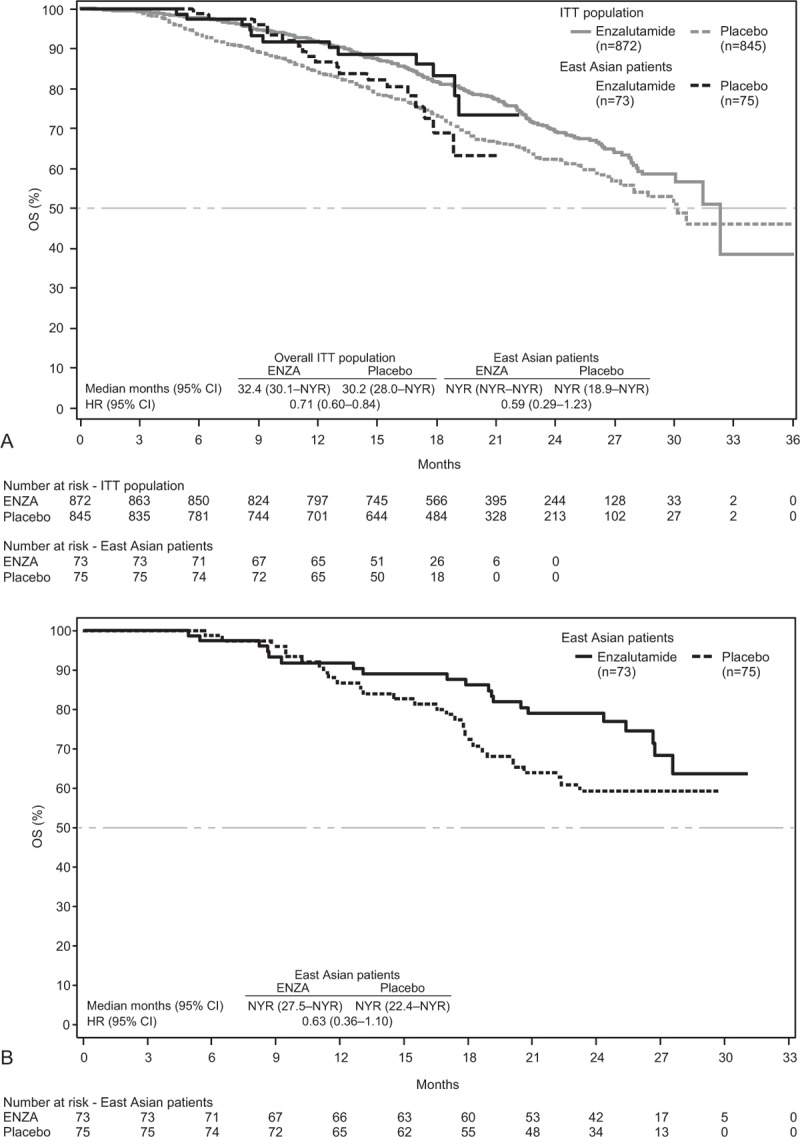
(A) Kaplan–Meier estimates of OS for East Asian patients and overall study population (data cutoff, September 16, 2013). (B) Kaplan–Meier estimates of updated OS for East Asian patients only (data cutoff, June 1, 2014). Dashed horizontal lines indicate median. Hazard ratios are based on unstratified Cox regression models with treatment as the only covariate, with values <1.00 favoring ENZA. CI = confidence interval, ENZA = enzalutamide, HR = hazard ratio, ITT = intent-to-treat, NYR = not yet reached, OS = overall survival.
Furthermore, treatment effects of ENZA versus placebo in East Asian patients reflected in the secondary endpoint results, including time to initiation of chemotherapy (HR, 0.33; 95% CI, 0.19–0.60), median time to PSA progression (HR, 0.32; 95% CI, 0.20–0.50), proportion of patients with a ≥50% decline in PSA from baseline (68.5% vs. 14.7%, respectively), and proportion of patients with objective soft-tissue response (60.0% vs. 7.7%, respectively), were consistent with results in the overall population (Table 2). In East Asian patients, median time to SRE was not reached (95% CI, 20.1 to not reached) among ENZA- or placebo-treated patients (HR, 1.35; 95% CI, 0.68–2.68) (Table 2).
Table 2.
Secondary endpoints.
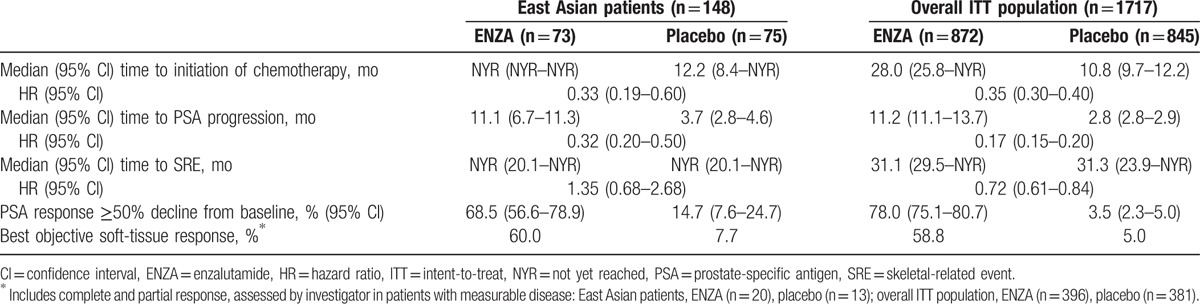
3.3. Subsequent antineoplastic and endocrine therapies
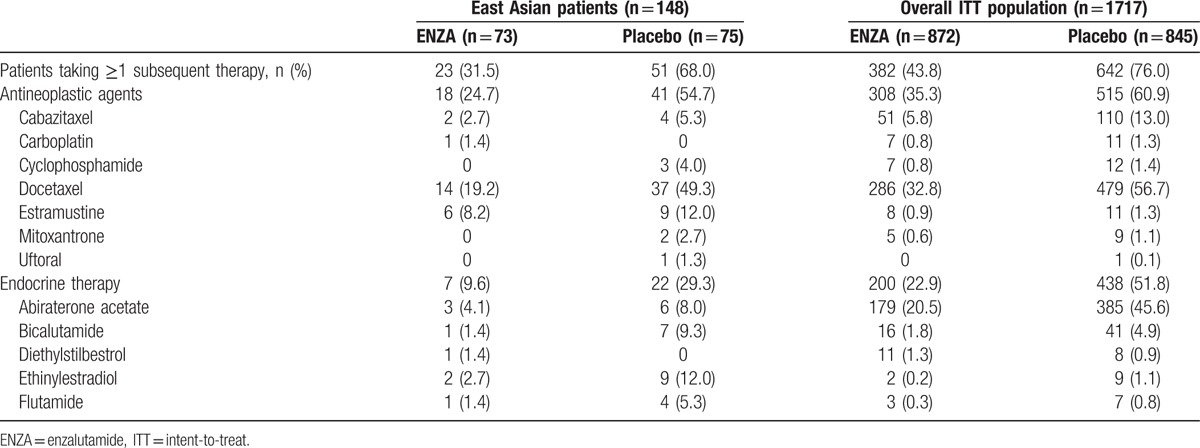
In East Asian patients, 31.5% of those treated with ENZA and 68.0% of those receiving placebo used subsequent therapies (Table 3). The most common therapies received by East Asian patients treated with ENZA were docetaxel (19.2%), estramustine (8.2%), and ethinylestradiol (2.7%). The most common therapies received by East Asian patients receiving placebo were docetaxel (49.3%), estramustine (12.0%), and ethinylestradiol (12.0%).
Table 3.
Subsequent antineoplastic and endocrine therapies.
3.4. Pharmacokinetics
The mean minimum plasma concentration of ENZA and the sum of ENZA and N-desmethyl ENZA, its active metabolite, were determined for East Asian and non-Asian patients (Table S1, Supplemental Content). When adjusted for body weight, the concentration of ENZA plus its active metabolite at weeks 5, 13, and 25 were similar in East Asian patients and non-Asian patients, with geometric mean ratios (East Asian vs. non-Asian) of 0.99, 1.01, and 1.03, respectively.
3.5. Safety
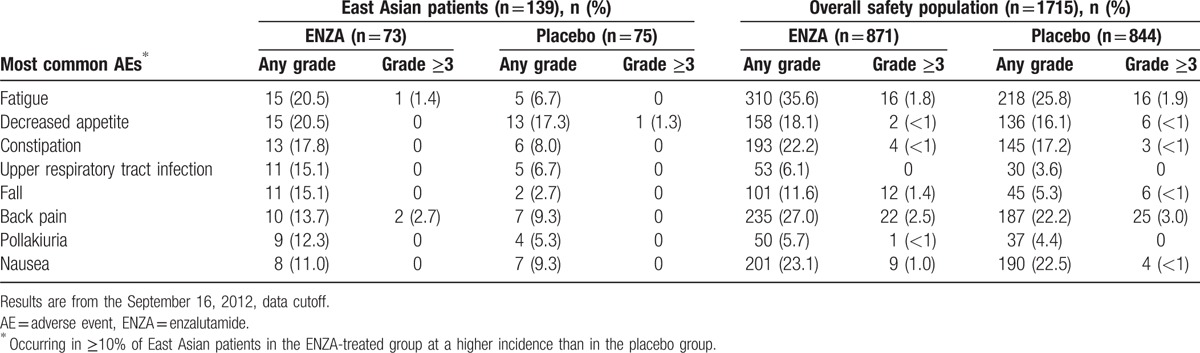
East Asian patients receiving ENZA had a more than 2-fold longer duration of therapy than those receiving placebo (Table 4). Among East Asian patients, the most frequent AEs (occurring in ≥10% of patients) were fatigue and decreased appetite, which were mostly grade ≤2 (Table 5). Grade ≥3 AEs were reported in 31.5% of East Asian patients receiving ENZA and in 22.7% of those receiving placebo. Treatment-related AEs grade ≥3 were reported in 1.4% of East Asian patients receiving ENZA and 2.7% of those receiving placebo. No seizures were reported among East Asian patients. Discontinuation of treatment because of an AE was reported by 3 (4.1%) ENZA- and 4 (5.3%) placebo-treated East Asian patients. One (1.4%) ENZA- and 2 (2.7%) placebo-treated East Asian patients required a dose reduction because of an AE. The overall incidence of AEs and serious AEs was generally consistent between East Asian patients and the overall population (Table 4), with few differences observed.
Table 4.
Overall AE summary.
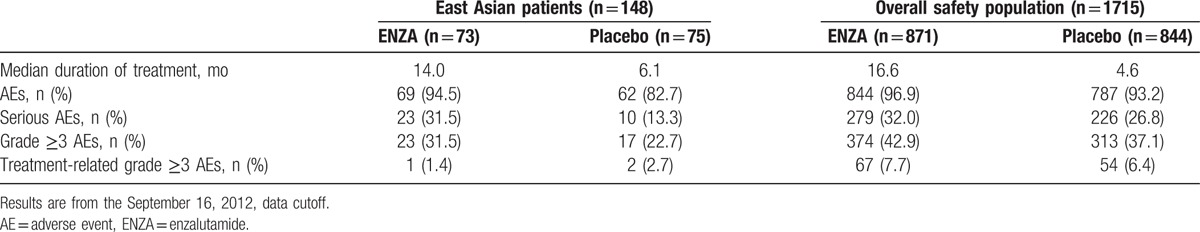
Table 5.
Most common AEs: Comparison with overall population.
4. Discussion
In this post hoc analysis of PREVAIL, treatment effects and safety in East Asian patients with asymptomatic or mildly symptomatic, chemotherapy-naïve mCRPC were generally consistent with those in the overall study population. Some of the observed differences in baseline disease characteristics of East Asian patients relative to the overall study population may be attributed to differences in clinical practice in Japan, Korea, and Singapore compared with Western countries. In Asian countries, patients with prostate cancer are often diagnosed at more advanced stages of disease, which may be related to less frequent PSA screening in Asian countries than in Western countries.[1,9,10] In PREVAIL, compared with the overall study population, a higher percentage of East Asian patients had a Gleason score ≥8 and a greater percentage had bone disease, suggesting a higher disease burden. However, at baseline, East Asian patients had lower median PSA levels and fewer had soft-tissue disease and pain. East Asian patients had an increased use of corticosteroids, commonly used to treat pain associated with bone disease, which may account for the larger proportion of patients with low baseline pain compared with the overall population. The percentage of East Asian patients who had received at least 2 antiandrogen therapies prior to treatment with ENZA was higher than in the overall study population. Notably, hormone therapy is widely used for treatment of early stage prostate cancer in Japan,[10] whereas radical prostatectomy is the preferred treatment in Korea.[3]
Some baseline disease characteristics may also be attributed to differences in ethnicity. It has previously been shown that, in general, Asian men have a lower baseline PSA than Caucasian men.[25–29] Studies of PSA screening in Korea,[29] Japan,[30] and Singapore[25] have identified increased percentages of patients with prostate cancer in groups with PSA levels below the commonly accepted 4.0 ng/mL threshold. Some groups have suggested that a lower PSA cutoff value should be used to recommend prostate biopsy for Asian patients.[27,29,31] In PREVAIL, ENZA-treated East Asian patients had a lower PSA response rate (68.5%) than those in the overall study population (78.0%). Similar results were obtained in other studies of Asian men with mCRPC, including phase II studies of abiraterone acetate and prednisolone, in which 43% to 60% of Asian patients had a PSA response.[32,33] Similarly, in studies of cabazitaxel in Japanese[34] and Korean,[35] patients with mCRPC previously treated with chemotherapy, PSA responses were achieved by 29% and 32% of patients, respectively.
AEs commonly reported by East Asian patients treated with ENZA in PREVAIL were similar to those reported by the overall study population. AEs that were more common among East Asian patients than in the overall study population included upper respiratory tract infection, pollakiuria, fall, and decreased appetite. Among ENZA-treated East Asian patients, common AEs (fatigue and back pain) reported at a grade level ≥3 were rare (occurring in <3% of patients).
The results from East Asian patients in PREVAIL should be interpreted with caution given the small number of Japanese, Korean, and Singaporean patients enrolled in the study. The heterogeneity of ethnicities across East Asia also precludes generalizing these results for the rest of Asia. In addition, PREVAIL was not designed to investigate the impact of any differences observed in baseline demographic or disease characteristics between East Asian patients and the overall population, nor was it powered to detect treatment effects or safety differences between ENZA and placebo in East Asian patients. These limitations impact median estimates of OS and rPFS in East Asian patients as well as the ability to detect differences in AEs between East Asian patients receiving ENZA or placebo. However, our analysis showed that results in East Asian patients were consistent with those in the overall population, which was sufficiently powered to detect treatment effects and the safety of ENZA compared with placebo.
In summary, this analysis showed that results in chemotherapy-naïve East Asian men with asymptomatic or minimally symptomatic mCRPC were consistent with those observed in the overall PREVAIL study population.
Supplementary Material
Footnotes
Abbreviations: AE = adverse event, AR = androgen receptor, CI = confidence interval, CRPC = castration-resistant prostate cancer, ECOG = Eastern Cooperative Oncology Group, ENZA = enzalutamide, HR = hazard ratio, mCRPC = metastatic castration-resistant prostate cancer, OS = overall survival, PSA = prostate-specific antigen, rPFS = radiographic progression-free survival, SRE = skeletal-related event.
The PREVAIL study was sponsored by the Astellas Pharma, Inc., and Medivation, Inc.; Medivation was acquired by Pfizer Inc in September 2016.
CSK, YDC, SEL, HML, TU, JY, TF, EC, WL, BT, TMB, and GK performed the research; BT and TMB designed the research study; CSK, YDC, SEL, HML, TU, JY, TF, EC, WL, BT, TMB, and GK contributed essential reagents or tools; and all authors analyzed the data and wrote the paper.
CSK, YDC, SEL, HML, and WL have nothing to disclose. TU has received lecture fees from Astellas. JY has received research funding from Astellas. TF has received research funding from Astellas. EC's institution has received research funding from Astellas. SA is an employee of Medivation. AT is an employee of Astellas. BT has received research funding from Astellas; honoraria from Astellas and Medivation; travel support from Astellas and Medivation; fees for consultancy, board membership, and speakers bureau participation from Amgen, Bayer, Ferring, and Sanofi. TMB has received research funding from Astellas, Janssen, and Medivation; fees for consultancy from Astellas and Janssen; and honoraria for educational programming from Astellas and Medivation. GK has received honoraria and research funding from Astellas, Bayer, GlaxoSmithKline, Novartis, Ono Pharmaceutical, Pfizer, and Takeda.
SA was an employee of Medivation at the time of manuscript development.
Medical writing and editorial support funded by both sponsor companies were provided by Nathan Yardley, PhD, and Shannon Davis, BA, of Ashfield Healthcare Communications (Middletown, CT).
Supplemental Digital Content is available for this article.
References
- [1].Ito K. Prostate cancer in Asian men. Nat Rev Urol 2014;11:197–212. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J, Soerjomataram I, Erik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed January 27, 2016. [Google Scholar]
- [3].Lee DH, Jung HB, Chung MS, et al. The change of prostate cancer treatment in Korea: 5 year analysis of a single institution. Yonsei Med J 2013;54:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haas GP, Delongchamps N, Brawley OW, et al. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol 2008;15:3866–71. [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization. Global Health Observatory (GHO) data; 2016. http://www.who.int/gho/en/. Accessed January 5, 2016. [Google Scholar]
- [6].Matsuda A, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2007: a study of 21 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2013;43:328–36. [DOI] [PubMed] [Google Scholar]
- [7].Vital Statistics in Japan, tabulated by Cancer Information Service. Cancer mortality from Vital Statistics in Japan (1958–2013). National Cancer Center; 2015. http://ganjoho.jp/en/professional/statistics/table_download.html. Accessed May 4, 2015. [Google Scholar]
- [8].Jung K-W, Won Y-J, Kong H-J, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat 2015;47:127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang L, Yang BX, Zhang HT, et al. Prostate cancer: an emerging threat to the health of aging men in Asia. Asian J Androl 2011;13:574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Namiki M, Akaza H, Lee SE, et al. Prostate Cancer Working Group report. Jpn J Clin Oncol 2010;40(suppl 1):i70–5. [DOI] [PubMed] [Google Scholar]
- [11].Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer 2001;1:34–45. [DOI] [PubMed] [Google Scholar]
- [12].Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst 2001;93:1687–97. [DOI] [PubMed] [Google Scholar]
- [13].Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res 2006;12:1665–71. [DOI] [PubMed] [Google Scholar]
- [14].Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 2005;23:8253–61. [DOI] [PubMed] [Google Scholar]
- [15].Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med 2004;10:33–9. [DOI] [PubMed] [Google Scholar]
- [16].Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res 2009;15:4792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 2008;26:242–5. [DOI] [PubMed] [Google Scholar]
- [18].Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol 2011;8:12–23. [DOI] [PubMed] [Google Scholar]
- [19].National Comprehensive Cancer Network. Prostate Cancer: Asia Consensus Statement (Version 2.2013). http://www.nccn.org/professionals/physician_gls/PDF/prostate-asia.pdf. Accessed December 23, 2015. [Google Scholar]
- [20].Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol 2015;16:509–21. [DOI] [PubMed] [Google Scholar]
- [22].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- [23].Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [25].Chia SE, Lau WK, Cheng C, et al. Prostate-specific antigen levels among Chinese, Malays and Indians in Singapore from a community-based study. Asian Pac J Cancer Prev 2007;8:375–8. [PubMed] [Google Scholar]
- [26].DeAntoni EP, Crawford ED, Oesterling JE, et al. Age- and race-specific reference ranges for prostate-specific antigen from a large community-based study. Urology 1996;48:234–9. [DOI] [PubMed] [Google Scholar]
- [27].Oesterling JE, Kumamoto Y, Tsukamoto T, et al. Serum prostate-specific antigen in a community-based population of healthy Japanese men: lower values than for similarly aged White men. Br J Urol 1995;75:347–53. [DOI] [PubMed] [Google Scholar]
- [28].Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA 1993;270:860–4. [PubMed] [Google Scholar]
- [29].Chung MS, Lee SH, Lee DH, et al. Practice patterns of Korean urologists for screening and managing prostate cancer according to PSA level. Yonsei Med J 2012;53:1136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ishidoya S, Ito A, Orikasa K, et al. The outcome of prostate cancer screening in a normal Japanese population with PSA of 2-4 ng/ml and the free/total PSA under 12%. Jpn J Clin Oncol 2008;38:844–8. [DOI] [PubMed] [Google Scholar]
- [31].Choi YD, Kang DR, Nam CM, et al. Age-specific prostate-specific antigen reference ranges in Korean men. Urology 2007;70:1113–6. [DOI] [PubMed] [Google Scholar]
- [32].Matsubara N, Uemura H, Satoh T, et al. A phase 2 trial of abiraterone acetate in Japanese men with metastatic castration-resistant prostate cancer and without prior chemotherapy (JPN-201 study). Jpn J Clin Oncol 2014;44:1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kwak C, Wu TT, Lee HM, et al. Abiraterone acetate and prednisolone for metastatic castration-resistant prostate cancer failing androgen deprivation and docetaxel-based chemotherapy: a phase II bridging study in Korean and Taiwanese patients. Int J Urol 2014;21:1239–44. [DOI] [PubMed] [Google Scholar]
- [34].Nozawa M, Mukai H, Takahashi S, et al. Japanese phase I study of cabazitaxel in metastatic castration-resistant prostate cancer. Int J Clin Oncol 2015;20:1026–34. [DOI] [PubMed] [Google Scholar]
- [35].Lee JL, Park SH, Koh SJ, et al. Effectiveness and safety of cabazitaxel plus prednisolone chemotherapy for metastatic castration-resistant prostatic carcinoma: data on Korean patients obtained by the cabazitaxel compassionate-use program. Cancer Chemother Pharmacol 2014;74:1005–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


