Abstract
Overlap syndrome of chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) leads to increased morbidity and mortality. There have been no reports available on the overlap syndrome for Koreans. Our primary aim was to identify prevalence and predictors of the overlap syndrome in Koreans.
This is a cross-sectional study with a community-based sample of 1298 participants (mean age, 59.7 ± 6.7) from the cohort of Korean Genomic and Epidemiologic Study during 2013 to 2014. OSA and COPD were assessed by apnea–hypopnea index (AHI) and the ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC < 70%), respectively, based on polysomnography and spirometry measurements. Using logistic regression with adjustment for the confounders identified by univariate analysis, odds ratio (OR) was estimated with 95% confidence interval (CI) of COPD among those with OSA.
The prevalence rate of OSA was 45.8%, of which 32.8% were moderate-to-severe (AHI ≥ 15); 10.8% of those having OSA also had COPD, that is, the overlap syndrome. The prevalence of COPD remained the same as 10.8% regardless of the presence of OSA. The mean ratio of FEV1/FVC for those with COPD was 0.77, regardless of OSA. The OR increased for age (OR, 1.1; 95% CI, 1.0–1.1) and smokers (OR, 3.6; 95% CI, 2.0–6.4), but decreased for body mass index (BMI) (OR, 0.84; 95% CI, 0.8–0.9) and overweight state (OR, 0.4; 95% CI, 0.2–0.7). Risk factors of the overlap syndrome differed by OSA severity, that is, BMI in those with moderate-to-severe OSA, whereas sex (OR, 4.7; 95% CI, 2.1–10.6) and age (OR, 1.1; 95% CI, 1.0–1.1) in those with mild OSA.
In a population study from Korea, 10.8% of OSA patients had an overlap syndrome with COPD. Although BMI is a well-known risk factor of OSA, it is likely that being overweight may be protective for moderate-to-severe OSA patients from the risk of COPD (i.e., overlap syndrome).
Keywords: COPD, moderate-to-severe OSA, overlap syndrome, overweight
1. Introduction
Obstructive sleep apnea (OSA) is the most common form of sleep apnea characterized by repeated episodes of obstructed hypopnea and apnea during sleep.[1–6] Chronic obstructive pulmonary disease (COPD) is the 4th-leading cause of death in the world.[7] OSA and COPD have been known to coexist,[8] and have common pathophysiological links such as tobacco smoking.[9,10] An increased body mass index (BMI) is a well-known risk factor for OSA.[11–13] On the other hand, BMI has been reported to protect from COPD.[14] Studies of BMI in the overlap syndrome of COPD with OSA are scant.
Although COPD is preventable and treatable,[15] the burden of COPD on morbidity and mortality is projected to increase in the coming decades due to continued exposure to COPD risk factors and population aging.[16] Seventeen percent of Korean adults aged 45 or older have at least mild COPD,[17] but the prevalence of the overlap syndrome of COPD with OSA among Korean people is unknown. This study aimed to investigate the prevalence of COPD with OSA among middle-aged and elderly Koreans, and to identify its predictors across the apnea severity spectrum.
2. Methods
2.1. Study participants and demographic data
In a cross-sectional study, we investigated the cohort of Korean Genome and Epidemiology Study, a community-based prospective study initiated in 2001 for the residents of Ansan city, aged between 40 and 70 years. The cohort participants took biennial examinations of demographic characteristics, medical history, health standing, and sleep-related factors. Details of the cohort design and recruitment have been reported elsewhere.[17,18] In the present study, we analyzed polysomnography (PSG) and spirometry measurements of a total of 1298 study participants evaluated during 2013 to 2014, in addition to their regular cohort examinations on demographic characteristics (age, sex, smoking and drinking status, height, weight, and circumference of neck), sleep-related factors (sleep quality, insomnia, and snoring), and health-related factors (BMI, blood pressure, use of medication for hypertension, diabetes mellitus, and presence of diabetes, cardiovascular diseases, cancers, and asthma). The institutional review board of Korea University Ansan Hospital approved the study protocol (approval number: AS0624). Written informed consent was obtained from all participants.
2.2. PSG and OSA severity
Participants underwent a standard overnight PSG examination using a comprehensive portable device (Embletta X-100; Embla Systems, Broomfield, CO) either at home or at the sleep laboratory onsite. PSG result was scored by a sleep technician following standard criteria.[19] Apneas (complete cessation of nasal and oral airflow) and hypopneas (discernible, >30% reduction in airflow on nasal pressure trace) were scored if occurring for 10 s or longer and accompanied by a 4% or greater reduction in oxyhemoglobin saturation. The OSA severity was categorized based on the apnea–hypopnea index (AHI) as normal (<5), mild (5–14.9), moderate (15–29.9), and severe (≥30).[20]
2.3. Spirometry
Lung function was measured by spirometry (VMAX2130, Sensormedics Corporation, Yorba, CA). Global initiative for chronic obstructive lung disease guidelines was adopted to define COPD with the ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC), <70%.[15,21,22] Spirometry was performed during the clinical cohort visit, and followed by PSG measurement within 4 months.
2.4. Questionnaire and other measurements
At the visit for pulmonary function tests, participants were required to complete a health statement questionnaire on their medical and physical status. BMI (kg/m2) based on height and weight was calculated to determine overweight (BMI, 25–29.9), obese (BMI ≥ 30), or underweight (BMI < 18.5). Information on age, gender, habitual snoring, current and past status of drinking and smoking, diabetes mellitus, hypertension, insomnia, cardiovascular disease, cancers, and asthma were queried for analysis as potential confounders. Poor subjective sleep quality was determined using the Pittsburgh Sleep Quality Index (PSQI) by the total score ≥ 5.
An overnight fasted blood sample was taken to determine glucose and blood lipid levels. Diabetes mellitus was identified based on the level of fasting blood glucose of at least 126 mg/dL, or use of antidiabetic medications. Systolic and diastolic blood pressures were measured 3 times with a sphygmomanometer, and their averages >140 and 90 mm Hg, respectively, or use of antihypertensive medication were considered as the presence of hypertension.
2.5. Statistical analyses
Summary statistics are mean ± standard deviation, number and percentage, as appropriate. Categorical data were analyzed using chi-squared test. Odds ratio (OR) of overlap syndrome of COPD among OSA was estimated with 95% confidence interval (CI) using multivariate logistic regression with stepwise variable selection. The distribution of AHI was skewed, and the median was examined. BMI was examined not only as a scale variable in the unit of kg/m2, but also as a nominal variable for the general classification of overweight or obese (≥25 kg/m2), versus either normal or underweight. Analysis of variance (ANOVA) with Bonferroni correction was used to compare BMI mean value between those with and without COPD across the strata of OSA severity either mild (AHI, 5–14.9) or moderate-to-severe (AHI ≥ 15). Statistical analyses were done using SPSS (IBM Statistics, version 20), and statistical significance was determined at the .05 level.
3. Results
3.1. Characteristics of study participants
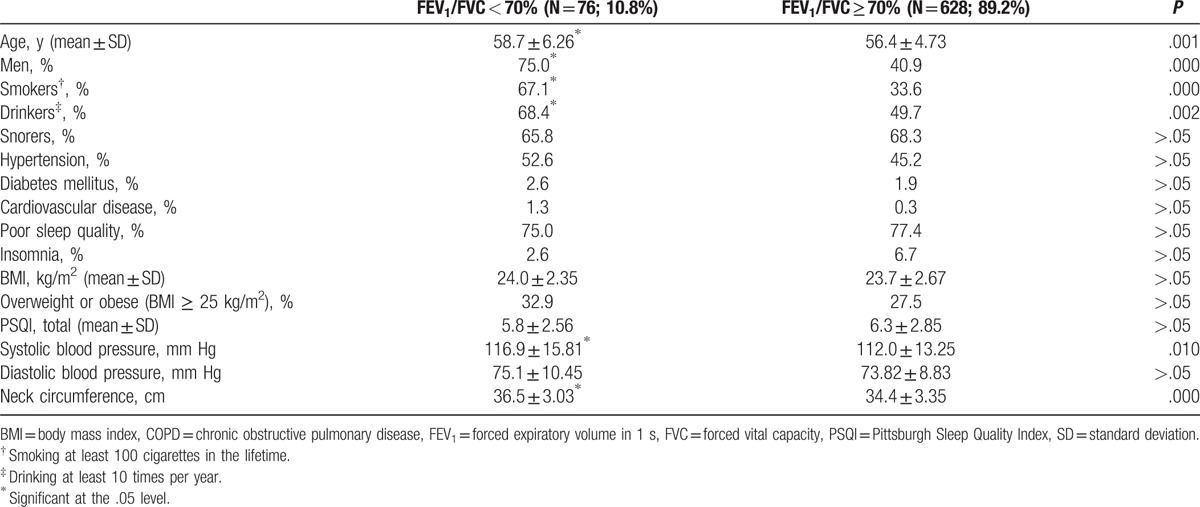
The overall mean of FEV1/FVC was 0.77 ± 0.06 for the study participants, and specifically 0.65 ± 0.05 for those having COPD and 0.79 ± 0.05 for those without COPD. The mean difference of the ratio was significant between those with and without COPD. Only 13 participants (1.0%) had the ratio, FEV1/FVC lower than 0.60 (Fig. 1). Table 1 presents the characteristics of study participants according to the evidence of COPD regardless of the presence of OSA. In Table 1, participants having COPD regardless of OSA were significantly older, more likely to be male, smoker, drinker, and likely to have a thicker neck and higher mean systolic blood pressure, but unlikely to have insomnia, compared with those not having COPD. Table 2 is similarly presented to Table 1, but without OSA patients, for those with and without COPD. The factors significant on COPD in Table 1, except insomnia, remained significant also in Table 2. There were only 2 participants found for insomnia among 76 COPD patients without OSA.
Figure 1.
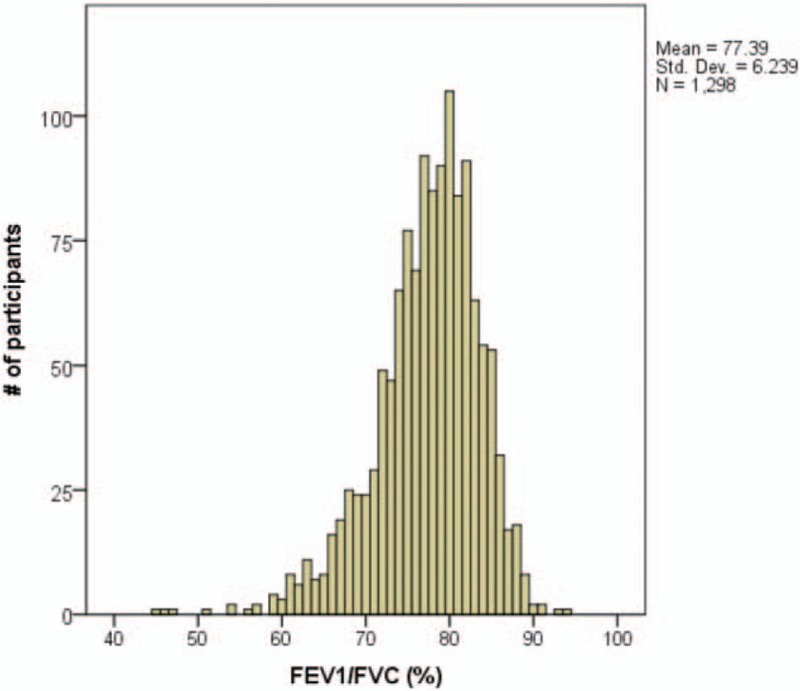
Distribution of FEV1/FVC. FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity.
Table 1.
Characteristics of the entire study population (n = 1298) by spirometry evidence of COPD.
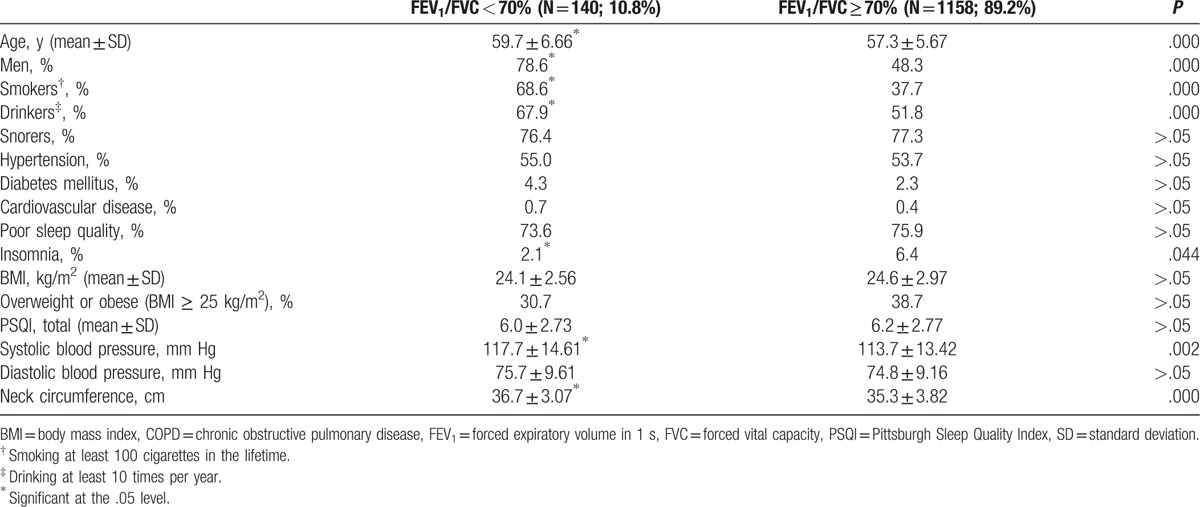
Table 2.
Characteristics of the study population by spirometry evidence of COPD, excluding those with obstructive sleep apnea.
3.2. Prevalence of overlap syndrome of COPD with OSA
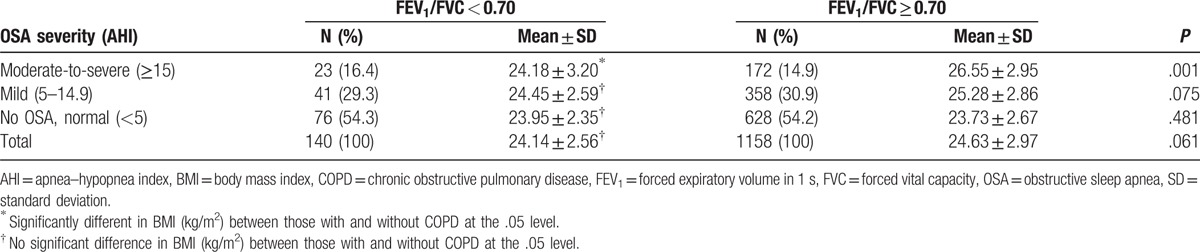
Mild OSA (AHI, 5–14.9) was common with 30.7% of the study participants (n = 1298). 10.8% (n = 64) of those having OSA (AHI ≥ 5; n = 594), specifically 10.3% of mild OSA (n = 399) and 11.8% of moderate-to-severe OSA (AHI ≥ 15; n = 195), had an overlap syndrome with COPD. The prevalence rate of COPD in the study participants (n = 1298) was 10.8%, regardless of OSA. In those without COPD (n = 1158), there were 358 (34.5%) participants with mild OSA and 172 (16.8%) participants with moderate-to-severe OSA. The distribution of the overlap syndrome of COPD with OSA is found in Table 3.
Table 3.
Comparison of BMI (kg/m2) between those with and without COPD after stratification by OSA severity.
3.3. Comparison of BMI after stratification by OSA severity
Being stratified by OSA severity, mild or moderate-to-severe, mean BMI (kg/m2) was compared between those with and without COPD. BMI mean value was lower for those with COPD than for the others without COPD (24.18 vs. 26.55 kg/m2; P = .001), and a significant difference was observed only for moderate-to-severe OSA patients. The difference in BMI mean value between those with and without COPD was not significant in the absence of OSA (Table 3).
Using ANOVA and post hoc comparison with Bonferroni correction, the mean BMI of those with COPD (FEV1/FVC < 0.70) was compared across the various severity levels of OSA, but no statistical significance was seen. On the other hand, in the subjects not having COPD (FEV1/FVC ≥ 0.70), BMI increased significantly with OSA severity (P = .001)—highest for moderate-to-severe OSA, followed by mild OSA, and the lowest for the absence of OSA. Without COPD, this result is consistent with other study findings that BMI is a significant risk factor for OSA. It was noted that our BMI range was generally lower than that seen in Caucasian, African, or Hispanic populations with OSA.[23]
3.4. Association of AHI and COPD
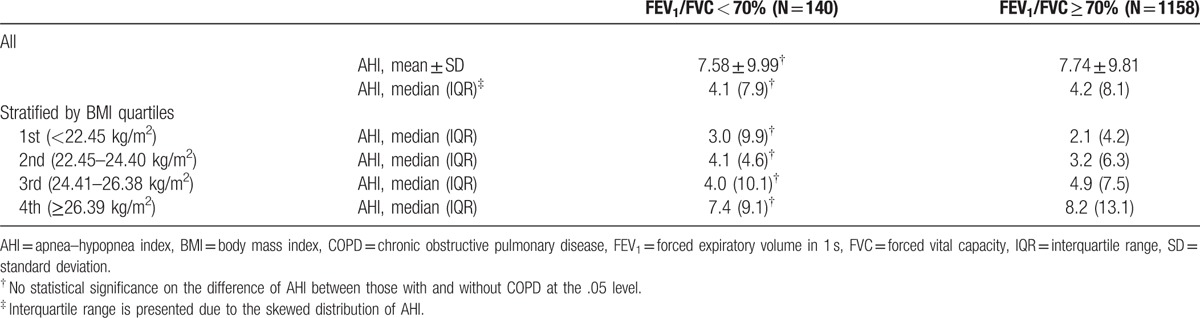
The distribution of AHI was skewed to the left (Fig. 1). The median of AHI was 4.10 per sleep hour in the presence of COPD, and 4.2 per sleep hour in the absence of COPD, but the difference was not significant. After being stratified by BMI quartiles—first, the lowest <22.45 kg/m2, second between 22.45 and 24.40 kg/m2, third between 24.41 and 26.38 kg/m2, and fourth, the highest 26.39 kg/m2 or above, AHI median values were compared between those with and without COPD, but the difference was not significant (Table 4). As BMI increased from the lowest to the highest quartile, the median of AHI increased from 3.0 to 7.4 events per sleep hour in those with COPD, and from 2.1 to 8.2 events per sleep hour in those without COPD, respectively (Table 4).
Table 4.
Apnea–hypopnea index by spirometry evidence of chronic obstructive pulmonary disease.
3.5. Adjusted OR of overlap syndrome at any level of OSA severity
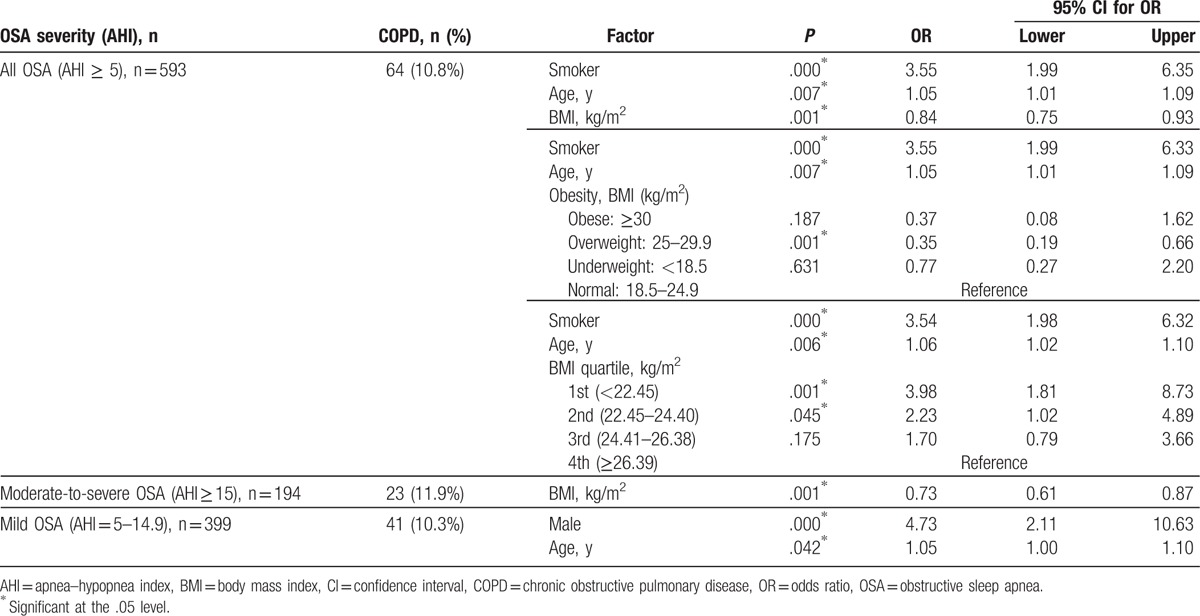
Adjusting for age, sex, BMI, current and past smoking and drinking status, hypertension, insomnia, cardiovascular disease, diabetes, and snoring, ORs of COPD with OSA at various severity was estimated with 95% CI using a logistic regression, and the factors identified significant by stepwise selection method are presented in Table 5. First, for those with OSA at any severity (i.e., AHI ≥5), the adjusted OR of overlap syndrome was 0.84 (95% CI, 0.75–0.93; P = .001) relating to an increase of BMI (kg/m2), that indicated a decreased risk of overlap syndrome with an increase of BMI. Compared to those with BMI in the normal range (18.5–24.9 kg/m2), the OR significantly reduced to 0.35 for overweight (BMI, 25–29.9 kg/m2), but for neither obese nor underweight. Compared to those in the highest quartile of BMI (≥26.39 kg/m2), the OR significantly increased to 3.98 (95% CI, 1.8–8.7; P = .001) for those in the lowest BMI quartile (<22.45 kg/m2) and 2.23 (95% CI, 1.0–4.9; P = .045) for those in the second lowest BMI quartile (BMI, 22.45–24.40 kg/m2), respectively. However, it was not significant for those in the second highest BMI quartile (24.41–26.38 kg/m2). In addition, the adjusted OR of overlap syndrome was significant for smokers (OR, 3.55; 95% CI, 2.0–6.4) and for aging in years (OR, 1.05; 95% CI, 1.0–1.1).
Table 5.
Significant predictors for overlap syndrome of COPD with OSA.
3.6. Adjusted OR of overlap syndrome by OSA severity
Particularly in those with moderate-to-severe OSA (AHI ≥ 15), OR of the overlap syndrome reduced to 0.73 (95% CI, 0.61–0.87; P = .001) as BMI increased. In those with mild OSA (AHI, 5–14.9), the OR significantly increased for males (OR = 4.73; 95% CI, 2.1–10.6; P = .000) and for aging in years (OR = 1.05; 95% CI, 1.0–1.1; P = .042), but not for the other factors including BMI (Table 5).
4. Discussion
The overlap syndrome of COPD with OSA has not been previously reported among Koreans. In a community-based Korean study, we identified that 10.8% of those with OSA (AHI ≥ 5 per sleep hour) also had COPD (FEV1/FVC < 0.70) (i.e., overlap syndrome) based on spirometry and PSG measurements. Our major findings also included the followings: AHI was not directly associated with COPD (Table 3). Mean BMI was lower for those with overlap syndrome than for those with OSA only, particularly at the moderate-to-severe OSA, without COPD (Table 3). Smoking, age, and BMI < 24.40 kg/m2 were significant risk factors for overlap syndrome of COPD among OSA at any level of severity (AHI ≥ 5). Being overweight (BMI, 25–29.9 kg/m2) but not obese was likely to be protective for OSA patients from the risk of overlap syndrome of COPD (Table 5). In those with moderate-to-severe OSA, BMI was an exclusive predictor for COPD, and the risk decreased by 27% for a unit increase of BMI (i.e., 1 kg/m2). Age, BMI, sex, smoking, drinking, hypertension, diabetes, cardiovascular disease, snoring, neck circumference, PSQI, and insomnia were all examined in logistic regression with stepwise variable selection, but only BMI was significant on the overlap syndrome of COPD among those with moderate-to-severe OSA.
Consistent with the literature, it was observed in the present study that OSA and COPD were not directly associated with each other (Table 4). In the present study, the BMI mean value of those with moderate-to-severe OSA was significantly lower for those with COPD than for those without COPD (Table 3). However, prior data have suggested that BMI was protective from COPD, but not in the coexistence with OSA. In a previous study,[24] pulmonary airway disease and OSA were considered to be associated by chance rather than by any pathophysiological linkage. It was suggested by Harish et al[25] that pulmonary function might not predict the occurrence of OSA in COPD. By contrast, it was also discussed that OSA exacerbated airway inflammation in COPD patients,[26] and a high prevalence of OSA was expected in patients with exacerbated COPD.[27] In a study by Turcani et al,[28] PSG performed in patients hospitalized for exacerbation of COPD indicated an increased prevalence of OSA compared with the general population and stable COPD patients. Our study participants were from the general population, thus exacerbated COPD patients were very unlikely. A longitudinal study should follow to suggest which one of COPD and OSA may be triggered or exacerbated by the other disease, or each of them may be exacerbated to coexist together by another independent risk factor.
In the present study, BMI was examined not only as a scale variable (kg/m2), but also as a nominal variable classified first by the general criteria of obesity, then by the BMI quartiles of the study participants, respectively (Table 5). It was particularly noted that being overweight but not obese was protective for OSA patients from COPD, and that BMI was a significant predictor of COPD but only in those with moderate-to-severe OSA (i.e., neither for COPD only without OSA nor for COPD with mild OSA). While an underlying mechanism of BMI protection for OSA patients from COPD is unknown, complex adipokine effects[29] or hypoxic preconditioning[30] may be plausible mechanisms.
Asthma has also been discussed frequently as an overlap syndrome with COPD,[31,32] but it was not analyzed in the present study, because only 3 participants (1 male and 2 females) were with asthma, and one of the females had FEV1/FVC lower than 70% (i.e., 69%) with moderate OSA (AHI, 15–29.9).
OSA prevalence in our study was 45.8%. In an earlier study that was reported in 2004, based on a younger cohort by about 10 years than ours, OSA prevalence rate was estimated to be 27% for the general population of Korea.[33] Although aging must account mainly for the increased prevalence, there may also be other possible contributors such as an overall increase of BMI or the increased number of overweight subjects, particularly in aged people and their related health complications. OSA prevalence may be higher among US Hispanic as well as in African American and other Asian populations.[34]
The strength of our study findings is that the evidence of OSA and COPD was based on PSG and spirometry results performed in a large cohort for the general population. Studying a large clinic patient sample may be very likely to give a different result, but selection bias effects come into play. Nevertheless, in the present study, we observed a significant association of COPD particularly with BMI among those with moderate-to-severe OSA. COPD and OSA have synergistic detrimental effects, and their comorbid association leads to compromised gas exchange (hypoxia and hypercapnia) and higher rates of morbidity and death.[35] As noted by Steveling et al,[36] standard clinical predictors cannot be used for patients with overlap syndrome, thus a high index of suspicion should be identified. Khatri and Ioachimescu[35] pointed out that considering the effect on health consequence and the quality of life, proper diagnosis, and appropriately tailored management in those with overlap syndrome is of high importance.
4.1. Potential limitations of the study
Excessive daytime sleepiness (ESS) was not examined in our study. However, Sanders et al[24] examined ESS together with OSA for overlap syndrome, but found no association between AHI and overlap syndrome. We also examined PSQI for its association with COPD, but did not observe any significance (Table 1). The PSQI questionnaire has not been validated yet in such a diseased population. In the present study, moderate OSA was not separately examined from severe OSA due to the very limited number (i.e., only 2) of study participants having both severe OSA and COPD together. A clinic-based sample is likely to have this extreme end of the overlap far more frequently. Although the study findings suggest that BMI is protective from COPD, particularly for those with moderate-to-severe OSA, this only raises a question for hypothesis generation. Finally, various populations of race and/or ethnicity may need to be investigated due to various distribution of BMI or obesity relating to the overlap syndrome.
5. Conclusion
In summary, this is the first report on the prevalence of overlap syndrome of COPD and OSA based on a community-based cohort study for Koreans. There was no association between COPD and AHI, whereas the risk of overlap syndrome of COPD among those with OSA increased for smoking and aging, but decreased for being overweight. It is likely that the overweight state may be protective for moderate-to-severe OSA patients from the development of overlap syndrome with COPD.
Acknowledgment
The authors thank research technicians in the Institute of Human Genomic Study and Ansan Hospital of Korea University for data collection.
Footnotes
Abbreviations: AHI = apnea–hypopnea index, BMI = body mass index, CI = confidence interval, COPD = chronic obstructive pulmonary disease, ESS = excessive daytime sleepiness, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, OR = odds ratio, OSA = obstructive sleep apnea, PSG = polysomnography, PSQI = Pittsburgh Sleep Quality Index.
K-MC had full access to the cohort data of the study and takes responsibility for the accuracy of the data analysis. CS obtained the funding of this study and takes responsibility for the accuracy of cohort data. K-MC and CS: study concept and design. K-MC: statistical analysis and drafting of the manuscript. All authors: acquisition of data, final approval of the manuscript submitted for publication, and accountability for all aspects of the study. K-MC, CS, and RJT: critical revision for intellectual content.
This study was supported by the grants from the Korea Center for Disease Control and Prevention under the Korea Ministry of Health and Welfare (2013-E71005-00 and 2014-E71003-00).
The authors have no conflicts of interest to disclose.
References
- [1].Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997;20:705–6. [DOI] [PubMed] [Google Scholar]
- [3].Olson LG, Hensley MJ, King MT, et al. A community study of snoring and sleep disordered breathing: health outcomes. Am J Respir Crit Care Med 1995;152:717–20. [DOI] [PubMed] [Google Scholar]
- [4].Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epid 2013;177:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fleetham J, Ayas N, Bradley D, et al. Canadian Thoracic Society guidelines: diagnosis and treatment of sleep disordered breathing in adults. Can Respir J 2006;13:387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kabir A, Ifteqar S, Bhat A. Obstructive sleep apnea in adults. Hosp Pract 1995;41:57–65. [DOI] [PubMed] [Google Scholar]
- [7].World Health Organization. World Health Report. 2000;Geneva: World Health Organization, Available from: http://www.who.int/whr/2000/en/statistics.htm. Accessed May 1, 2016. [Google Scholar]
- [8].Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985;6:651–61. [PubMed] [Google Scholar]
- [9].Wetter DV, Young TB, Didwell TR, et al. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med 1994;154:2219–24. [PubMed] [Google Scholar]
- [10].Davis RM, Novotny TE. The epidemiology of cigarette smoking and its impact on chronic obstructive pulmonary disease. Am Rev Respir Dis 1989;140:S82–4. [DOI] [PubMed] [Google Scholar]
- [11].Lopez PP, Stefan B, Schulman CI, et al. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg 2008;74:834–8. [PubMed] [Google Scholar]
- [12].Wall H, Smith C, Hubbard R. Body mass index and obstructive sleep apnoea in the UK: a cross-sectional study of the over-50 s. Prim Care Respir J 2012;21:371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Poulain M, Doucet M, Major GC, et al. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ 2006;174:1293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cao C, Wang R, Wang J, et al. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS ONE 2012;7:e43892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Global Initiative for Chronic Obstructive Lung Disease (GOLD): global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2014 update. [Google Scholar]
- [16].Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397–412. [DOI] [PubMed] [Google Scholar]
- [17].Kim H, Yun CH, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep 2013;36:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kwon AM, Baik I, Thomas RJ, et al. The association between leukocyte telomere lengths and sleep instability based on cardiopulmonary coupling analysis. Sleep Breath 2015;19:963–8. [DOI] [PubMed] [Google Scholar]
- [19].Iber C, Ancoli-Israel S, Chesson A, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st edWestchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- [20].American Academy of Sleep Medicine Task Force (AASMTF). Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999;22:667–89. [PubMed] [Google Scholar]
- [21].Knudson RJ, Lebowitz MD, Holberg CJ, et al. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 1983;127:725–34. [DOI] [PubMed] [Google Scholar]
- [22].American Thoracic Society (ATS). Standardization of spirometry. 1994 update. Am J Respir Crit Care Med 1995;152:1107–36. [DOI] [PubMed] [Google Scholar]
- [23].Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015;38:877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med 2003;167:7–14. [DOI] [PubMed] [Google Scholar]
- [25].Harish M, Shine Shukoor R, Dharmic S. Comparative study of sleep apnoea in chronic bronchitis and emphysema. World J Med Sci 2014;10:50–5. [Google Scholar]
- [26].Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med 2015;16:1123–30. [DOI] [PubMed] [Google Scholar]
- [27].Soler X, Gaio E, Powell FL, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc 2015;12:1219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Turcani P, Skrickova J, Pavlik T, et al. The prevalence of obstructive sleep apnea in patients hospitalized for COPD exacerbation. Biomed Papers 2015;159:422–8. [DOI] [PubMed] [Google Scholar]
- [29].Tkacova R, Ukropec J, Skyba P, et al. Effects of hypoxia on adipose tissue expression of NFkappaB, ikappaBalpha, IKKgamma and IKAP in patients with chronic obstructive pulmonary disease. Cell Biochem Biophys 2013;66:7–12. [DOI] [PubMed] [Google Scholar]
- [30].Burtscher M, Gatterer H, Szubski C, et al. Effects of interval hypoxia on exercise tolerance: special focus on patients with CAD or COPD. Sleep Breathing 2010;14:209–20. [DOI] [PubMed] [Google Scholar]
- [31].Global Initiative for Asthma: Diagnosis of Diseases of Chronic Airflow Limitation: Asthma COPD and Asthma—COPD Overlap Syndrome (ACOS); 2015. [Google Scholar]
- [32].Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med 2015;373:12. [DOI] [PubMed] [Google Scholar]
- [33].Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med 2004;170:1108–13. [DOI] [PubMed] [Google Scholar]
- [34].Kripke DF, Ancoli-Israel S, Klauber MR, et al. Prevalence of sleep-disordered breathing in ages 40–64 years: a population-based survey. Sleep 1997;20:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Khatri SB, Ioachimescu OC. The intersection of obstructive lung disease and sleep apnea. Cleve Clin J Med 2016;83:127–40. [DOI] [PubMed] [Google Scholar]
- [36].Steveling EH, Clarenbach CF, Miedinger D, et al. Predictors of the overlap syndrome and its association with comorbidities in patients with chronic obstructive pulmonary disease. Respiration 2014;88:451–7. [DOI] [PubMed] [Google Scholar]


