Abstract
Background:
Obsessive-compulsive disorder (OCD) is a chronic neuropsychiatric disorder with a 2% to 3% lifetime prevalence; in addition, 10% of OCD patients are resistant to conventional therapy. Deep brain stimulation (DBS) has been an effective treatment for treatment resistant OCD patients (TROCD). We aimed to determine the cost-effectiveness of DBS for TROCD.
Methods:
We used a Markov model to estimate the cost-effectiveness of DBS compared to conventional treatment for TROCD with a 10-year time horizon. Published data were used to estimate the rates of treatment response and complications. Costs were calculated from the perspective of the third-party payer. Data on quality of life were obtained from a literature review and a survey of OCD patients. We applied the model separately to Korea and the United Kingdom (UK) to enhance the validity.
Results:
Base-case analysis showed an incremental cost-effectiveness ratio of US$37,865 per quality-adjusted life-year in Korea and US$34,462 per quality-adjusted life-year in the UK. According to the World Health Organization's criteria, DBS for TROCD was “cost-effective” in Korea (<3x GDP per capita) and “highly cost-effective” in the UK (<GDP per capita). One-way sensitivity analysis showed consistent cost-effectiveness results for most variables with the exception of short-term duration of treatment effect (<4 years in Korea; <3 years in the UK).
Conclusion:
The results showed that DBS is a cost-effective treatment for TROCD in both the countries. Our findings provide economic evidence on the applicability of DBS for patients, health care service providers, and payers
Keywords: cost-effectiveness, deep brain stimulation, economic evaluation, neuromodulation, obsessive-compulsive disorder, treatment-resistant obsessive-compulsive disorder
1. Introduction
Obsessive-compulsive disorder (OCD) is a psychiatric disorder characterized by the presence of intrusive and senseless thoughts (obsessions) and/or excessive repetitive behaviors (compulsions). OCD has a lifetime prevalence of 2% to 3%[1] worldwide, and runs a chronic and disabling course that compromises an individual's functioning and well-being, ultimately having a negative effect on the lives of both patients and their families.[2] Cognitive behavioral therapy (CBT) and pharmacotherapy, including the use of serotonin reuptake inhibitors, have been proven to be effective for treating OCD. However, an estimated 10% of OCD patients remain resistant to these therapies and suffer from severe symptoms that lead to substantial functional impairment.[3]
Deep brain stimulation (DBS) is a minimally invasive neurosurgical procedure used for a range of neuropsychiatric disorders[4] that inhibits or functionally overrides hyperactivity in target brain areas through high-frequency stimulation. In the case of OCD, DBS was approved for treatment by the US FDA in 2009.[5] In OCD patients, DBS modulates abnormal activity in circuits related to the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and striatum.[6] A recent meta-analysis demonstrated the treatment effect of DBS in severe, treatment-refractory OCD (TROCD) patients, showing that 60.0% (95% confidence interval [CI]) = 49.0%–69.0%) of OCD patients receiving DBS experienced a reduction in symptom severity >35%.[7] In addition, previous studies reported a notable impact of DBS on the quality of life (QoL) of OCD patients.[8,9] Given these findings, DBS has received greater clinical attention as a last option for TROCD patients.
Although researchers have investigated the clinical effectiveness and impact of DBS on patients’ QoL, the evidence of the cost-effectiveness of DBS for TROCD was very limited except only one previous study.[10] Considering the significant cost and clinical impact of DBS on TROCD, cost-effectiveness can be a critical factor in decision-making for payers, health care providers, and patients. Accordingly, this study aimed to evaluate the cost-effectiveness of DBS for TROCD patients. We hypothesized that within a 10-year time horizon, DBS for TROCD would be a cost-effective treatment option. In particular, we investigated the cost-effectiveness of DBS in Korea and the United Kingdom (UK), which have distinct health care systems, to enhance the validity of the findings.
2. Methods
2.1. Model
We built a Markov model to assess the cost-effectiveness of DBS compared with conventional treatment (medication and CBT) for TROCD patients. The schematic model structure is described in Figure 1. A 10-year time horizon and 1-year cycle length is applied with half cycle correction. We assumed that 10-year time horizon would be generalizable to naturalistic clinical settings, based on the follow-up period of previous studies[9,11] and relatively young age of TROCD patients receiving DBS.[7]
Figure 1.
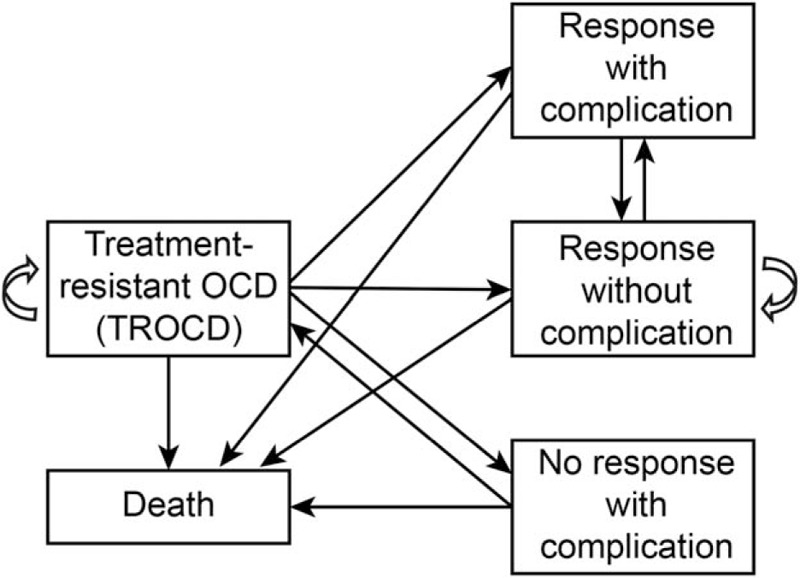
Structure of the Markov model. According to the presence of a treatment effect and/or complications after DBS, TROCD patients are classified into 1 of 5 statuses after DBS treatment: response with complication, response without complication, no response with complication, TROCD (no response without complication), and death. Patients who continue conventional OCD treatment (CBT and pharmacotherapy) are assumed to remain in TROCD status. CBT = cognitive behavioral therapy, DBS = deep brain stimulation, OCD = obsessive-compulsive disorder, TROCD = treatment-resistant obsessive-compulsive disorder.
As clinical status of OCD has detrimental impact on patient's QoL and mortality,[8,9,12] we included patient's QoL and mortality improvement as health outcome of DBS and conventional treatment in the model. The result was presented as the incremental cost per quality-adjusted life-year (QALY). QALY is a generic measure of state of health of a person or group in which the benefits, in terms of length of life, are adjusted to reflect the QoL. One QALY means 1 year of life in perfect health.[13]
In accordance with a previous meta-analysis,[7] the target study population consisted of men and women with TROCD aged 35 to 40 years. TROCD patients in this study was defined as OCD patients who did not respond to at least 5 years of sufficient conventional treatment and who still presented a Yale–Brown Obsessive Compulsive Scale (YBOCS) score >30. TreeAge Pro 2011 software (TreeAge Software, Inc., Williamstown, MA) was used to build the model.
In this model, all patients started from TROCD status. TROCD patients who received DBS could either remain as no response without complication (TROCD) or could switch to response with complication, response without complication, no response with complication or death. Responsiveness was defined as patients who showed a reduction of >35% in YBOCS score within a year of DBS implantation. Faster battery depletion of DBS is assumed in OCD due to higher frequency stimulation than Parkinson disease, in which the battery change occurs every 4 years.[6] Therefore, we postulated that OCD patients would need a battery change every 3 years.
A longitudinal investigation of severe OCD patients reported a very limited response to conventional treatment after the first 5 years.[14] As the indications for DBS include limited response after at least 5 years of conventional treatment, we assumed that the treatment as usual (TAU) group would maintain TROCD status without clinical improvement, even with continuous conventional treatment. We also assumed that patients who responded to DBS in year 1 would remain in the response group during the following 10 years unless they died. Similarly, patients who did not respond to DBS in year 1 remained in the no-response group during the following 10 years unless they died. We applied the model to populations in Korea and the UK to enhance the validity of the models, as these countries have different national health insurance systems that provide health care services with relatively uniform quality and cost, respectively.
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital. Informed consent was waived by the IRB because this study involved no more than minimal risk to the participants, and could not practicably be carried out without the waiver.
2.2. Transition probabilities
The response rate to DBS was assumed to be 60% (95% CI: 49.0%–69.0%) in OCD patients, based on a previous meta-analysis.[7] The risk of complications was reflected in year 1 and every 3 years after DBS implantation (battery change years). As no papers have reported on the DBS complication rate in Korea, the complication rate used for the Korean model was obtained from the previous study that most comprehensively reviewed the complication rates from DBS in the United States.[15] In the UK model, we used the complication rate in a paper that analyzed cost-effectiveness of DBS for Parkinson disease in the UK.[16] The complication rates after initial DBS implantation were 0.194 in Korea and 0.282 in the UK. CNS-related complications (e.g., seizure, subdural hematoma) were accounted for in the DBS implantation procedure only, not in battery changes.
OCD patients were reported to have a 2.14 (95% CI: 1.76–2.57) times increased mortality when compared with the general population.[12] However, the effect of OCD severity on mortality was not investigated. We arbitrarily postulated that TROCD patients may have a similar mortality risk than patients with average severity. Therefore, we applied a mortality rate 2.14 times higher than that of the general population (Korea and the UK, respectively) to the DBS no-response group and the TROCD group, and the mortality rate of the general Korean and UK population to the DBS response group. The age-specific mortality data in Korea were obtained from the 2014 statistical database of the Korean Statistical Information Service,[17] and the mortality data for the UK were obtained from the 2010 to 2012 UK office for national statistics report.[17]
2.3. Costs
2.3.1. Korea
This analysis accounted for the following direct medical costs from the perspective of the Korean third-party payer (national health insurance system): the costs of DBS devices, the cost of surgery and admission for DBS (implantation or battery change), the cost of preoperation evaluation, and the cost of outpatient visits and medications (Table 1). The cost included both out-of-pocket burden for patients and the subsidiary from the Korean national health insurance system.
Table 1.
Base case inputs (Korea).
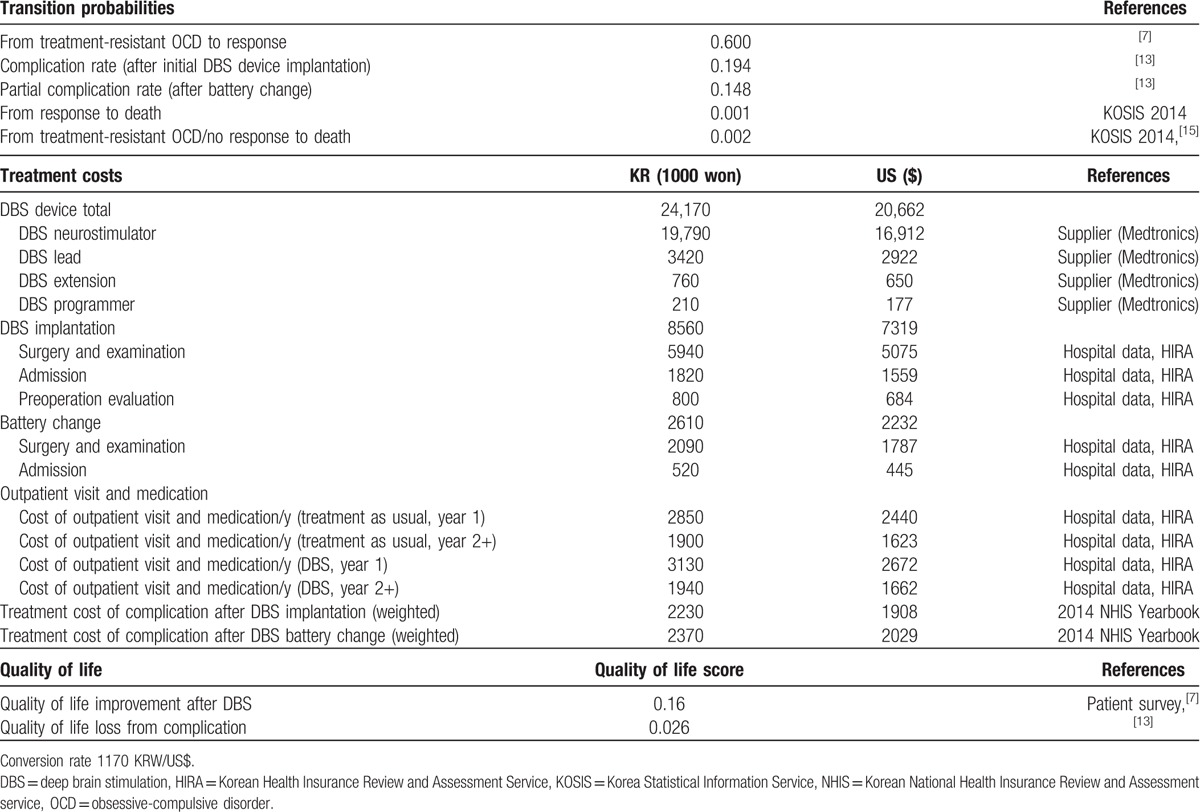
Unit cost was obtained from the Korea Health Insurance Review and Assessment Service (HIRA), billing data from Seoul National University Hospital and the DBS device supplier (Medtronics).
We calculated the weighted average cost of complications based on the specific medical expenses for the disease category from the 2014 National Health Insurance Statistical Yearbook published by the Korea National Health Insurance Service and HIRA.[18] As the complications related to DBS implantation or battery change are transient,[7] the cost of complication was adapted to a 1-year time frame. All costs were discounted by 5% each year in accordance with the Korean HIRA guidelines. The cost inputs and results are all converted in US$ with currency exchange rate of 1170 KRW/US$.
2.3.2. The UK
The cost data included in the UK model were obtained through a literature review. For device-related costs and complication-related costs, we applied the values from a DBS cost-effectiveness study with Parkinson disease.[16] The unit costs for drug and outpatient visits were obtained from a health technology assessment report published by the UK National Institute for Health Research in 2016.[19] The authors of the UK national institute study collected pharmaceutical cost data from British National Formulary estimates as well as outpatient clinic costs from the Personal Social Services Research Unit. However, there was year gap with cost year used in the Eggington et al and the cost year used in the UK National Institute study (2013). Therefore, cost uplifts were applied to inflate to current cost values. As in the Korean model, all costs were discounted by 5% each year. The cost inputs and results are all converted in US$ with currency exchange rate of 0.811 UKP/US$.
2.4. QoL
The EuroQol five-dimension questionnaire (EQ-5D) has been widely used in cost-effectiveness analyses to measure QoL.[20,21] Although a meta-analysis reported YBOCS score reduction by DBS in TROCD patients,[7] no studies have evaluated the changes in EQ-5D-based QoL scores due to DBS in TROCD patients in Korea or in the UK. Therefore, to calculate the QoL changes after DBS, we needed to determine the correlation between YBOCS score and QoL score. A direct patient survey was thus performed to measure the EQ-5D-based QoL score of OCD patients. We recruited 73 OCD patients who visited the Seoul National University Hospital OCD clinic and measured the QoL score of each patient using questionnaires in the EQ-5D-5L developed by the EuroQol group.[19,22] Based on these data, regression analysis was performed between the YBOCS score and QoL score. The analyses showed that the QoL score decreased by 0.0107 (95% CI: −0.0150 to −0.0063) for each unit increase in YBOCS score (P <.0001) (The findings from the survey are being prepared for publication as a separate study.).
According to the meta-analysis, the mean YBOCS score of the TROCD patients before DBS was 33.2, and the mean YBOCS decreased by 45.1% to 18.2 in the treatment response group.[7] When the results of the regression analysis were applied to these YBOCS scores, the QoL score improvement in DBS treatment was calculated to be 0.160 (95% CI: 0.001–0.224) (Tables 1 and 2). As the complications related to DBS are transient,[7] we reduced the QoL score improvement by 0.026 during the intervention year for patients with complications.[15]
Table 2.
Base case inputs (the UK).
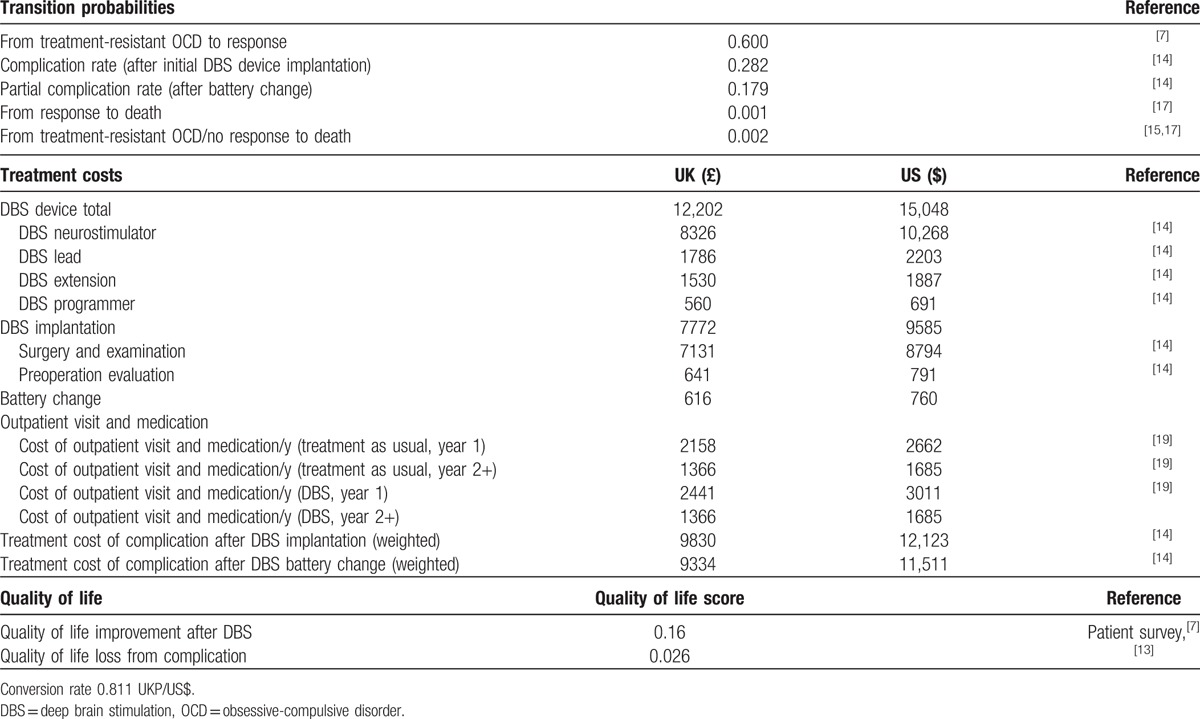
3. Results
3.1. Base case analysis
The base case inputs are described in Tables 1 and 2, including transition probabilities, treatment costs, and QoL. The DBS device cost, which accounted for the largest portion of the cost, was US$20,662 in Korea and US$15,048 in the UK. The costs of the initial DBS implantation and of battery changes, which occurred every 3 years, were US$7319 and US$2232 in Korea and US$9585 and US$760 in the UK, respectively. The costs of outpatient visits and medications were in the range of US$1623 to US$2672 per year in Korea and US$1685 to 3011 in the UK.
Table 3 provides the results of the base case cost-effectiveness analysis. Considering the 5% discount, in Korea, the 10-year costs of TAU and DBS for a TROCD patient were US$10,447 and US$44,672, respectively. The total QALY gain for TAU and DBS was 5.53 and 6.45 QALYs, respectively. Therefore, the incremental cost-effectiveness ratio (ICER) of DBS was US$37,865 per QALY.
Table 3.
Base case cost-effectiveness analysis of deep brain stimulation for treatment-resistance obsessive-compulsive disorder in Korea and in the UK.
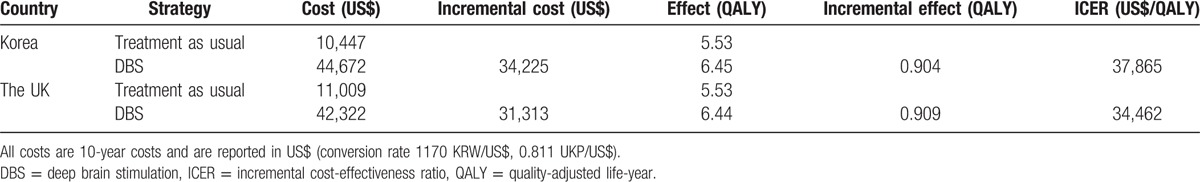
In the UK, the 10-year costs of TAU and DBS for TROCD patients were US$11,009 and US$42,322, respectively. The total QALY gain for TAU and DBS was 5.53 and 6.44 QALYs, respectively. The ICER of DBS was US$34,462 per QALY in the UK.
3.2. Sensitivity analysis
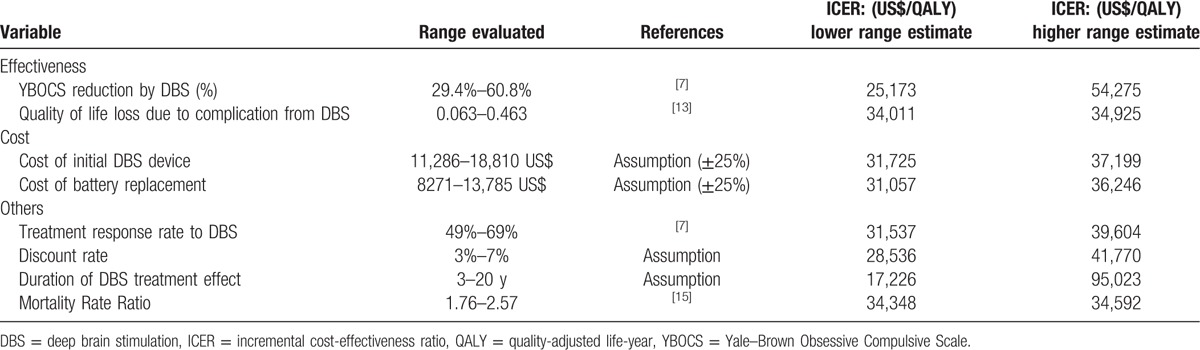
One-way sensitivity analyses were performed for the key variables in the model across the ranges specified in Tables 4 and 5. The costs of the DBS device and neurostimulator replacement varied by ±25%. The discount rate varied between 3% and 7%. The percentage of reduction in YBOCS score by DBS, the treatment response rate, QoL loss related to complications from DBS and the mortality rate ratio of OCD patients varied by the 95% CIs obtained in the literature search.[7,12,15]
Table 4.
One-way sensitivity analyses (Korea).
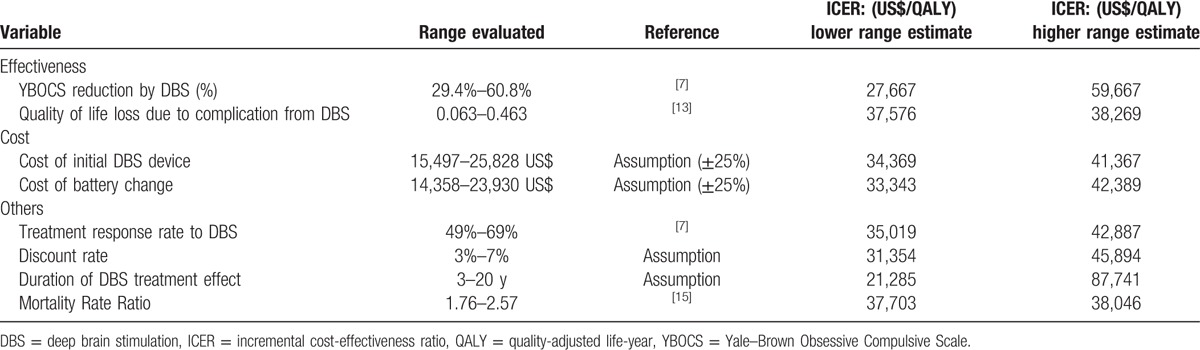
Table 5.
One-way sensitivity analyses (the UK).
3.2.1. Korea
For most variables except for duration of DBS treatment effect, the ICER remained under US$59,667 per QALY (Table 4), which is considered cost-effective by World Health Organization's (WHO) criteria (<3× gross domestic product [GDP] per capita, US$81,669 per capita). Of the variables investigated, the ICER was the most sensitive to the duration of DBS treatment effect. The ICER ranged from US$218,124 per QALY for the first year to US$61,395 per QALY at 5 years and US$37,865 per QALY at 10 years. DBS for TROCD can be considered cost-effective in Korea, assuming the treatment effect lasts 4 years or more (Fig. 2).
Figure 2.
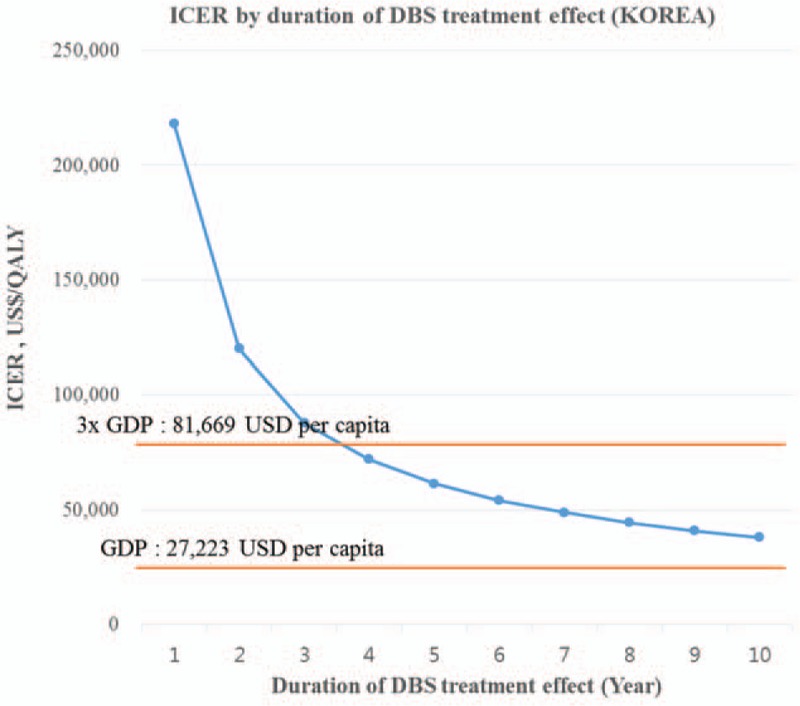
Univariate sensitivity analysis for duration of DBS treatment effect (Korea). DBS = deep brain stimulation, ICER = incremental cost-effectiveness ratio, QALY = quality-adjusted life-year.
3.2.2. The UK
For most variables except for percentage of YBOCS reduction by DBS and duration of DBS treatment effect, the ICER remained under US$41,770 per QALY, which is considered highly cost-effective (<GDP per capita, US$43,876 per capita). As in the Korean model, the UK model results were the most sensitive to the duration of DBS treatment effect. The ICER ranged from US$279,280 per QALY for the first year to US$62,110 per QALY at 5 years and US$34,462 per QALY at 10 years after surgery. DBS for TROCD can be considered cost-effective in the UK (<3× GDP per capita, US$131,628 per capita) if the treatment effect lasts 3 years or more (Fig. 3). The reduction in YBOCS by DBS in the response group was a key driver of the ICER in the UK model. The ICER ranged from US$25,173 per QALY to US$54,275 per QALY within the confidence interval of the YBOCS reduction by DBS (29.4%–60.8%).[7] However, DBS still remained cost-effective (<3× GDP per capita), while the rate of YBOCS reduction varies.
Figure 3.
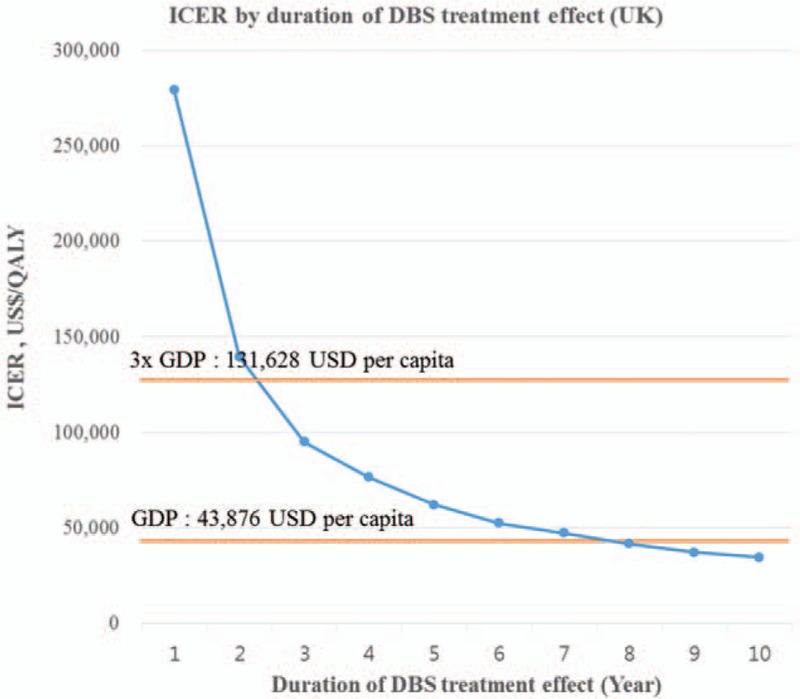
Univariate sensitivity analysis for duration of DBS treatment effect (UK). DBS = deep brain stimulation, ICER = incremental cost-effectiveness ratio, QALY = quality-adjusted life-year.
4. Discussion
To our knowledge, this is the first study investigating the cost-effectiveness of DBS in TROCD patients through Markov model in 2 countries. Our analysis showed an ICER of US$37,865 per QALY in Korea and US$34,462 per QALY in the UK. According to the cost-effectiveness criteria outlined by the WHO, DBS in Korea is a “cost-effective” and DBS in the UK a “highly cost-effective” treatment option for TROCD patients. In this study, sensitivity analyses showed consistent cost-effectiveness results for most variables with the exception of short-term duration of treatment effect (<4 years in Korea; <3 years in the UK).
WHO uses the GDP per capita as a reference indicator of cost-effectiveness. Highly cost-effective interventions are those with a cost per QALY that is less than the GDP per capita; cost-effective interventions range between 1 and 3 times the GDP per capita; and cost-ineffective interventions are those that are >3 times the GDP per capita.[23] As Korea's GDP per capita in 2015 was US$27,223 and the UK's GDP per capita in 2015 was US$43,876,[24] the ICER of US$37,865 per QALY in Korea was cost-effective and the ICER of US$34,462 per QALY in the UK was a highly cost-effective intervention. However, other international standards have been used to determine cost-effectiveness. For example, US$50,000 per QALY gained is generally used as a conventional threshold for assessing the cost-effectiveness of an intervention.[25] According to this criterion, DBS for TROCD patient is a cost-effective treatment option for the 2 countries assessed. The National Institute for Health and Clinical Excellence (NICE) in the UK uses more stringent criteria, applying a cost-effectiveness threshold ranging between 20,000 UKP and 30,000 UKP.[26] According to the NICE criterion, the cost-effectiveness of US$34,462 per QALY (25,125 UKP per QALY) for DBS in the UK was in the cost-effectiveness range. Taken together, the results of this study indicate that DBS is a cost-effective option for TROCD based on all available criteria.
The sensitivity analysis showed that the duration of treatment effectiveness of DBS was a key factor in determining cost-effectiveness. As the majority of equipment costs occur in year 1; the longer the DBS treatment effect lasted, the higher the cost-effectiveness of DBS was in this study. The WHO's threshold for highly cost-effective interventions (<GDP per capita) was reached at year 9 in the UK but was not attained in Korea within the 10-year horizon. In addition, using US$50,000 per QALY gained as the threshold for cost-effectiveness, the treatment effect had to last at least 7 years to consider DBS cost-effective in Korea and the UK.
There is only one cost-effectiveness study of DBS for TROCD.[10] Unlike our study based on Markov model in Korea and in the UK, this study is a 2-year prospective, open cost-effectiveness study in the Netherlands. The base case outcome of this study (141,446 EUR per QALY) is much higher than our result mainly due to shorter time horizon (2 vs 10 years). However, comparable ICER could be achieved by assuming the same 2-year time horizon in our model (109,855 EUR per QALY in Korea, 127,636 EUR per QALY in the UK). Several studies have analyzed the cost-effectiveness of DBS for late-stage Parkinson disease. A recent systematic review reported an average incremental cost utility ratio of US$41,932 per additional QALY gained[27] for DBS for a patient with Parkinson disease. The ICERs of this study (US$37,865 per QALY in Korea, US$34,462 per QALY in the UK) are akin to the ICER of DBS for patients with late-stage Parkinson disease. There are several differences between DBS for Parkinson disease and OCD to consider. First, the battery change interval would be shorter in OCD, as the higher energy demand is expected to cause faster battery depletion in OCD (average 100–130 Hz)[7] than in Parkinson disease (average 15–60 Hz).[28] The shorter battery change interval lead lower cost-effectiveness in OCD. However, longer time frame used in OCD than Parkinson disease (10 vs 3–5 years) primarily contributes to the higher cost-effectiveness, and makes ICER of DBS for OCD similar to ICER of Parkinson disease. The shorter time frame in Parkinson disease is possibly due to the older age of Parkinson disease patients and the rapidly progressing nature of late-stage Parkinson disease. Further studies are needed to more directly compare the cost-effectiveness of DBS in patients with Parkinson disease and with TROCD.
In this study, the ICER of DBS in Korea (US$37,865 per QALY) was approximately 10% higher than that in the UK (US$34,462 per QALY). The main factor differentiating the 2 countries were the UK's lower DBS neurostimulator cost, which occurred at year 1 and every 3 years for battery replacement. The DBS neurostimulator cost US$16,912 in Korea but US$10,268 in the UK, representing an approximately 40% cheaper price in the UK. This cost gap is due to the different DBS device models introduced in each country. The model introduced in Korea requires 2 neurostimulators for each patient; however, the UK model has dual channels and thus requires one neurostimulator per patient. In addition, rechargeable DBS devices have recently been developed, and the cost-effectiveness of DBS would likely be more robust with this device, as the cost of DBS maintenance would decrease with broader use of this rechargeable model.[29]
5. Limitations
This study has several limitations that should be considered. We adopted the complication rates and data on complication-related QoL loss from previous studies.[15,16] Although these studies extensively reviewed the complications of DBS, the majority of the data were obtained from studies related to DBS for Parkinson disease. Parkinson disease patients tend to be older and could have more complications than OCD patients. Therefore, we may have overestimated the impact of DBS-related complications by applying the results from studies in which DBS was used for Parkinson disease.
Second, our assumptions regarding the no-response group were conservative. Although the YBOCS improvement in the no-response group was not clinically significant (<35%), these patients may have experienced considerable improvement in QoL. Our assumption that there was no QoL improvement in the no-response group may have led to underestimations of the overall impact of DBS on QoL improvement.
Lastly, we assumed that the response or no-response group was determined according to the treatment response in year 1 and lasted for 10 years. Although limited data show that treatment response status could change after year 1,[11] there was insufficient data on the time-course of treatment response over 10-year time horizon. Therefore, we used the criteria of 1-year, as a conservative assumption. Future studies that directly measure changes in symptoms and QALY after DBS over longer time period will more precisely incorporate the QALY improvement generated by DBS for both the response and no-response groups.
6. Conclusions
In summary, this study indicates that DBS could be considered a cost-effective treatment option for TROCD patients in Korea and the UK. To meet the cost-effectiveness criteria suggested by the WHO, the effects of DBS need to last >4 years in Korea and 3 years in the UK. As the first paper analyzing the cost-effectiveness of DBS in patients with TROCD through Markov model in 2 different countries, this paper provides a foundation for evaluating the applicability of DBS not only for patients and health care service providers but also for private and public payers.
Footnotes
Abbreviations: ACC = anterior cingulate cortex, CBT = cognitive behavioral therapy, DBS = deep brain stimulation, GDP = gross domestic product, HIRA = Korean Health Insurance Review and Assessment Service, ICER = incremental cost-effectiveness ratio, OCD = obsessive-compulsive disorder, OFC = orbitofrontal cortex, QALY = quality-adjusted life-year, QoL = quality of life, TAU = treatment as usual, TROCD = treatment-resistant obsessive-compulsive disorder, UK = the United Kingdom, WHO = World Health Organization, YBOCS = Yale–Brown Obsessive Compulsive Scale.
SNK and SP equally contributed to this manuscript.
This study was supported by a grant from the Korean Mental Health Technology R&D Project, Ministry of Health & Welfare in Republic of Korea (HM15C1233). The statistical analyses were supported by the medical research collaborating center (MRCC) of Seoul National University Hospital.
The authors have no conflicts of interest to disclose.
References
- [1].Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010;15:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Subramaniam M, Soh P, Vaingankar JA, et al. Quality of life in obsessive-compulsive disorder: impact of the disorder and of treatment. CNS Drugs 2013;27:367–83. [DOI] [PubMed] [Google Scholar]
- [3].Denys D. Pharmacotherapy of obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Psychiatr Clin North Am 2006;29:553–84. xi. [DOI] [PubMed] [Google Scholar]
- [4].Naesström M, Blomstedt P, Bodlund O. A systematic review of psychiatric indications for deep brain stimulation, with focus on major depressive and obsessive-compulsive disorder. Nordic J Psychiatry 2016;70:483–91. [DOI] [PubMed] [Google Scholar]
- [5].FDA. Humanitarian Device Exemption (HDE): deep brain stimulator for OCD, US Food and Drug Administration; 2009. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=H050003. Accessed March 30, 2017. [Google Scholar]
- [6].Haynes WIA, Mallet L. High-frequency stimulation of deep brain structures in obsessive-compulsive disorder: the search for a valid circuit. Eur J Neurosci 2010;32:1118–27. [DOI] [PubMed] [Google Scholar]
- [7].Alonso P, Cuadras D, Gabriëls L, et al. Deep brain stimulation for obsessive-compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLoS One 2015;10:e0133591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huff W, Lenartz D, Schormann M, et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: outcomes after one year. Clin Neurol Neurosurg 2010;112:137–43. [DOI] [PubMed] [Google Scholar]
- [9].Ooms P, Mantione M, Figee M, et al. Deep brain stimulation for obsessive-compulsive disorders: long-term analysis of quality of life. J Neurol Neurosurg Psychiatry 2014;85:153–8. [DOI] [PubMed] [Google Scholar]
- [10].Ooms P, Blankers M, Figee M, et al. Cost-effectiveness of deep brain stimulation versus treatment as usual for obsessive-compulsive disorder. Brain Stimul 2017;Online first. [DOI] [PubMed] [Google Scholar]
- [11].Luyten L, Hendrickx S, Raymaekers S, et al. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry 2016;21:1272–80. [DOI] [PubMed] [Google Scholar]
- [12].Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry 2016;73:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].NICE (National Institute for Health and Care Excellence). NICE glossary; 2017. Available at: https://www.nice.org.uk/glossary?letter=q. Accessed June 2, 2017. [Google Scholar]
- [14].Garnaat SL, Boisseau CL, Yip A, et al. Predicting course of illness in patients with severe obsessive-compulsive disorder. J Clin Psychiatry 2015;76:e1605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pisapia JM, Halpern CH, Williams NN, et al. Deep brain stimulation compared with bariatric surgery for the treatment of morbid obesity: a decision analysis study. Neurosurg Focus 2010;29:E15. [DOI] [PubMed] [Google Scholar]
- [16].Eggington S, Valldeoriola F, Chaudhuri KR, et al. The cost-effectiveness of deep brain stimulation in combination with best medical therapy, versus best medical therapy alone, in advanced Parkinson's disease. J Neurol 2014;261:106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].KOSIS (Korean Staistical Information Services). Mortality rate and number of death by gender, age, and cause of death in Korea; 2015. Available at: http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B34E01&conn_path=I3. Accessed April 6, 2017. [Google Scholar]
- [18].Sung SC, Son MS. 2014 National health insurance statistical yearbook. Korea National Health Insurance Service, Korea Health Insurance Review & Assessment Service; 2015. [Google Scholar]
- [19].Skapinakis P, Caldwell D, Hollingworth W, et al. A systematic review of the clinical effectiveness and cost-effectiveness of pharmacological and psychological interventions for the management of obsessive-compulsive disorder in children/adolescents and adults. Health Technol Assess 2016;20:1–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Andersson E, Ljótsson B, Hedman E, et al. Internet-based cognitive behavior therapy for obsessive compulsive disorder: a pilot study. BMC Psychiatry 2011;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Subramaniam M, Abdin E, Vaingankar JA, et al. Impact of psychiatric disorders and chronic physical conditions on health-related quality of life: Singapore Mental Health Study. J Affect Disord 2013;147:325–30. [DOI] [PubMed] [Google Scholar]
- [22].Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].WHO (World Health Organization). Choosing interventions that are cost-effective; 2016. Available at: http://www.who.int/choice/en/. Accessed May 1, 2016. [Google Scholar]
- [24].The World Bank. GDP per capita (current US$) data. World Bank national accounts data; 2017. Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD?year_high_desc=true. Accessed March 30, 2017. [Google Scholar]
- [25].Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res 2008;8:165–78. [DOI] [PubMed] [Google Scholar]
- [26].McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733–44. [DOI] [PubMed] [Google Scholar]
- [27].Becerra JE, Zorro O, Ruiz-Gaviria R, et al. Economic analysis of deep brain stimulation in parkinson disease: systematic review of the literature. World Neurosurg 2016;93:44–9. [DOI] [PubMed] [Google Scholar]
- [28].Golestanirad L, Elahi B, Graham SJ, et al. Efficacy and safety of Pedunculopontine Nuclei (PPN) deep brain stimulation in the treatment of gait disorders: a meta-analysis of clinical studies. Can J Neurol Sci 2016;43:120–6. [DOI] [PubMed] [Google Scholar]
- [29].Perez J, Gonzalez V, Cif L, et al. Rechargeable or nonrechargeable deep brain stimulation in dystonia: a cost analysis. Neuromodulation 2017;20:243–7. [DOI] [PubMed] [Google Scholar]


