Abstract
Rationale:
Nivolumab (Nivo) is an immune checkpoint inhibitor that has been used to treat advanced melanoma, nonsmall cell lung carcinoma, and renal cell carcinoma since 2015. Nivo is associated with several side effects, including hepatitis, pneumonitis, acute renal failure, endocrine disorder, and other immune-related adverse events. Here, we describe the case of a 65-year-old man with squamous cell lung carcinoma who developed myasthenia gravis (MG) after a third Nivo infusion.
Patient concerns:
A 65-year-old man with advanced squamous cell lung carcinoma developed ptosis, diplopia, drop head, and general weakness 5 days after a third Nivo infusion.
Diagnoses, interventions, and outcomes:
We diagnosed him with Nivo-related MG and myositis based on clinical symptoms, elevation of muscle enzymes, negativity for autoantibodies and exclusion of other diagnoses. Steroid treatment with methylprednisolone 1 mg/kg/d and pyridostigmine 60 mg twice a day was administered beginning at admission; however, the patient's condition progressively worsened, despite treatment. Respiratory failure developed 2 weeks after admission, and his family declined the use of a mechanical ventilator. The patient died on day 27 after the third Nivo infusion.
Lessons:
Nivo-related MG should be highly suspected in patients who develop ptosis, diplopia, and general weakness. The corresponding treatments include discontinuation of Nivo and steroid treatment with plasmapheresis. The disease course may be rapid and fatal. This report stresses the importance of awareness of this rare and lethal adverse effect while using nivolomab immunotherapy.
Keywords: case report, myasthenia gravis, nivolumab, squamous lung carcinoma
1. Introduction
Nivolumab (Nivo) is a human IgG4 immune checkpoint inhibitor antibody that binds to programed death-1 (PD-1) receptor. PD-1 is an inhibitory immunoreceptor predominately expressed on the surface of activated T cells and plays an important role in immune tolerance and tumor escape from the immune system.[1] Use of Nivo has been shown to suppress the inhibitory activity of T cells, leading to an increased host immune response to the tumor. Nivo has been used to treat various advanced cancers, including melanoma, nonsmall cell lung carcinoma (NSCLC), and renal cell carcinoma.[1] The known side effects of Nivo include hepatitis, pneumonitis, acute renal failure, endocrine disorder, and other immune-related adverse events (irAEs).[2] The current treatment of adverse effects may dependent on the organ system and grade if the condition is severe; it may be appropriate to withdraw Nivo.[1] In case of severe irAEs, immunosuppressive drugs are sometimes needed.
Here we described the case of a 65-year-old man with advanced squamous cell lung carcinoma who developed myasthenia gravis (MG) after Nivo treatment.
2. Case report
A 65-year-old man presented to our hospital with progressive ptosis, diplopia, and general weakness for 1 week. He was a heavy smoker (2–3 packs/d for 50 years) with chronic obstructive pulmonary disease and had been diagnosed with poorly differentiated squamous cell carcinoma of the left-upper lobe of the lung (size about 6 cm), with encasement of the thoracic aorta, left hilar structures, and subcarinal mediastinal lymphadenopathy (cT4N2M0, stage IIIB) 12 months prior. Concurrent chemoradiotherapy was performed after diagnosis; however, the disease progression and a series of chemotherapy regimens with cisplatin and paclitaxel, cisplatin and gemzar, and vinorelbine had been used within the previous 1 year. Despite these therapies, the disease continued to progress. Thus, the patient underwent immune therapy with Nivo 3 mg/kg every 2 weeks beginning 2 months prior. The final Nivo treatment was 12 days before admission. The patient reported weakness of four extremities 5 days after Nivo infusion. Upon physical examination, the patient had difficulty opening his eyes and had limited eye movement with a notable drop head. The muscle power in the four extremities was found to be grade 4. The laboratory data showed elevated levels of creatine kinase (2216 U/L), aspartate aminotransferase (153 U/L), alanine aminotransferase (110 U/L), lactate dehydrogenase (484 U/L), and troponin-I (2.62 ng/mL). Magnetic resonance imaging of the brain revealed no obvious stroke or brain metastases. Abdominal ultrasonography showed normal liver size and gallbladder polyps of about 0.9 cm. Transthoracic echocardiography showed normal left ventricular systolic function and no impairment of wall motion. These data suggested the presence of myositis and neuromuascular junction disorder. Autoimmune antibodies related to myositis, including antinuclear, anti-Jo1, and anti-Mi-2 antibodies, were negative. Nerve conduction velocity tests indicated polyneuropathy involving the median, ulnar, peroneal, tibial, and sural nerves. Electromyography showed no significant decrease in compound muscle action potential on low-frequency stimulation to the left face. High-frequency repetitive stimulation tests of the trapezius showed no incremental changes in compound muscle action potential. Acetylcholine receptor antibody (AChR ab) was not detected, and botulism serology and stool culture were normal. Peripheral blood lymphocyte analysis showed 21.74% CD4 T cells, 31.24% CD8 T cells, and elevation of the CD8/CD4 ratio (1.4; normal range 0.5–1). AChR ab-related MG, lambert Eton myasthenia syndrome, and botulism intoxication were ruled out. Nivo-related MG was strongly suspected, and the patient was given methylprednisolone 1 mg/kg/d since admission and pyridostigmine 60 mg oral twice/d 2 days after admission. However, he failed to respond to the intervention. He developed drooling with dysphagia and difficulty moving 14 days after admission. We planned to performed plasma exchange for him and discussed the possibility of respiratory failure and the use of a mechanical ventilator with his family. Conscious disturbance was found the next morning, and venous blood gas analysis showed a pH of 7.229, partial pressure of carbon dioxide of 119.2 mm Hg, and HCO3 of 48.7 mm Hg. His families refused the use of a mechanical ventilator. The patient died due to Nivo-related MG and myositis with hypercapnia respiratory failure on day 27 after the third infusion of Nivo.
The patient was diagnosed with Nivo-related MG and myositis based on the symptoms and timing of drug exposure and the exclusion of any other causes of myasthenic syndrome and myositis.
3. Discussion
Nivo has been used to treat NSCLC since 2015.[3] Adverse events consistent with immune-related causes have been observed, and grade 3 or 4 drug-related adverse events occur in 14% to 17% of patients.[1,2] The known adverse events are fatigue, pneumonitis, and diarrhea.[2] However, neurological, respiratory, musculoskeletal, and cardiac adverse events have also been reported following anti-PD1 treatment, including Nivo.[4–7]
In this study, we reported a patient diagnosed with Nivo-related MG. Based on the findings from our patient and a review of the literature (Table 1) on Nivo-related MG, we identified 7 cases.[5,8–12] Two of these 7 cases showed stabilization of MG following administration of regular medication before Nivo administration and presented with acute exacerbation after Nivo was given. Two of the 7 cases showed negative anti-AChR-Ab. Three of the 7 patients died despite medical treatment, including steroids, immunoglobulin (IVIG), and pyridostgmin. In these 3 cases, 2 patients died due to respiratory failure without the use of a mechanical ventilator, and the other patient died due to complete heart block, sepsis, and duodenal ulcer bleeding. All 7 cases developed MG or acute exacerbation after the first 3 infusions of Nivo.
Table 1.
Summary of seven cases of Nivo-related MG.
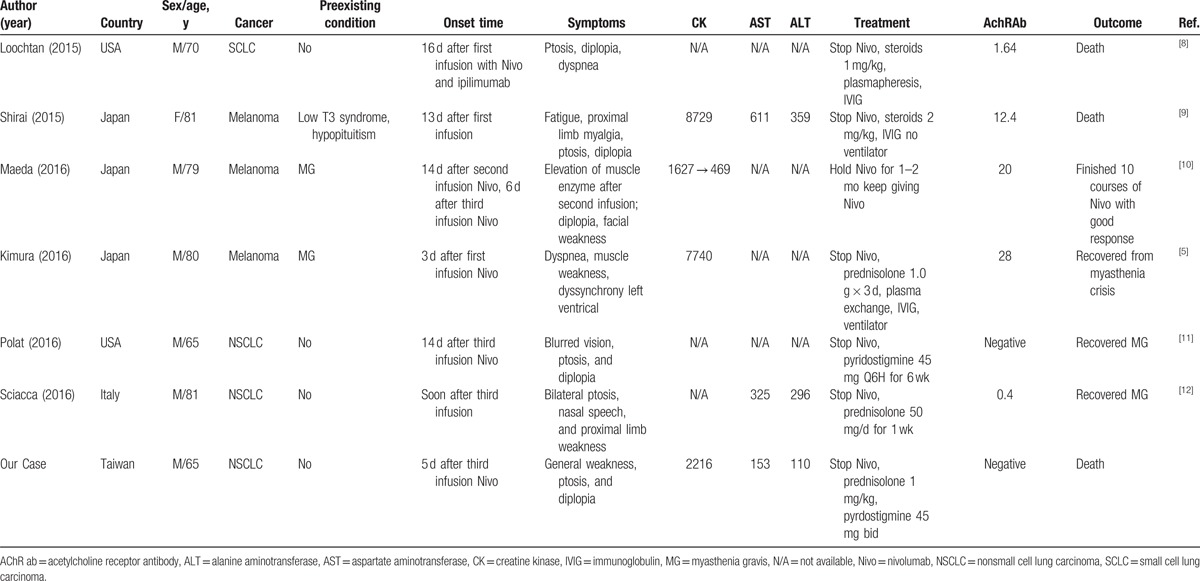
MG can be divided according to the distinct clinical feature and antibody specificity. Our patient may belong to the seronegative subgroups. However, antimuscle-specific receptor tyrosine kinase, antilow-density lipoprotein receptor-related protein 4, and pathogenic antibodies against other postsynaptic membrane antigens (interacting with acetylcholine receptors) were not measured in this patient. The diagnosis should be reassessed, and antibody tests should be repeated after 6 to 12 months.[13] Electromyography has 75% to 80% sensitivity of diagnosing MG. Further, electromyography in acute severe generalized disease may not be detected in less than 4 weeks.[14] Our patient developed MG only for 1 week, thus the electromyography might show negative result. A similar case of MG who had negative anti-AChR-Ab and electromyography after Nivo therapy has been reported.[11]
Kimura et al[5] reported an 80-year-old man with a history of MG who received Nivo for metastatic melanoma. In their study, the patient exhibited Nivo-related myocarditis, myositis, and myasthenic crisis with a rapid increase in anti-AChR antibody within 2 weeks after the first dose. In addition, after Nivo infusion, the CD8/CD4 T lymphocyte ratio increased, as demonstrated by skeletal muscle biopsy. The patient's peripheral blood mononuclear cell analysis after Nivo infusion showed decreases expression of FOXP3, CD3, and CD4 genes and increased expression of the CD8 gene compared with those before Nivo infusion. Similarly, in our case, elevation of the CD8/CD4 T-cell ratio was also noted. Blocking of the PD-1 receptor by Nivo may enhance the antitumor activity of T cells, causing elevation of the CD8/CD4 ratio and decreasing the numbers of regulatory T cells (Tregs).[15] However, the PD1 pathway and Tregs are also important for self-tolerance and autoimmunity.[16] Blockade of this pathway is thought to cause severe irAEs owing to T-cell activation, and cautious monitoring during the administration of immunotherapy with anti-PD1 agents is crucial.
The treatments for Nivo-related MG include stopping Nivo immediately, pulsing steroids, plasmapheresis, and IVIG treatments. Supportive treatment with a ventilator is necessary when respiratory failure develops. Since Nivo is often used to treat patients with advanced NSCLC, our case report presents a rare but lethal complication of myathenia gravis induced by Nivo and provides information regarding a potential treatment strategy. One limitation of this case report was that the patient did not receive further plasma exchange and IVIG; thus, we cannot know whether the patient would have responded to immune absorption treatment. The recommended dosage of Nivo is once every 2 weeks for NSCLC treatment; however, there are no biological marker available indicating irAEs. Further investigations of the potential adverse effects of Nivo are strongly recommended.
4. Conclusion
Nivo-related MG should be highly suspected in patients with symptoms including ptosis, diplopia, and general weakness. The corresponding treatments include discontinuation of Nivo and pulse steroid treatment with plasmapheresis. The disease course is fast and fatal. This report stresses the importance of the awareness of this rare and lethal adverse effect while using the anti-PD1 drug nivolomab as immunotherapy.
Footnotes
Abbreviations: AChR ab = acetylcholine receptor antibody, irAE = immune-related adverse event, IVIG = immunoglobulin, MG = myasthenia gravis, Nivo = nivolumab, NSCLC = nonsmall cell lung carcinoma, PD-1 = programed death-1, Treg = regulatory T cell.
Ethical approval was not necessary. Patient's anonymity can be maintained in written text and without the use of photographs.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Min L, Hodi FS. Anti-PD1 following ipilimumab for mucosal melanoma: durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res 2014;2:15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kimura T, Fukushima S, Miyashita A, et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci 2016;107:1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bilen MA, Subudhi SK, Gao J, et al. Acute rhabdomyolysis with severe polymyositis following ipilimumab-nivolumab treatment in a cancer patient with elevated anti-striated muscle antibody. J Immunother Cancer 2016;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yoshioka M, Kambe N, Yamamoto Y, et al. Case of respiratory discomfort due to myositis after administration of nivolumab. J Dermatol 2015;42:1008–9. [DOI] [PubMed] [Google Scholar]
- [8].Loochtan AI, Nickolich MS, Hobson-Webb LD. Myasthenia gravis associated with ipilimumab and nivolumab in the treatment of small cell lung cancer. Muscle Nerve 2015;52:307–8. [DOI] [PubMed] [Google Scholar]
- [9].Shirai T, Sano T, Kamijo F, et al. Acetylcholine receptor binding antibody-associated myasthenia gravis and rhabdomyolysis induced by nivolumab in a patient with melanoma. Jpn J Clin Oncol 2016;46:86–8. [DOI] [PubMed] [Google Scholar]
- [10].Maeda O, Yokota K, Atsuta N, et al. Nivolumab for the treatment of malignant melanoma in a patient with pre-existing myasthenia gravis. Nagoya J Med Sci 2016;78:119–22. [PMC free article] [PubMed] [Google Scholar]
- [11].Polat P, Donofrio PD. Myasthenia gravis induced by nivolumab therapy in a patient with non-small-cell lung cancer. Muscle Nerve 2016;54:507–1507. [DOI] [PubMed] [Google Scholar]
- [12].Sciacca G, Nicoletti A, Rampello L, et al. Benign form of myasthenia gravis after nivolumab treatment. Muscle Nerve 2016;54:507–9. [DOI] [PubMed] [Google Scholar]
- [13].Gilhus NE. Myasthenia gravis. N Engl J Med 2016;375:2570–81. [DOI] [PubMed] [Google Scholar]
- [14].Liik M, Punga AR. Repetitive nerve stimulation often fails to detect abnormal decrement in acute severe generalized myasthenia gravis. Clin Neurophysiol 2016;127:3480–4. [DOI] [PubMed] [Google Scholar]
- [15].Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014;27:1–7. [DOI] [PubMed] [Google Scholar]
- [16].Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


