Abstract
The myeloperoxidase (MPO) gene 463G/A and 129G/A polymorphisms have been reported to be associated with coronary artery disease (CAD), but the results remain inconclusive. This meta-analysis was designed to clarify these controversies.
PubMed, EMBASE, and the Cochrane Library were used to retrieve the relevant literature up to March 2015 according to keywords. A total of 8 case–control studies, including 3491 cases and 7293 controls, were included in this meta-analysis. Summary odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were calculated.
There was strong evidence of an association between the MPO 463G/A polymorphism and CAD. The data revealed that only the dominant model was associated with CAD (dominant model: OR = 0.872, 95% CI = 0.77–0.99). Regarding the 129G/A gene polymorphism, the pooled OR for the genotype AA + AG versus GG was 0.906 (95% CI = 0.74–1.10).
This meta-analysis suggested an association between the MPO 463G/A polymorphism and the risk of CAD, but there is no significant association between the MPO 129G/A gene polymorphism and CAD risk.
Keywords: coronary artery disease, meta-analysis, myeloperoxidase, polymorphism
1. Introduction
Coronary artery disease (CAD) is a multifactorial disease that is pathologically based on atherosclerosis and thrombogenesis in the artery. CAD is influenced by genetic polymorphisms and environmental factors, and genetic differences in molecules have been linked to the susceptibility to this disease.[1]
Evidence has accumulated that indicates that an unbalanced production of oxidized molecules within the vascular wall is one of the mechanisms that is involved in the onset and progression of atherosclerosis and CAD.[2] Myeloperoxidase (MPO) is a member of the heme peroxidase super family of neutrophils and monocytes and has been proposed to be a novel risk indicator for future coronary events in healthy people and a prognostic marker in CAD patients.[3–5] Elevated serum MPO levels have been reported to be associated with a higher risk for CAD.[6] A number of polymorphisms have been identified in the promoter of the MPO gene and the coding regions.[7] Two single-nucleotide polymorphisms (SNPs) in the MPO gene, that is, 463G/A and 129G/A, have been demonstrated to the regulate MPO protein level and activity.[8] The A-allele at the MPO gene position 129 and homozygosity for a G at position 463 result in lower MPO concentrations.[9] The A-allele at the MPO gene position 129 and homozygosity for a G at position 463 result in lower MPO concentrations.[10,11] Therefore, we performed a comprehensive meta-analysis with the aim of determining whether there are significant associations of the MPO 463G/A and 129G/A polymorphisms with the susceptibility to CAD.
2. Methods
2.1. Search strategy
We searched the PubMed, EMBASE, and Cochrane Library platforms up to December 2015 for observational studies that evaluated the associations of the 129G/A and 463G/A polymorphisms of the MPO gene with the risk of CAD. The following terms were used for the search: “myeloperoxidase” or “MPO,” “polymorphism” or “variation” or “mutation” combined with “coronary artery disease” or “coronary heart disease” or “ischemic heart disease” or “myocardial infarction” or “CAD” or “CHD (coronary heart disease)”. The search results were limited to articles published in the English language. Two investigators (YW and XZ) screened each of the titles, abstracts, and full texts to independently determine inclusion, and disagreements were followed by discussion until a consensus was reached. All analyses were based on previous published studies, thus ethical approval and patient consent were not required.
2.2. Study selection
The inclusion criteria were as follows: evaluation of the association of the MPO 463G/A and 129G/A polymorphisms with CAD risk, case–control studies, the MPO 129G/A or 463G/A frequencies were provided, and the CAD diagnoses were appropriate (i.e., angiographically confirmed or elevations of cardiac enzymes), changes in electrocardiographic and clinical symptoms according to the WHO criteria were present. Studies were excluded as follows: duplicate publications (the studies with smaller data sets were excluded), review articles, laboratory or animal studies, and other studies clearly unrelated to the MPO 129G/A and 463G/A polymorphisms or CAD.
2.3. Data extraction
Two reviewers (YW and XZ) independently performed the data extraction from all of the eligible publications using a redesigned form. The following data were collected from each study: the first author's name; the year of publication; the study population (ethnicity, sex, and age); the percentage of males among the cases and controls; the mean ages and number of cases and controls; and the numbers of individuals with the GG, AG, and AA genotypes in both cases. The results were compared, and disagreements were settled by consensus.
2.4. Assessment of study quality
The qualities of the included studies were assessed using the Newcastle–Ottawa Quality Assessment Scale for case–control studies. A study can be granted a maximum of 1 star for each numbered item within the selection and exposure categories. A maximum of 2 stars can be awarded for comparability. The total quality scores ranged from 0 to 9 stars.
2.5. Statistical analysis
The statistical analyses were performed with the STATA software version 11.0 (STATA Corp., College Station, TX). To measure the strengths of the genetic associations of the MPO 129G/A and 463G/A polymorphism with the risk of CAD, the pooled odds ratios (ORs) and 95% confidence interval (CI) were calculated. A χ2 test was used to check whether the frequencies of the genotypes deviated from the Hardy–Weinberg equilibrium (HWE). The heterogeneity among studies was analyzed using the Q statistic test. P < .10 was considered statistically significant.[12] If significant heterogeneity was observed, the DerSimonian–Laird method was used to evaluate the pooled ORs (95% CIs) in random effects model. If no significant heterogeneity was observed, the Mantel–Haenszel test was used to calculate the pooled ORs (95% CIs) in fixed effects models.[13] To explore the effect of heterogeneity among the studies, subgroup analyses were performed. Sensitivity analysis was performed by omitting each study to assess the quality and consistency of the results. We also performed Egger test to examine publication bias, and P < .05 was considered representative of statistical significance.[14]
3. Results
3.1. Study characteristics
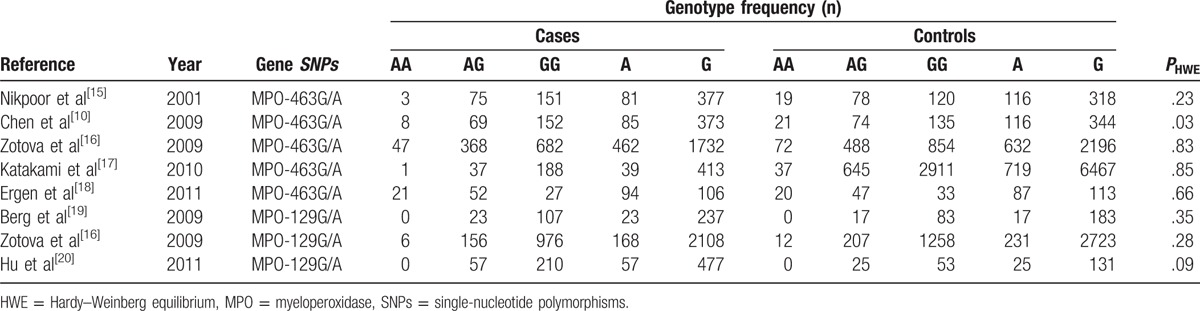
The process of study selection and exclusion with specific reasons is illustrated in Fig. 1. Two SNPs in the MPO gene, that is, MPO 129G/A and MPO 463G/A, were considered. Five studies[10,15–18] including 1881 cases and 5554 controls investigated the association between the MPO 463G/A genotype and the CAD risk. Three studies[16,19,20] comprising 1610 patients and 1739 controls addressed the association between the MPO 129G/A polymorphism and CAD and were included in this meta-analysis. For 1 study[16] that involved 2 stage groups, each group was analyzed separately. The quality scores of all of the included studies were greater than 5 stars. The main characteristics of all of the case–control studies are listed in Table 1. In addition, all of the studies used blood samples for deoxyribonucleic acid (DNA) extraction, and the polymerase chain reaction, TaqMan (Applied Biosystems, Foster City, CA), or DNA sequencing methods were used for the genotyping. The controls were mainly matched for sex and age. All of the studies were included in analysis of the association of the MPO 463G/A polymorphism and CAD, although 1 study exhibited evidence of a departure from the HWE. The distributions of the genotypes of the MPO 129G/A polymorphism in all of the control groups were found to be consistent with the HWE. The genotype frequencies of the studies included in the meta-analysis are listed in Table 2.
Figure 1.
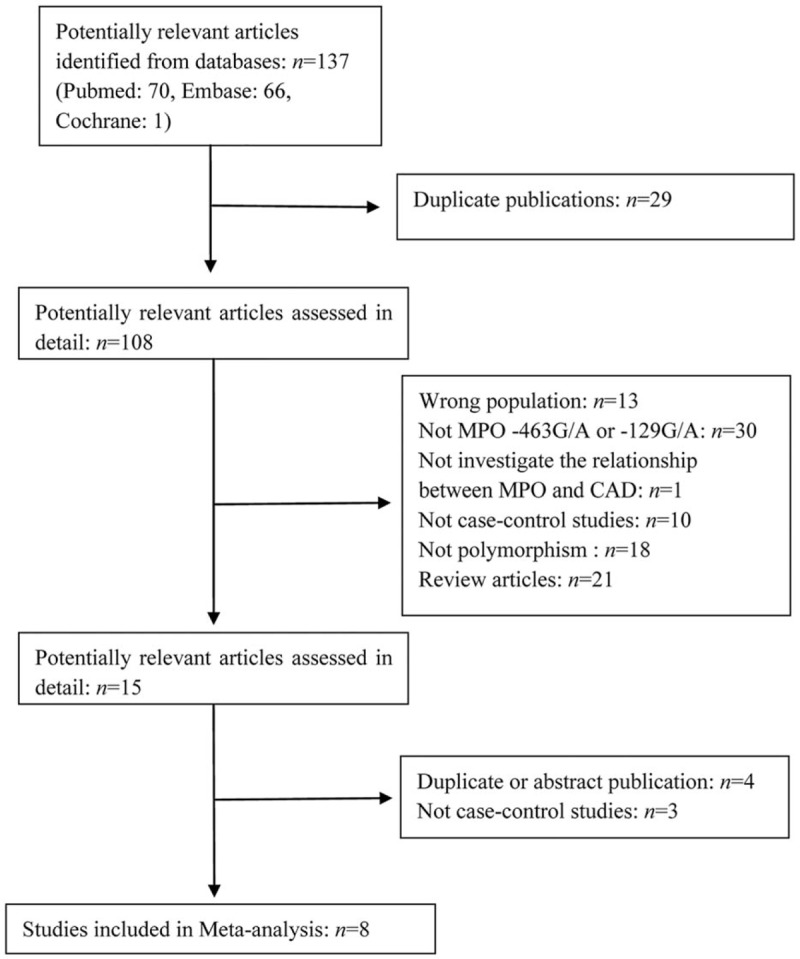
Flow diagram of study selection process.
Table 1.
Characteristics of the studies included in the meta-analysis.
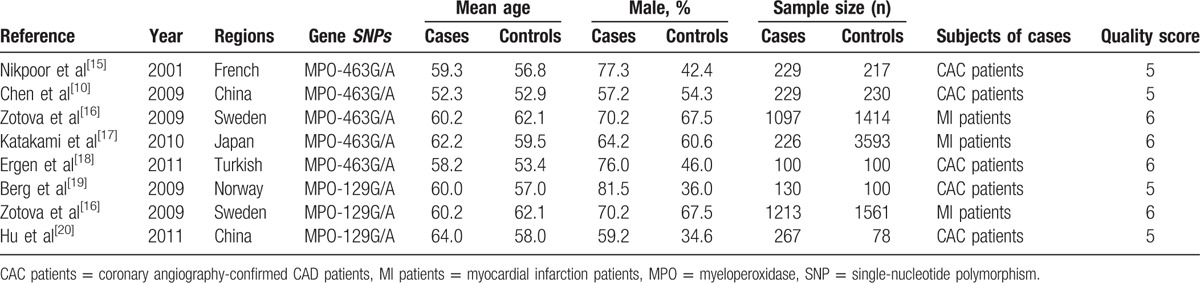
Table 2.
Genotype frequencies in the studies included in the meta-analysis.
3.2. Meta-analysis results
3.2.1. Association between the MPO 463G/A polymorphism and CAD risk
Table 3 lists the main results of the meta-analysis of the association between the MPO 463G/A polymorphism and CAD risk. When all studies were pooled in the meta-analysis, only the dominant model was associated with CAD, and the data indicated that the subjects with the AA + AG genotypes exhibited a 12.8% decrease in the risk of CAD relative to the subjects with the GG genotype (dominant model: OR = 0.872, 95% CI = 0.77–0.99). However, there was noteworthy heterogeneity between the studies. Hence, we then performed subgroup analysis. Through stratified analyses, the heterogeneities of the subgroups were notably reduced. In the subgroup analysis of ethnicity, heterogeneity almost completely disappeared in Asian populations, and we discovered that the 463G/A polymorphism was significantly associated with a decreased CAD risk in Asians under the AA versus GG model (OR = 0.353, 95% CI = 0.16–0.78) and the recessive model (OR = 0.372, 95% CI = 0.17–0.81). Interestingly, a conflicting association was found in the A allele versus G allele model (OR = 1.326, 95% CI = 1.05–1.67). In addition, no significant associations were detected between MPO 463G/A and the risk of CAD in Caucasians in all the genetic model (Table 3). In the subgroup analysis of subjects, different subjects were categorized as coronary angiography-confirmed CAD patients (CAC group) and myocardial infarction patients (MI group), shown in Table 1. We discovered that the 463G/A polymorphism was significantly associated with a decreased CAD risk in the CAC group under the dominant model (OR = 0.76, 95% CI = 0.59–0.97; Fig. 2C), but no associations were found in the MI group (Fig. 3).
Table 3.
Summary ORs and 95% CIs of the association of the MPO 463G/A polymorphism with CAD risk.
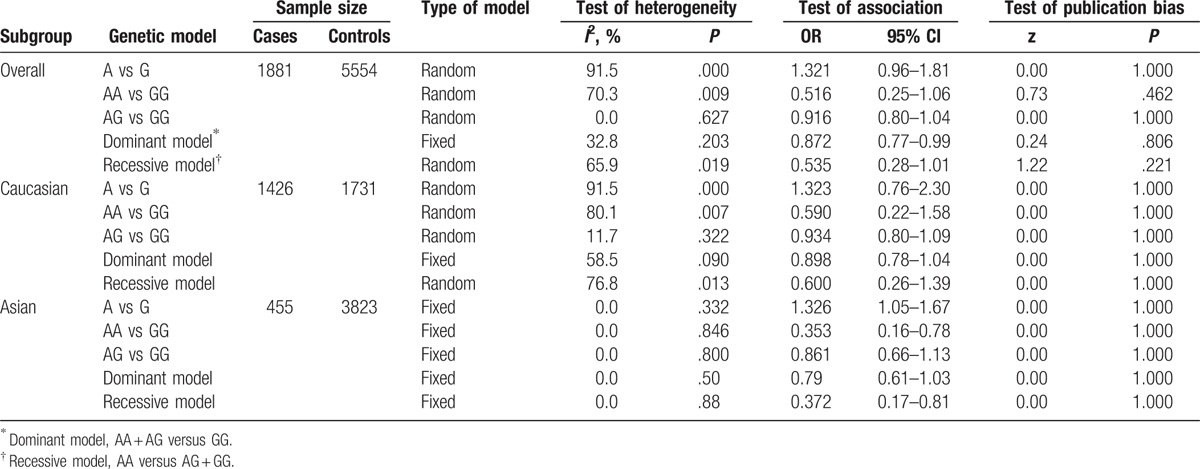
Figure 2.
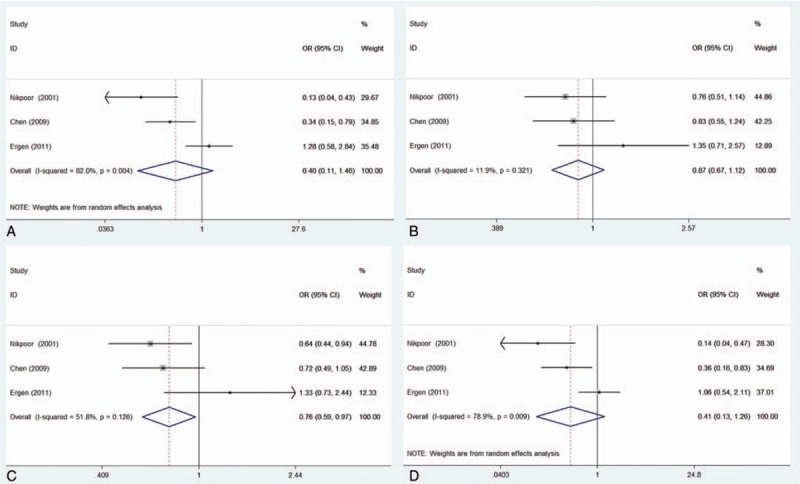
Forest plot of the association of the myeloperoxidase 463G/A polymorphism with coronary artery disease (CAD) risk stratified by subjects in the CAC group. (A) AA versus GG; (B) AG versus GG; (C) dominant model, AA + AG versus GG; (D) recessive model, AA versus AG + GG. CAC group = group of coronary angiography-confirmed CAD patients.
Figure 3.
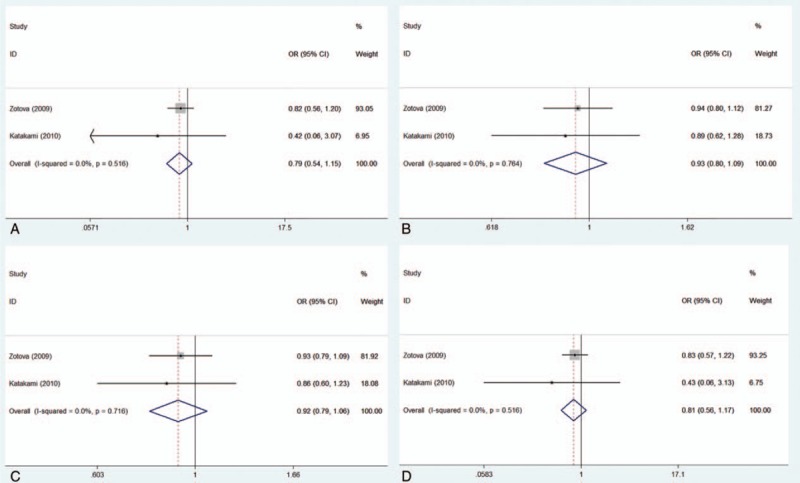
Forest plot of the association of the myeloperoxidase 463G/A polymorphism with the coronary artery disease risk stratified by subjects in the myocardial infarction group. (A) AA versus GG; (B) AG versus GG; (C) dominant model, AA + AG versus GG; (D) recessive model, AA versus AG + GG. MI group = group of myocardial infarction patients.
3.2.2. Association between the MPO 129G/A polymorphism and CAD risk
No comparison between alleles or other genotypes were mentioned, so we only illustrated the relationship between MPO 129G/A AA + AG/GG and the risk of CAD. The heterogeneity was not obvious (I2 = 31.5%, P = .232); thus, a fixed-effects model was applied. Nevertheless, the MPO 129G/A genotype polymorphism did not present any statistical association with the risk of CAD (AA + AG vs GG: OR: 0.906, 95% CI = 0.74–1.10; Table 4).
Table 4.
Summary ORs and 95% CIs of the association of the MPO 129G/A polymorphism with CAD risk.

3.3. Publication bias
Eggers’ tests were performed to assess the publication biases of the included studies. The results suggest no evidence of publication biases among the studies (P > .05) in any of the genetic models (Tables 3 and 4).
3.4. Sensitivity analysis
Sensitivity analyses were conducted to evaluate the component of study quality. First, we performed a sensitivity analysis based on the sample sizes. The study with the largest sample[16] was removed to reflect the influence of this study on the result. The pooled ORs in all models were not significantly altered, which indicated the reliability of our results (Fig. 4).
Figure 4.
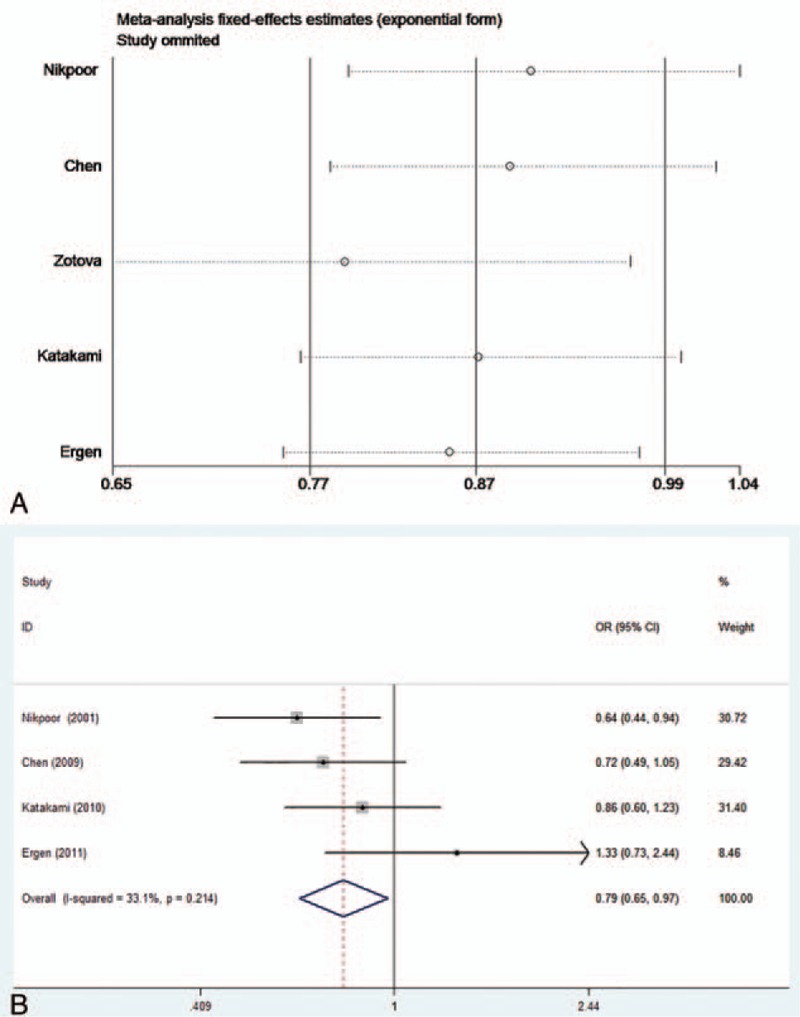
(A) Sensitivity analysis of the association of the coronary artery disease (CAD) risk with the myeloperoxidase (MPO) 463G/A polymorphism under the dominant model. (B) Forest plot of the association of the MPO 463G/A polymorphism with the CAD risk stratified under the dominant model after the study with the largest sample was removed.
4. Discussion
CAD is a multifactorial disease which is influenced by genetic polymorphisms and environmental factors, and genetic polymorphisms have been linked to the susceptibility to this disease.[1] In recent years, genome-wide association studies have identified SNPs are associated with some complex diseases.[21] MPO, a member of the heme peroxidase super family of neutrophils and monocytes, is strongly associated with CAD. Two SNPs in the MPO gene, that is, 463G/A and 129G/A, have been demonstrated to the regulate MPO protein level and activity, but the associations between MPO polymorphisms and CAD risk are conflicting. Therefore, we performed a meta-analysis to combine a substantial number of cases and controls for a better understanding of the associations of the MPO 463G/A and 129G/A polymorphisms with the risk of CAD.
A growing body of evidence demonstrates that the MPO might play a crucial role in the pathogenesis of CAD. Oxidative stress and inflammation play important roles in the pathogenesis of CAD, and MPO is an important oxidant generating enzyme that has been found to alter the incidence of CAD via its abilities to oxidize low-density lipoproteins[22] and consume nitric oxide.[23] MPO 463G/A and 129G/A have been reported to regulate the MPO protein level and activity; thus, it is rational to hypothesize that polymorphisms in this gene may be related to the CAD risk. Piedrafita et al found that the A allele at the 129 locus of the MPO gene can destroy the MPO gene's specificity protein 1 (SP1)-binding site. SP1 is a strong transcription factor; thus, the A allele is associated with lower MPO expression.[9] Thus, we thought that MPO polymorphisms might be involved in CAD via their influences on the activities of SP1-binding site.
In our study, the incidences of gene polymorphisms varied substantially between different racial populations. Therefore, we performed an analysis stratified by ethnicity. Interestingly, different associations of the MPO 463G/A and 129G/A polymorphisms were found. Regarding the 463G/A polymorphism, the GG genotype was associated with an increased CAD risk in Asians but was not associated with CAD in Caucasians. Regarding the 129G/A polymorphism, associations between the GG genotype and the risk of CAD were not found in either Asians or Caucasians. The reasons underlying these results are unclear, but some factors may account for these results. First, different genetic backgrounds and environmental factors may account for the differences between ethnic groups. Second, the sample size is still relatively small and may not provide sufficient power to estimate the association between MPO gene polymorphism and CAD risk, especially the MPO 129A/G. In addition, this meta-analysis was based on only published studies in English, publication bias, selection bias, and different matching criteria between studies may play roles.
Different CAD subtypes are associated with different pathophysiological mechanisms. So we conducted stratified analysis by subjects. In the CAC group, studies designed by Chen et al[10] and Ergen et al,[18] coronary angiography revealed 50% stenosis in at least 1 major coronary vessel, and the research of Nikpoor et al[15] took the presence of one or more coronary artery stenoses obstructing the lumen diameter by 30% or more to define an individual as a case. Other researchers have focused on MI patients. The heterogeneity was decreased after the subgroup analysis, although there was no significant association between the MPO 463A/G gene polymorphism and CAD risk. Regrettably, we did not have adequate data or the relevant studies to explain this nonconformity, but it confirmed our speculation that the subjects could have substantially influenced the initial heterogeneity.
Furthermore, several potential limitations of the present meta-analysis should be considered. First, this meta-analysis was based only on papers published in English. Although an Egger test did not find evidence for a publication bias in the present study, publication bias may have occurred. Second, our results are based on unadjusted estimates, whereas more precise analyses could have been conducted if all of the individual raw data were available and would allow for adjustments for other covariants, including cigarette consumption, alcohol drinking, family history, and lifestyle. Third, CAD is a multifactorial disease, which interactions between many factors including age, sex, and so on. So a single gene factor cannot impact CAD susceptibility alone, but data for young adults were limited, and data were not available to perform subgroup analyses based on non-Caucasian ethnicity or female sex. Fourth, our populations included only Asians and Caucasians, and other ethnic populations, such as Africans, should be involved in future studies. Moreover, the sample size was still relatively small. In particular, there were only 2 studies with Asian populations, and the exploration of moderator variables was limited by the low number of studies. The number of studies and participants included in this meta-analysis for MPO 129G/A study is small and may not have provided sufficient power to estimate the associations between the MPO 129G/A gene polymorphisms and CAD risk.
Despite these limitations, the meta-analysis still has some merits, and the MPO 436A variant genotype may be a risk factor in the etiology of CAD, especially in Asians. Studies involving larger sample sizes should be conducted to further understand the relationship of the MPO 129G/A gene polymorphism with CAD risk. Additional well-designed studies, including Caucasians as well as other ethnic populations, are needed to further elucidate the association of the MPO 463G/A polymorphism with CAD risk.
Footnotes
Abbreviations: A = adenine, CAC = coronary angiography-confirmed CAD, CAD = coronary artery disease, CI = confidence interval, G = guanine, HWE = Hardy–Weinberg equilibrium, MI = myocardial infarction, MPO = myeloperoxidase, OR = odds ratio, SNP = single-nucleotide polymorphism, SP1 = specificity protein 1.
XZ and XC have contributed equally to the article.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Humphries SE, Morgan L. Genetic risk factors for stroke and carotid atherosclerosis: insights into pathophysiology from candidate gene approaches. Lancet Neurol 2004;3:227–36. [DOI] [PubMed] [Google Scholar]
- [2].Griendling KK, Sorescu D, Lassègue B, et al. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 2000;20:2175–83. [DOI] [PubMed] [Google Scholar]
- [3].Meuwese MC, Stroes ES, Hazen SL, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol 2007;50:159–65. [DOI] [PubMed] [Google Scholar]
- [4].Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005;25:1102–11. [DOI] [PubMed] [Google Scholar]
- [5].Zhang R, Brennan M-L, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 2001;286:2136–42. [DOI] [PubMed] [Google Scholar]
- [6].Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 2003;108:1440–5. [DOI] [PubMed] [Google Scholar]
- [7].Chevrier I, Tregouet D-A, Massonnet-Castel S, et al. Myeloperoxidase genetic polymorphisms modulate human neutrophil enzyme activity: genetic determinants for atherosclerosis? Atherosclerosis 2006;188:150–4. [DOI] [PubMed] [Google Scholar]
- [8].Rutgers A, Heeringa P, Giesen JE, et al. Neutrophil myeloperoxidase activity and the influence of two single-nucleotide promoter polymorphisms. Br J Haematol 2003;123:536–8. [DOI] [PubMed] [Google Scholar]
- [9].Piedrafita FJ, Molander RB, Vansant G, et al. An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J Biol Chem 1996;271:14412–20. [DOI] [PubMed] [Google Scholar]
- [10].Chen Z, Yin Q, Ma G, et al. Myeloperoxidase gene-463G > A polymorphism and premature coronary artery disease. Genet Mol Biol 2009;32:260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lusis AJ, Mar R, Pajukanta P. Genetics of atherosclerosis. Annu Rev Genomics Hum Genet 2004;5:189–218. [DOI] [PubMed] [Google Scholar]
- [12].Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [13].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [14].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nikpoor B, Turecki G, Fournier C, et al. A functional myeloperoxidase polymorphic variant is associated with coronary artery disease in French-Canadians. Am Heart J 2001;142:336–9. [DOI] [PubMed] [Google Scholar]
- [16].Zotova E, Lyrenäs L, de Faire U, et al. The myeloperoxidase gene and its influence on myocardial infarction in a Swedish population: protective role of the −129A allele in women. Coron Artery Dis 2009;20:322–6. [DOI] [PubMed] [Google Scholar]
- [17].Katakami N, Kaneto H, Matsuoka TA, et al. Accumulation of gene polymorphisms related to oxidative stress is associated with myocardial infarction in Japanese type 2 diabetic patients. Atherosclerosis 2010;212:534–8. [DOI] [PubMed] [Google Scholar]
- [18].Ergen A, İsbir S, Timirci Ö, et al. Effects of myeloperoxidase −463 G/A gene polymorphism and plasma levels on coronary artery disease. Mol Biol Rep 2011;38:887–91. [DOI] [PubMed] [Google Scholar]
- [19].Berg K, Madsen HO, Garred P, et al. The additive contribution from inflammatory genetic markers on the severity of cardiovascular disease. Scand J Immunol 2009;69:36–42. [DOI] [PubMed] [Google Scholar]
- [20].Hu J, Xu J, Zhou X, et al. Correlation between MPO 129 A/G polymorphism and severity of coronary artery disease. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2011;27:306–10. [PubMed] [Google Scholar]
- [21].Arcidiacono T, Terranegra A, Biasion R, et al. Calcium kidney stones. Diagnostic and preventive prospects. G Ital Nefrol 2006;24:535–46. [PubMed] [Google Scholar]
- [22].Podrez EA, Schmitt D, Hoff HF, et al. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest 1999;103:1547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem 2000;275:37524–32. [DOI] [PubMed] [Google Scholar]


