Abstract
Background:
Prostate cancer (PCa) patients initiating androgen deprivation therapy (ADT) are suffering from adverse effects; exercise has been proposed as a treatment to relieve adverse effects of ADT, available meta-analysis has proved exercise improves quality of life, and therapy caused fatigue; recently, some high-quality trials have been conducted in order to get more assessment; we conduct an updated meta-analysis to evaluate feasibility that exercise relieves adverse effects in PCa patients initiating ADT.
Materials and methods:
A systematic article search was performed from Cochrane Library, MEDLINE, EMBASE, and PubMed databases up to March 10, 2017. Outcomes included changes in body composition, physical function, bone health and cardiometabolic changes. We conduct subgroup analysis to analyze the duration and type of exercise correlated with the effect and calculated using standard mean difference (SMD) and corresponding 95% confidence intervals (CI).
Result:
Fifteen studies involving 1135 patients were included in our meta-analysis, and significant positive effects were found in body strength (leg press (SMD: 0.78 (95%CI: 0.57–0.99, P <.00001, I2 = 0%)), chest press (SMD: 0.71 (95%CI: 0.50–0.92, P <.00001, I2 = 0%)), exercise tolerance (VO2 peak SMD: 0.35 (95%CI: 0.04–0.66, P = .03, I2 = 0%) in 6 months and SMD: 0.59 (95%CI: 0.16–1.03, P = .007, I2 = 0% over 6 months)), fatigue (SMD: 0.84 (95%CI: −1.43 to 3.10, P = .85, I2 = 51%) in 6 months and SMD: −9.3 (95%CI: −16.22 to −2.39, P = .0030, I2 = 49%) over 6 months)), ADT-caused obesity (body mass index SMD: −0.33 (95%CI: −0.55 to −0.12, P = .002, I2 = 38% in 6 months and SMD: −0.59 95%CI: −1.02 to 0.17, P = .006, I2 = 25% over 6 months)), and sex function (SMD: 0.66 (95%CI: 0.35–0.97, P <.00001, I2 = 2%). There were no evidence of benefit for cardiometabolic changes and bone health. No systematic difference was observed between resistance exercise training (RET) and aerobic exercise training (AET) in ADT-caused obesity, fatigue, and exercise tolerance
Conclusion:
Exercise can significantly improve the upper and lower muscle strength, increase exercise tolerance, help PCa patients receiving ADT control their body fat mass, BMI, and keep the sex function. ADT-related fatigue is correlated with exercise duration time. No differences were observed in LBM, bone mineral density, and any other metabolic blood markers. Available data show that there is no difference between AET and RET.
Keywords: adverse effect, androgen depression therapy (ADT), exercise, prostate cancer patients
1. Introduction
Prostate cancer (PCa) is the most common diagnosed cancer among aged men in developed countries.[1] Because of cancer cell inertness, the 5-year relative survival rate is approaching 100% in some countries. Androgen deprivation therapy (ADT) is a basic treatment for PCa patients which can prolong survival rate and reduce disease-related morbidity.[2] However, patients initiating ADT suffer from adverse effects including reducing quality of life,[3] decreasing in physical function, body composition, bone mineral density (BMD) loss,[4,5] and increasing risk of metabolic dysfunction and cardiovascular diseases,[6] and these mental and physical changes in the end will have a bad effect on patient health.
Exercise has been proposed as a treatment to relieve adverse effects of ADT. However, although recent meta-analysis has reported the benefit of exercise therapy on the cancer-specific quality of life and fatigue[7] in PCa patients, available data about the feasibility of exercise is still inconsistent. The purpose of this meta-analysis is to evaluate the role of exercise in control mental and physical changes in PCa patients receiving ADT and analyze whether the duration and the type of exercise correlate with the effect.
2. Material and method
2.1. Evidence search
The primary outcomes of this study were the effects on body composition, physical function, and fatigue; secondary outcomes included cardiometabolic changes in biochemical markers, bone health, and sexual health.
We searched Cochrane Library, MEDLINE, EMBASE, and PubMed database date up to March 10, 2017. Search terms we use included “prostate cancer” or “prostate neoplasm” or “prostatic neoplasm” or “cancer of prostate” and “exercise” or “training” or “aerobic” or “resistance”; we also expanded our search to recent review and Clinicaltrials.gov.
2.2. Inclusion and exclusion criteria
Articles are required to be published in English papers. We only included trial on PCa patients initiating ADT (include ADT only, adjutant ADT after radical prostatectomy, or accompanied with radiotherapy). Interventions were aerobic exercise training (AET) or resistance exercise training (RET) or 2 types combined. Eligibility was assessed independently by 2 authors, with differences resolved by discussion. Trials were excluded when involving other cancer patients or combining other intervention (e.g., diet plan) unless outcome could be isolated. If the studies were published from the same center or from same author, we only included the studies containing maximum cases and outcome assessment, unless the study outcomes were different from others. The selection process was conducted followed by Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram (Fig. 1).
Figure 1.
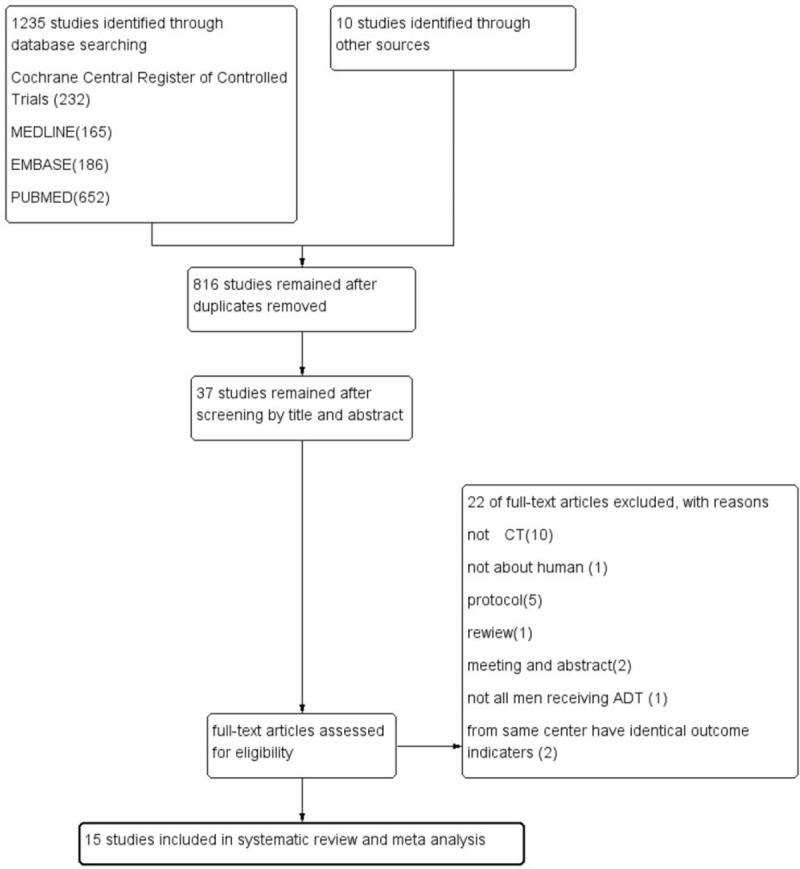
Flow diagram of selection of articles.
2.3. Data extraction and synthesis
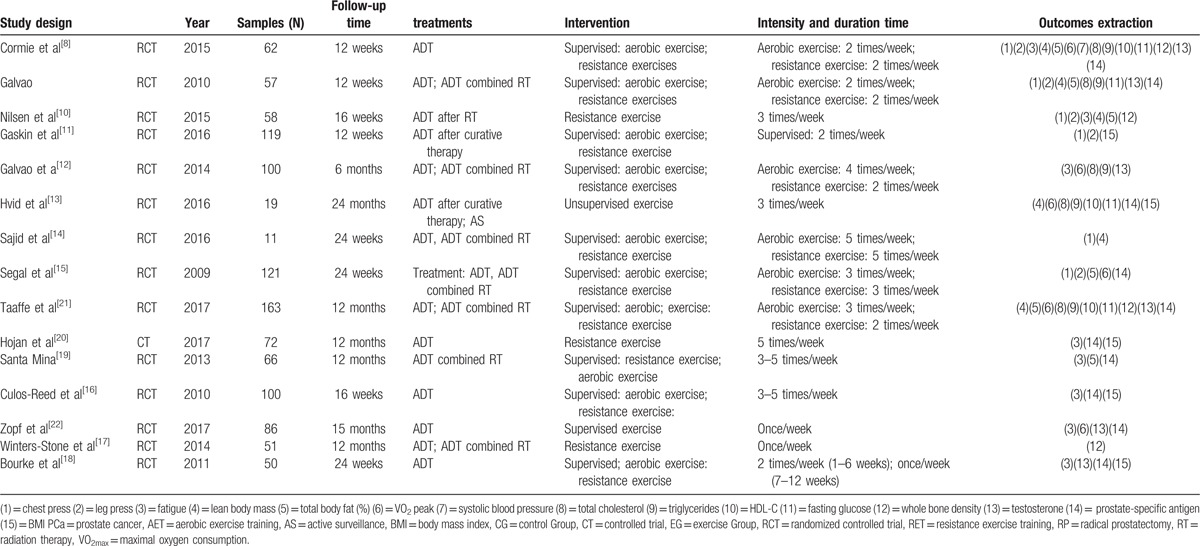
We documented study characteristics and outcomes as follows: study design and year, samples, follow-up time, participants and treatments, intervention details, key outcomes, outcomes extraction (Table 1). If available data were sufficient and appropriate to conduct a meta-analysis, we did it by using Review Manager software. As for the continuous outcomes, we calculated the standard mean difference (SMD) or standard difference (SD); heterogeneity and inconsistency across studies were tested by Q statistics (P >.1) and I2 <50%. If statistical heterogeneity was observed, meta-analysis was performed using a random-effect model; otherwise, fixed-effect model was performed. If meta-analysis cannot be conducted, we provided a narrative review.
Table 1.
Characteristic and result of 15 trials.
2.4. Risk of bias assessment
The risk of bias was conducted using the Cochrane Collaboration tool (Fig. 2); 2 authors conducted the risk of bias difference were resolved by discussion.
Figure 2.
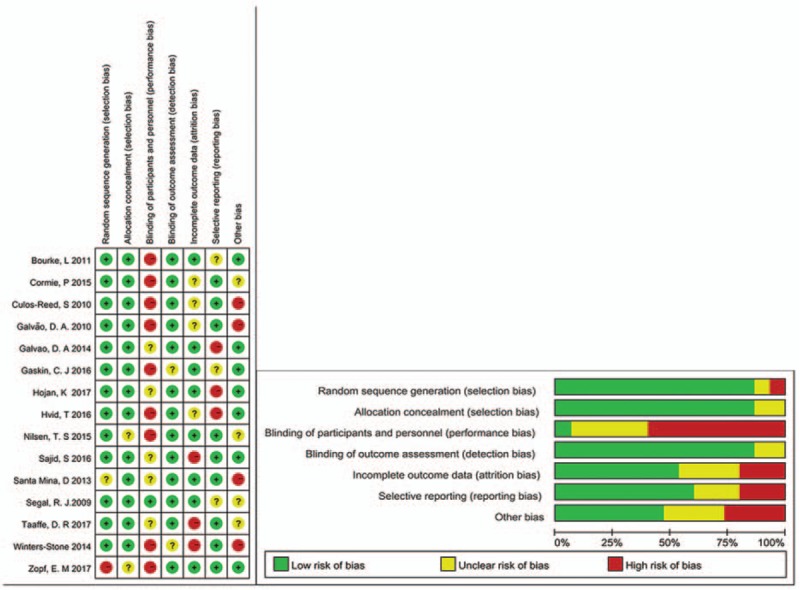
Risk of bias assessment (graph and summary).
2.5. Ethics statement
There is no need to seek consent from patients, as in this study all the data were collected from the published data and analyzed anonymously without any potential harm to the patients.
3. Result
3.1. Literature search
There are 1235 studies searched from database and 10 additional studies from review and Clinicaltrials.gov search; with 816 studies remaining after duplicates removed; 779 studies were excluded after reading the title and abstract, then 22 studies were excluded after reading full article, through inclusion and exclusion criteria, 15 studies[8–22] involving 1135 patients initiating ADT remained for our meta-analysis (including 14 randomized controlled trial and 1 controlled trial). All patients were undergoing ADT (ADT only or ADT after curative therapy). Exercise intervention contains supervised or unsupervised or mixed training, exercise type contains AET or RET, or both combined, with duration time lasting from 1.5 to 24 months; detailed information is recorded in Table 1.
3.2. Review outcome
3.2.1. Body composition
In our review, 11 studies reported the effect on body composition among PCa patients initiating ADT.[8–16,18,20] The outcome indicators we extracted contained total lean body mass (LBM) (kg),[8–11,13] total body fat (%),[8–10,12,15] and body mass index (BMI).[10,11,13,16,18,20] BMI was available in 6 studies, the pooled SMD was −0.33 (95%CI: −0.55 to −0.12, P = .002, I2 = 38% in 6 months and SMD: −0.59 95%CI: −1.02 to 0.17, P = .006, I2 = 25% over 6 months) (Fig. 3A) indicating that there is a significant difference between exercise group and control group; 4 studies reported LBM changes, the pooled SMD was 0.08 (95%CI: −0.20 to 0.30, P = .57, I2 = 0%) (Fig. 3B), with no positive effect was observed; 5 studies involving 359 patients measured the total body fat (%), with a moderate positive effect was found (SMD: −0.22 (95%CI: −0.42 to 0.01, P = .04, I2 = 0%)) (Fig. 3C).
Figure 3.
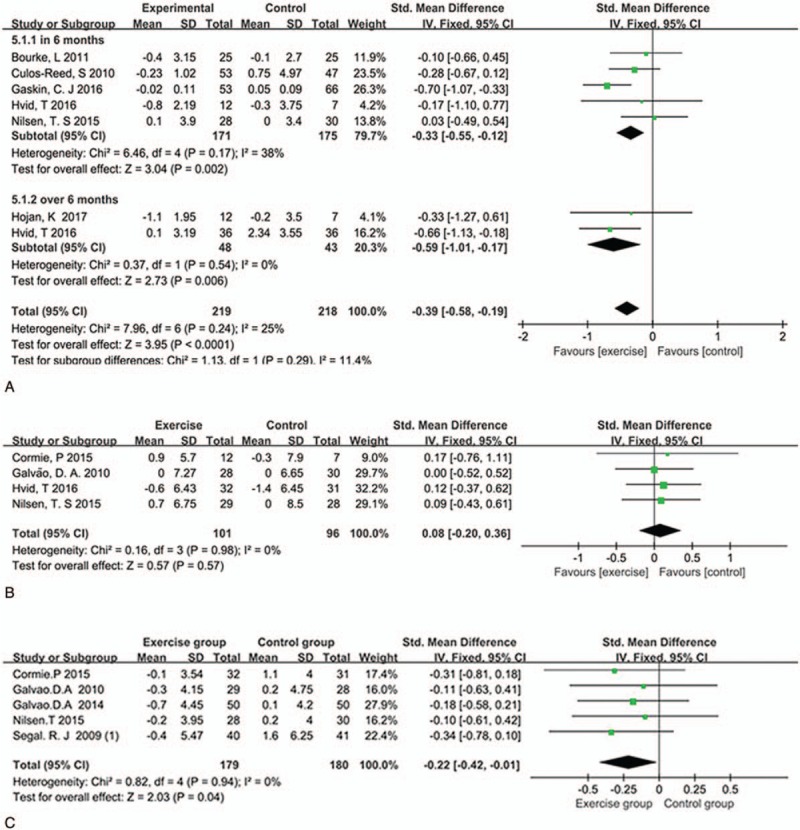
Plot A is the change in body BMI compared to exercise group and control group in 6 months and over 6 months, plot B is the change in LBM, and plot C is the change in total body mass. BMI = body mass index, LBM = lean body mass.
3.2.2. Physical function
Eight studies reported the physical function behaviors[8–11,14,15,18,22]; we indicated leg press, chest press and VO2 peak for meta-analysis; 6 studies involving 389 patients reported the chest press (the pooled SMD was 0.71 (95%CI: 0.50–0.92, P <.00001, I2 = 0%)) (Fig. 4A)) and 5 studies reported leg press changes (the pooled SMD was 0.78 (95%CI: 0.57–0.99, P <.00001, I2 = 0%)) (Fig. 4B), this means exercise has a significant change in upper and lower muscle strength. Four studies including 261 patients used the VO2 peak as a marker for exercise tolerance, the outcomes showed exercise has a moderate positive effect in 6 months, (SMD:0.35 (95%CI: 0.04–0.66, P = .03, I2 = 0%)) and has a significant effect over 6 months (SMD: 0.59 (95%CI: 0.16–1.03, P = .007, I2 = 0%)) (Fig. 4C).
Figure 4.
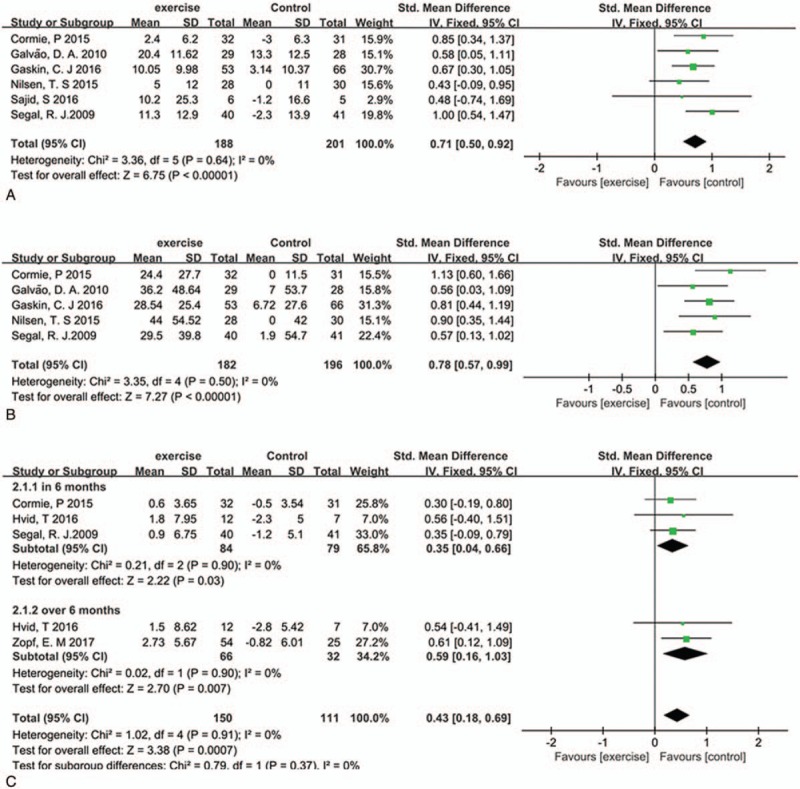
Plot A is the change in chest press compare exercise group and control group, plot B is the change in leg press, and plot C is change in VO2 peak in 6 months and over 6 months. VO2 = oxygen consumption.
3.2.3. Cardiometabolic changes
Seven studies reported the cardiometabolic changes in biochemical markers. We extracted total serum cholesterol, triglyceride, high-density lipoprotein (HDL), fasting glucose, and systolic blood pressure for meta-analysis. The pooled SMD in total serum cholesterol was 0.35 (95%CI: 0.1–0.61, P = .007, I2 = 0%) (Fig. 5A). This means that exercise has a positive effect on improving total serum cholesterol, but we did not found any difference in triglyceride (4 studies involving 239 patients, SMD: 0.27 95%CI: −0.5 to 1.03, P = .5, I2 = 87%) (Fig. 5B) and HDL (3 studies involving 139 patients, SMD: 0.21 95%CI: −0.13 to 0.55, P = .08, I2 = 0%) (Fig. 5C), as well as fasting glucose (4 studies involving 239, patients, SMD: −0.30 95%CI: −0.64 to 0.04, P = .30, I2 = 0%) (Fig. 5D).
Figure 5.
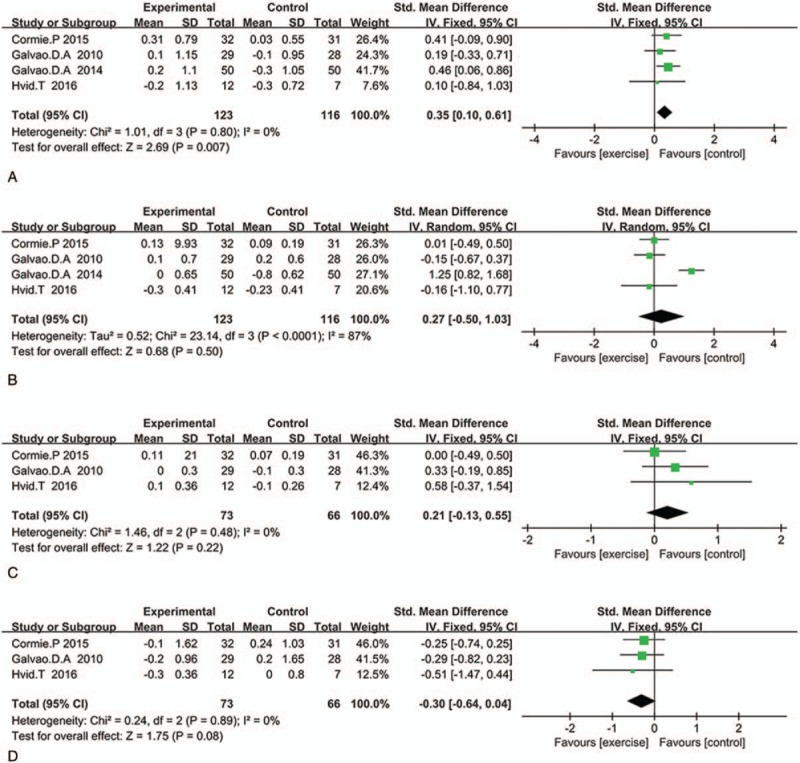
Plot A is the change in total serum cholesterol compared exercise group and control group, plot B is the change in triglyceride, plot C is the change in HDL, and plot D is the pooled outcome in fasting glucose. HDL = high-density lipoprotein.
3.2.4. Fatigue
Seven studies involving 641 patients reported the ADT-related fatigue by using the FACIT-Fatigue or EORTC QLQ-C30 questionnaire. The pooled SMD in 6 months was 0.84 (95%CI: −1.43 to 3.10, P = .85, I2 = 51%) (Fig. 6). This means that there was no significant difference between exercise and control group, but when exercise duration lasts over 6 months, exercise has a positive effect (SMD:−9.3 95%CI: −16.22 to −2.39, P = .0030, I2 = 49%).
Figure 6.
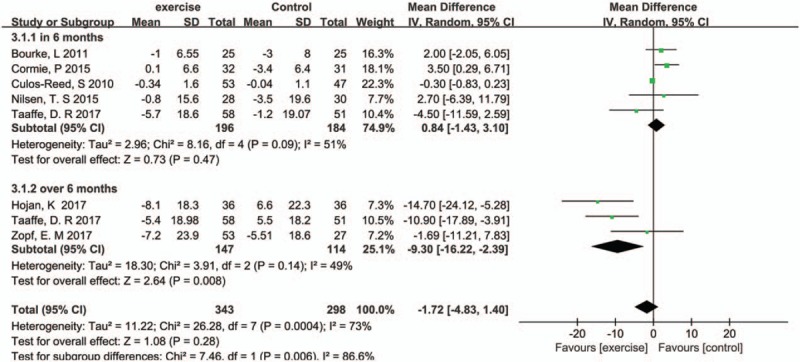
Pooled outcome of fatigue (duration in 6 months and over 6 months).
3.2.5. Bone health
Only 3 small studies[8,14,18] reported the BMD change. Pooled SMD was −0.03 (95%CI: −0.07 to 0.01, P = .12, I2 = 0%) (Fig. 7). Analysis indicated that there was no significant difference in BMD change.
Figure 7.
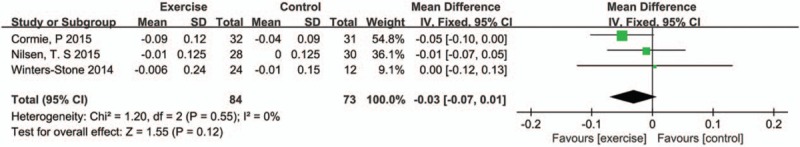
Pooled outcome of BMD. BMD = bone mineral density.
3.2.6. Sexual health
Three studies reported the sex function with pooled SMD being 0.66 (95%CI: 0.35–0.97, P <.00001, I2 = 2%) (Fig. 8).
Figure 8.
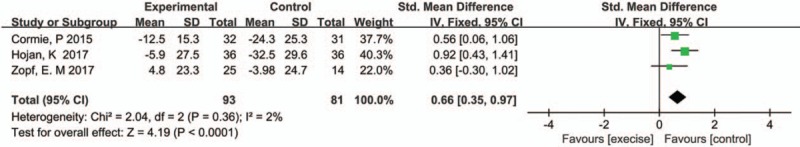
Pooled outcome of sex function.
3.2.7. Effect of 2 types of exercise models
One RCT and 2 three-armed RCT recorded 2 types of exercise models (AET and RET), and the pooled SMD for fatigue score, body fat mass, and VO2 peak was 0.09 (95%CI: −0.27 to 0.44, P = .63, I2 = 51%) (Fig. 9A), −0.14 (95%CI: −0.47 to 0.18, P = .60, I2 = 51%) (Fig. 9B), and −0.12 (95%CI: −0.44 to 0.21, P = .63, I2 = 0%) (Fig. 9C), respectively. This means that there was no difference in effect of AET and RET; heterogeneity (I2 = 51%) was observed, and further studies are needed to explain it.
Figure 9.
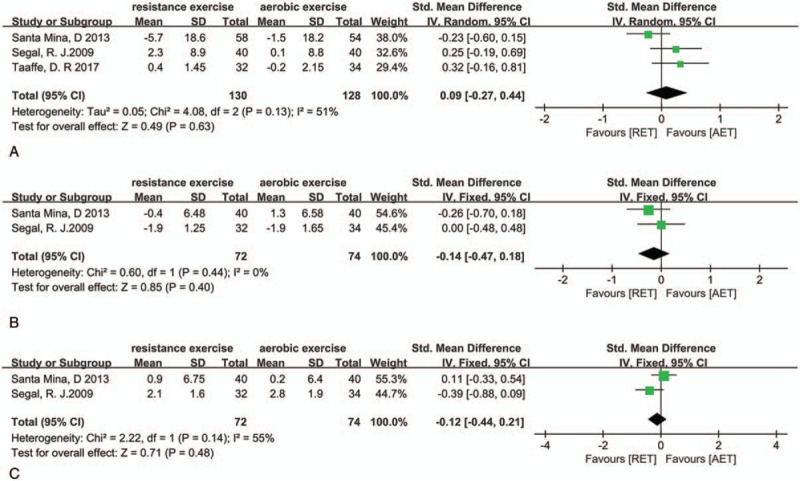
Plot A is the pooled outcome about fatigue between AET and RET, plot B is the outcome about fat mass, and plot C is the pooled outcome about VO2 peak. AET = aerobic exercise training, RET = resistance exercise training, VO2 = oxygen consumption.
4. Discussion
This meta-analysis provides a comprehensive summary about exercise alleviate adverse effects on PCa patients initiating ADT and this is the first meta-analysis that analyzes whether the duration and the type of exercise correlate with the effect. Firstly, we evaluated the effect of exercise in many aspects, which is more extensive than available meta-analysis[7]; secondly, if dates were available for us to go on a subgroup analysis about exercise duration time, we did a subgroup meta-analysis based on duration time (lasting in 6 months or over 6 months); thirdly, we did a meta-analysis to compare the effects of AET and RET. Consequences indicate that the exercise group showed improvement in mental and physical health compared with the usual care group.
ADT-related obesity and muscle loss are severe side-effects among PCa patients.[26,27] Changes in body composition include but not limited to decrease in LBM, increase in body fat mass, and decline in functional capacity.[23] Androgen play a vital role in this process[24]; moreover, with obvious side effect on body composition in the first 6 months.[25] Exercise leads a positive effect on body composition among cancer patients. One study involving 57 PCa patients initiating ADT was randomized to a 12 weeks exercise therapy group or usual care group. The exercise group had a higher adjusted mean LBM compared with controls (P = .047),[9] but after combining results of 4 studies, no significant effect is observed between exercise and control groups; there maybe some reason to explain it; on the one hand, age of participant ranged from 50 to 80 years old. For aged PCa patients, it is difficult to achieve the training standard, this may affect the outcome; on the other hand, as PCa patients suffer hypoandrogenism after ADT, exercise may not be efficient in ADT-related muscle loss. More trials need to be investigated to evaluate the effect. Through our analysis, we found that exercise leads to a great improvement in upper and lower muscle strength and endurance, at the same time, exercise also does some help to pulmonary function. These changes are important because they can contribute to improve the ability of independent living and decrease the risk of fracture, which happens with other healthy aged men going on a exercise plan.[28] Moreover, consequence shows that exercise has a moderate positive effect on body fat mass (%) and BMI, at the same time, subgroup analysis shows that there is no difference observed when compared exercise in 6 months and over 6 months. This indicates that exercise can help PCa patients receiving ADT keeping out from ADT-related obesity, as recent studies showed that BMI and serum levels of testosterone were correlative with disease progression in patients with low-risk PCa;[29,30] exercise, on the other hand, will help these patients avoid these risk factors.
Our meta-analysis also proves a statistically significant difference that exercise can alleviate ADT-related fatigue over 6 months but with no difference in 6 months between the exercise group and the control group. This outcome indicates that exercise is correlated with the duration of exercise, but the heterogeneity of pooled fatigue cannot be ignored (I2 = 51% in 6 months and I2 = 49% over 6 months). There may be some reason for these difference; first of all, some studies recorded fatigue by using Fatigue Subscale Questions From The European Organization for Research and Treatment of Cancer quality of Life Questionnaire-core 30 (EORTC QLQ-C30) questionnaire, and others recorded fatigue by using Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-fatigue) questionnaire, this may cause a big difference; secondly, the questionnaire score is correlated with personal subjective feeling; different patients may have different feeling about fatigue; thirdly, before patients initialing ADT, they may already have received surgery or radiotherapy; bias appears in this procedure. This will increase the heterogeneity of outcome and affect the validity of the study.
As for cardiometabolic changes, recent trials reported that PCa patients receiving ADT is associated with an increased risk of metabolic syndrome compared with those not receiving ADT. They found that ADT will cause an increase in fasting glucose level, total serum cholesterol, and triglyceride.[31] Exercise has been proved to be a good way to reduce these changes among healthy people.[32] In out meta-analysis, we found that the exercise group showed improvement in total serum cholesterol compared with the control group, but there was no difference in HDL and triglyceride levels. Consequences indicate that exercise cannot change the blood lipid metabolism compared with the usual care group. Studies have observed that there exist a risk of incident of diagnosing diabetes mellitus during ADT among PCa patients,[33] but no trials used incident of diagnosing diabetes mellitus as outcomes. We only found 4 trials that measured fasting glucose as a outcome, the follow-up time ranging from 12 weeks to 24 months. There is no significant difference between the exercise group and the control group. More trials are required to be undertaken to investigate the glucose and insulin sensitivity as well as incident of diagnosing diabetes mellitus.
It has been proved that PCa patients receiving ADT would have a decrease in BMD and an increasing risk of incident of fracture.[34,35] In our review, 3 studies recorded the BMD as a marker for bone health, and 1 trial[18] (involving 51 men, 12-month follow-up time) showed a slight positive effect on L4 spine BMD; but the pooled outcome of these 3 trials showed that there is no difference in total BMD between the exercise group and the controls.
Because of the hypoandrogenism in PCa patients initialing ADT, patients receiving ADT suffer from low sex function and erectile dysfunction. From our analysis, the pooled sex function score assessed by the QLQ-PR25 differed significantly between 2 groups. Outcome indicates that exercise has a beneficial effect on sexual health among PCa patients receiving ADT, with a less of decline in function compared with the control group; finally, as there is no difference in prostate-specific antigen change between the exercise group and the controls, exercise will not cause recurrence or progression of disease in PCa patients receiving ADT.
In our analysis, we also compared 2 types of exercise models and compared their effects on body composition, fatigue, and exercise tolerance. The pooled outcome showed that there was no difference between 2 exercise model (AET and RET). As the available data provide a limited information about comparison of AET and RET, more research needs to be conducted.
There are some limitations in our meta-analysis, Firstly, included trial 1 is a nonrandomized controlled trial; baseline factors such as smoking, age, etc will influence the exercise efficacy after treatment. This also affects the accuracy of our meta-analysis. Secondly, when patients going on ADT, they may already receive surgery or radiotherapy, bias appears in the procedure; finally, due to the small sample size studies enrolled, results may lack statistical accuracy.
5. Conclusion
In our systematic review and meta analysis, exercise can significantly improve the upper and lower muscle strength, increase the exercise tolerance, help PCa patients receiving ADT control their body fat mass, BMI, and keep the sex function. ADT-related fatigue is correlated with exercise duration time, and no differences were observed in LBM, BMD, and any other metabolic blood markers. Furthermore, available data show that there is no difference between AET and RET.
Footnotes
Abbreviations: ADT = androgen deprivation therapy, AET = aerobic exercise training, BMD = bone mineral density, BMI = body mass index, CI = confidence intervals, CT = controlled trial, EORTC QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-core 30, HDL = high-density lipoprotein, LBM = lean body mass, PCa = prostate cancer, PSA = prostate-specific antigen, RCT = randomized controlled trial, RET= resistance exercise training, RP = radical prostatectomy, RT = radiotherapy, SMD = standard mean difference.
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- [2].Pagliarulo V, Bracarda S, Eisenberger MA, et al. Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol 2012;61:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Singh J, Trabulsi EJ, Gomella LG. The quality-of-life impact of prostate cancer treatments. Curr Urol Rep 2010;11:139–46. [DOI] [PubMed] [Google Scholar]
- [4].Bourke L, Boorjian SA, Briganti A, et al. Survivorship and improving quality of life in men with prostate cancer. Eur Urol 2015;68:374–83. [DOI] [PubMed] [Google Scholar]
- [5].Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015;67:825–36. [DOI] [PubMed] [Google Scholar]
- [6].Keating NL, O’Malley A, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2012;104:1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bourke L, Smith D, Steed L, et al. Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol 2016;69:693–703. [DOI] [PubMed] [Google Scholar]
- [8].Cormie P, Galvão DA, Spry N, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int 2015;115:256–66. [DOI] [PubMed] [Google Scholar]
- [9].Galvão DA, Taaffe DR, Spry N, et al. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010;28:340–7. [DOI] [PubMed] [Google Scholar]
- [10].Nilsen TS, Raastad T, Skovlund E, et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol 2015;54:1805–13. [DOI] [PubMed] [Google Scholar]
- [11].Gaskin CJ, Fraser SF, Owen PJ, et al. Fitness outcomes from a randomised controlled trial of exercise training for men with prostate cancer: the ENGAGE study. J Cancer Surviv 2016;10:972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Galvao DA, Spry N, Denham J, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol 2014;65:856–64. [DOI] [PubMed] [Google Scholar]
- [13].Hvid T, Lindegaard B, Winding K, et al. Effect of a 2-year home-based endurance training intervention on physiological function and PSA doubling time in prostate cancer patients. Cancer Causes Control 2016;27:165–74. [DOI] [PubMed] [Google Scholar]
- [14].Sajid S, Dale W, Mustian K, et al. Novel physical activity interventions for older patients with prostate cancer on hormone therapy: A pilot randomized study. J Geriatr Oncol 2016;7:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol 2009;27:344–51. [DOI] [PubMed] [Google Scholar]
- [16].Culos-Reed SN, Robinson JW, Lau H, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Support Care Cancer 2010;18:591–9. [DOI] [PubMed] [Google Scholar]
- [17].Winters-Stone KM, Dobek JC, Bennett JA, et al. Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc 2014;46:1482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bourke L, Doll H, Crank H, et al. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers Prev 2011;20:647–57. [DOI] [PubMed] [Google Scholar]
- [19].Santa Mina D, Alibhai SM, Matthew AG, et al. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. J Aging Phys Activity 2013;21:455–78. [DOI] [PubMed] [Google Scholar]
- [20].Hojan K, Kwiatkowska-Borowczyk E, Leporowska E, et al. Inflammation, cardiometabolic markers, and functional changes in men with prostate cancer: A randomized controlled trial of a 12-month exercise program. Polskie Archiwum Medycyny Wewnetrznej 2017;127:25–35. [DOI] [PubMed] [Google Scholar]
- [21].Taaffe DR, Newton RU, Spry N, et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year-long randomised controlled trial. Eur Urol 2017;S0302-2838:30108–12. [DOI] [PubMed] [Google Scholar]
- [22].Zopf EM, Bloch W, Machtens S, et al. Effects of a 15-month supervised exercise program on physical and psychological outcomes in prostate cancer patients following prostatectomy: the prorehab study. Integr Cancer Ther 2017;14:409–18. [DOI] [PubMed] [Google Scholar]
- [23].Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol 2005;63:280–93. [DOI] [PubMed] [Google Scholar]
- [25].van Londen GJ, Levy ME, Perera S, et al. Body composition changes during androgen deprivation therapy for prostate cancer: a 2-year prospective study. Crit Rev Oncol/Hematol 2008;68:172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kohrt WM, Bloomfield SA, Little KD, et al. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc 2004;36:1985–96. [DOI] [PubMed] [Google Scholar]
- [27].Galvao DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int 2008;102:44–7. [DOI] [PubMed] [Google Scholar]
- [28].Bosco C, Crawley D, Adolfsson J, et al. Quantifying the evidence for the risk of metabolic syndrome and its components following androgen deprivation therapy for prostate cancer: a meta-analysis. PloS One 2015;10:e0117344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Cobelli O, Terracciano D, Tagliabue E, et al. Body mass index was associated with upstaging and upgrading in patients with low-risk prostate cancer who met the inclusion criteria for active surveillance. Urol Oncol 2015;33:201.e1–201.e8. [DOI] [PubMed] [Google Scholar]
- [30].Ferro M, Lucarelli G, Bruzzese D, et al. Low serum total testosterone level as a predictor of upstaging and upgrading in low-risk prostate cancer patients meeting the inclusion criteria for active surveillance. Oncotarget 2017;8:18424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Storer TW, Miciek R, Travison TG. Muscle function, physical performance and body composition changes in men with prostate cancer undergoing androgen deprivation therapy. Asian J Androl 2012;14:204–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bagley L, Slevin M, Bradburn S, et al. Sex differences in the effects of 12 weeks sprint interval training on body fat mass and the rates of fatty acid oxidation and VO2max during exercise. BMJ Open Sport Exerc Med 2016;2:e000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Silveira H, Moraes H, Oliveira N, et al. Physical exercise and clinically depressed patients: a systematic review and meta-analysis. Neuropsychobiology 2013;67:61–8. [DOI] [PubMed] [Google Scholar]
- [34].Wadhwa VK, Weston R, Mistry R, et al. Long-term changes in bone mineral density and predicted fracture risk in patients receiving androgen-deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU Int 2009;104:800–5. [DOI] [PubMed] [Google Scholar]
- [35].Alibhai SM, Duong-Hua M, Cheung AM, et al. Fracture types and risk factors in men with prostate cancer on androgen deprivation therapy: a matched cohort study of 19,079 men. J Urol 2010;184:918–23. [DOI] [PubMed] [Google Scholar]


