Abstract
Paclitaxel is a medicinal ingredient with high anticancer activity and widely used in hospitals and clinics. In this study, we isolate endophytic fungi efficiently producing paclitaxel from yew for the purpose of paclitaxel manufacture.
The bark of Taxus wallichiana var. mairei was surface sterilized and then inoculated in potato dextrose agar culture medium to isolate endophytic fungi. The paclitaxel in the fungal culture was extracted with mixture of chloroform and the same amount of methanol. The content of paclitaxel in the extract was determined and identified with LC-MS. The endophytic fungus efficiently producing paclitaxel was species identified with ITS rDNA and 26S D1/D2 rDNA sequencing.
There were 528 endophytic fungal strains were isolated from the bark of T wallichiana var. mairei in total. There was only a strain efficiently producing paclitaxel in these endophytic fungi. The unique strain was identified as Phoma medicaginis. The paclitaxel contents in whole potato dextrose broth (PDB) culture, spent culture medium from this strain and that in dry mycelium is 1.215 mg/L, 0.936 mg/L, and 20 mg/kg, respectively.
An endophytic fungus efficiently producing paclitaxel was isolated from T wallichiana var. mairei. This isolated endophytic fungus can be used as a producing strain for paclitaxel manufacture.
Keywords: endophytic fungus, paclitaxel, Phoma medicaginis, Taxus wallichiana var. mairei
1. Introduction
Paclitaxel was a kind of chemical component found in yew at first. The high anticancer activity a of paclitaxel is widely accepted and used in hospitals and clinics.[1,2] The content of paclitaxel in yew was very low.[3] Lots of yew have been seriously damaged even destroyed to extract paclitaxel in recent years. Some endophytic fungi producing paclitaxel have been found in yew.[4–12] But, these paclitaxel productivities in endophytic fungi are so low that they cannot be useful for industrialization until now. We have isolated about 528 endophytic fungal strains from T wallichiana var. mairei distributing in Taihang Mountain in Henan Province of China over the past 2 years to find endophytic fungus efficiently producing paclitaxel. A strain that can efficiently produce paclitaxel was found among these isolated endophytic fungal strains. The paclitaxel extracted from the unique strain was authenticated with LC-MS. This discovery is very important for paclitaxel manufacture and protection of yew resources.
2. Materials and methods
2.1. Ethics statement
This study did not involve any human or animal. The ethical approval is not necessary.
2.2. Materials
Materials: The live yew branches were collected from branches of T wallichiana var. mairei (Lemée et H. Lév.) L. K. Fu et Nan Li growing in the Taihang Mountain, Henan Province, China in March 2014. The branches were 4 to 10 cm in diameter.
Reagents: Standard paclitaxel (99.5%) purchased from Sigma Company (St. Louis, MO). Ethanol (AR). Methanol (AR). Acetonitrile (HPLC grade). Potato dextrose agar (PDA) culture medium. Potato dextrose broth culture medium (PDB).
Instrument: HPLC instrument (Shimadzu GC-2010). HPLC column (Shimadzu C18 reverse phase column, 5 μm, 250 mm × 4.6 mm). LCQ Advantage LC-MS. Ultrasonator. Rotary evaporator (RE-52AA). Super-clean bench (SJ-CJ-2FQ). Shake culture box (ZDX-150).
2.3. Methods
2.3.1. Isolate and purificate endophytic fungus
The live barks were cut from yew branch with blade and their withered outer layer were removed with blade. The bark was immersed and shocked in 75% (v/v) ethanol for 2 minutes, 0.1% mercuric chloride solution for 8 minutes sequentially to surface sterilize. The surface sterilized bark was immersed and shocked in sterilized water for 1 minute in each of 3 times to wash the disinfectant. Then the surface sterilized bark were cut into pieces in approximately 4 cm × 4 cm with flame-sterilized blade. These pieces of bark were inoculated in PDA culture medium in petri dish for culture at 20 to 25°C for 4 to 5 days. The uninoculated PDA culture medium were taken as control. The hyphal tips of endophytic fungus growing out from the bark were islolated with blade and cultured on PDA plates to purificate. All these experiments were done in the super-clean bench.
2.3.2. Screen and determine endophytic fungi efficiently producing paclitaxel
Submerged ferment endophytic fungi: The hypha with PDA pieces (approximately 4 mm × 4 mm) were inoculated into 60 mL PDB culture medium in culture flask for incubation at 120 rpm and 20 to 25°C for 4 days. Five milliliters of liquid culture medium containing hyphae fung was inoculated into 60 mL PDB culture medium in culture flask and cultured at 120 rpm and 20 to 25oC for 8 days. The uninoculated PDB culture medium in culture flask was taken as control.
Prepare extract: Liquid culture medium containing hyphae were filtered with filter paper at first. Then the hyphae and the fungal culture medium were extracted respectively. The hyphae were dried at 45°C and ground with quartz sand. The ground hyphae were immersed in 30 mL mixture of chloroform and methanol (1:1, v/v) in an ultrasonic bath and ultrasonic shocked for 30 minutes. The residue was extracted once again after the extract was filtered with filter paper. The combined filtrate of 2 extractions was evaporated in rotary vacuum evaporator in reduced pressure at 40°C. The residue in the evaporator was dissolved in 30 mL chloroform and then mixed with 30 mL water. The organic phase of mixture was collected and evaporated in rotary vacuum evaporator at 40°C. The residue of evaporation was dissolved in 5 mL methanol and filtered with 0.22 μm filter. The filtrate was taken as hyphae extract. Filtered spent culture medium was evaporated in the rotary vacuum evaporator in reduced pressure at 70°C. The residue in the evaporator was extracted with the same method of ground hyphae extraction. The extract solution of filtered spent culture medium was taken as spent culture medium extract. Liquid culture medium containing hyphae from other culture flasks was filtered. The filtered spent culture medium was evaporated in rotary vacuum evaporator in reduced pressure at 70°C. The residue in the evaporator was mixed with the ground hyphae (filter residue of liquid culture medium containing hyphae). The mixture was extracted with the same method of ground hyphae extraction. The extract solution of liquid culture medium containing hyphae was taken as whole culture extract.
Determine paclitaxel: Determination of paclitaxel in extract was done with HPLC. Ten-microliter extract was injected into HPLC column. The column temperature was set as 35°C. The wavelength for determination in recorder was set at 228 nm. The mobile phase consisted of acetonitrile and water. The content of acetonitrile in the mobile phase gradiently varied along with time, namely from 27% (v/v) to 30% in 0 to 15 minutes, from 30% to 37% in 15 to 30 minutes, from 37% to 42% in 30 to 40 minutes, from 42% to 47% in 40 to 60 minutes, and from 47% to 48% in 60–72 minutes. The flow rate of mobile phase was 0.8 mL/minute. 0.0005, 0.001, 0.004, 0.01, 0.02, and 0.05 mg/mL standard paclitaxel solutions were respectively prepared. All these standard paclitaxel solutions were analyzed with the above HPLC method. The standard curve was analyzed according to chromatography peak areas of standard paclitaxel and their content with SPSS (Statistical Product and Service Solutions) (Chicago). Paclitaxel in all extracts were determined with the same HPLC method described above. The contents of paclitaxel in all extracts were analyzed according to their chromatography peak areas and the standard curve.
Spectroscopic analysis of extracts: The specific endophytic fungus producing paclitaxel determined with HPLC was cultured in PDB culture medium repeatedly. The whole culture extract of these fungal cultures was prepared and determined with HPLC. The paclitaxel sample in extract was collected from HPLC column during the retention time (from 67.7 to 68.7 minutes). The fungal paclitaxel sample and standard paclitaxel were analyzed with electrospray ionization-tandem mass spectrometry (ESI-MS/MS). ESI was applied as ion source scanning with negative ion mode. The temperature of the capillary cone was 350°C. The spray voltage was 5.0 kV. N2 was taken as atomization gas and auxiliary gas in 0.2 μL/minute spray flow.
2.3.3. Identification of endophytic fungi
The endophytic fungus producing paclitaxel was identified by Taihegene Biotechnology Co Ltd (Beijing, China) with the similarity between the 26S rDNA D1/D2 sequence (and ITS1/ITS2 rDNA sequence) of endophytic fungus and that of known species in gene pool.
3. Results
About 528 endophytic fungal strains were isolated and purified from T wallichiana var. mairei. All of the extracts from the cultures of these strains were determined with HPLC. The retention time of a chromatography peak in the chromatograms of whole culture extract, hyphael extract, and spent culture medium extract prepared from an endophytic fungus (No. 218) was identical with that of standard paclitaxel (Fig. 1). Peak was not found in that retention time or arounding in the chromatograms of extracts from any other strains and the blank culture sample. The paclitaxel sample collected from the extract of strain No. 218 and standard paclitaxel were analyzed with electrospray ionization-tandem mass spectrometry (ESI-MS/MS). Some moleculars or ions, which possessed same molecular weight as that of moleculars or ions in the mass spectrum of standard paclitaxel, were found in the mass spectrum of the collected paclitaxel sample (Fig. 2). The mass spectrometry analysis of the collected paclitaxel sample verified that there was paclitaxel in the culture of strain No. 218. The contents of paclitaxel in extracts of whole culture and spent culture medium of strain No. 218 were 0.0146 and 0.0112 mg/mL, respectively, according to the standard curve (Table 1). Therefore, the paclitaxel content in whole PDB culture and that in spent culture medium from strain No. 218 is 1.215 and 0.936 mg/L, respectively. The paclitaxel content in dry mycelium is 20 mg/kg.
Figure 1.
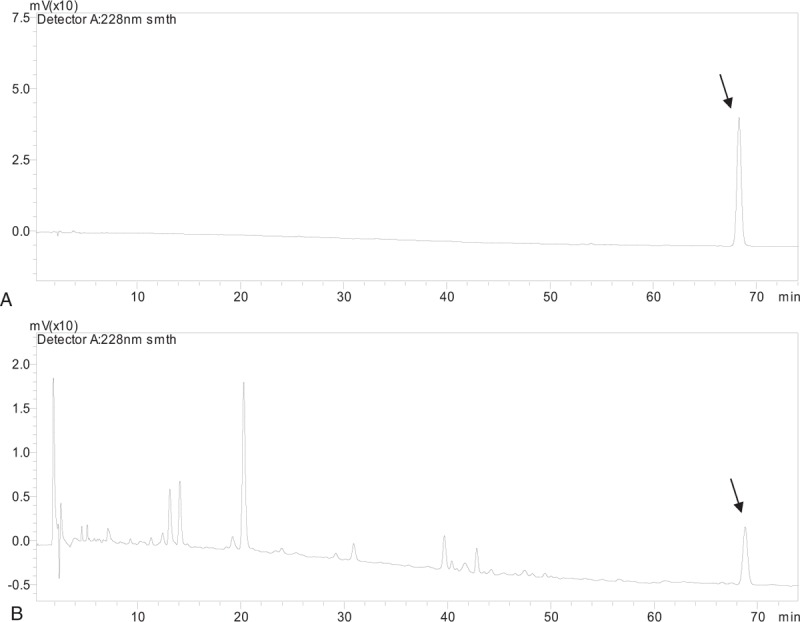
HPLC of paclitaxel standard (A) and paclitaxel in extraction from mixture of mycelia and spent culture medium (B).
Figure 2.
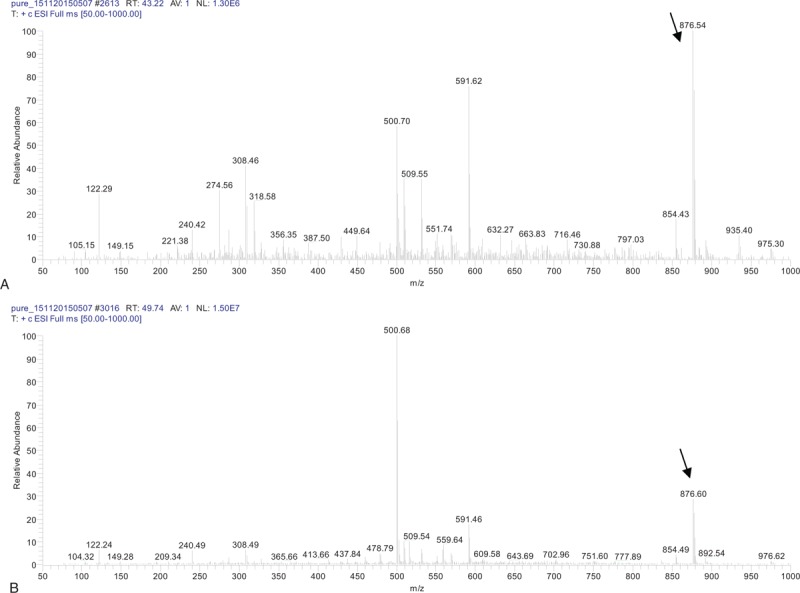
MS spectra of paclitaxel standard (A) and paclitaxel in extraction from mixture of mycelia and spent culture medium (B).
Table 1.
Standard curve and paclitaxel contents in extracts and culture.

The similarity between 26S D1/D2 rDNA sequence (accession number: KT906672) of strain No. 218 and that of Phoma medicaginis was 100%, higher than that between it and any other genus. The similarity between the ITS1/ITS2 rDNA sequence (accession number: KT906673) of strain No. 218 and that of P medicaginis was 99%, also higher than that between it and any other species. Therefore, strain No. 218 was identified as P medicaginis. This strain grew well on PDA at 20 to 24oC with thick white hypha.
4. Discussion
The isolation of an endophytic fungus producing paclitaxel is widely studied and possesses significant to paclitaxel manufacture and resource protection of taxus. Researchers have isolated more than 100 strains producing taxol in more than 20 genera that until now.[3,13,14] Although there are much of reports on the isolation of endophytic fungi producing paclitaxel, but all of the contents of paclitaxel in the reported isolates were too low to be used in immediate commercialization.[3]
Phoma is a group that produce abundant secondary metabolites even antibiotic. Some strains of P medicaginis or other species in Phoma producing antibiotic even anticancer agents have been reported previously.[15–21] The P medicaginis was also found as endophytic fungi in Taxus globosa.[22] Although the contents of the anticancer agent in the strain of P medicaginis previously reported were also very low,[21] but these results show that Phoma is a very valuable genera that can be used in bioengineering to produce medicine.
There are lots of endophytic fungi in yew. But very seldom strain produce paclitaxel.[23] We have reported that there was only an endophytic fungus producing paclitaxel in very low efficiency in the 435 fungal strains isolated in our last study.[24] In our 528 endophytic fungal strains isolated in this study, only one strain efficiently produces paclitaxel. This shows that there is diversity in endophytic fungi of yew, but the endophytic fungus efficiently producing paclitaxel is very rare. This endophytic fungus strain producing efficiently paclitaxel isolated from T wallichiana var. mairei in the Taihang Mountain in China was identified as P medicaginis according to similarity between the 26S rDNA D1/D2 sequence (and ITS sequence) of endophytic fungus and that of known species in gene pool. The paclitaxel yield of this strain possesses potentiality for paclitaxel manufacture.
The contents of paclitaxel in whole culture medium, hyphae, and spent culture medium of this strain were determined, respectively. The content of paclitaxel in spent culture medium is close to that in whole culture. This phenomenon indicate that most paclitaxel synthesized in hypha is secreted into culture medium.
5. Conclusion
An endophytic fungus efficiently producing paclitaxel was isolated from T wallichiana var. mairei. This isolated endophytic fungus can be used as a producing strain for paclitaxel manufacture.
Footnotes
Abbreviations: HPLC = high performance liquid chromatography, PDA = Potato dextrose agar, PDB = potato dextrose broth.
Funding: This study was supported by Science & Technology Research Project of Henan Province and Education Department Key Project of Science & Technology Research in Henan Province.
The authors have no conflicts of interest to disclose.
References
- [1].Lasala JM, Stone GW, Dawkins KD, et al. An overview of the taxus; express, paclitaxel-eluting stent clinical trial program. J Interv Cardiol 2006;19:422–31. [DOI] [PubMed] [Google Scholar]
- [2].Nicholas HO, David JK. Camptothecin and taxol: historic achievements in natural products research. J Nat Prod 2004;67:129–35. [DOI] [PubMed] [Google Scholar]
- [3].Visalakchi S, Muthumary J. Taxol (anticancer drug) producing endophytic fungi an overview. Int J Pharma Bio Sci 2010;1:1–9. [Google Scholar]
- [4].Caruso M, Colombo AL, Crespi-Perellino N, et al. Studies on a strain of Kitasatospora sp. paclitaxel producer. Ann Microbiol 2000;50:89–102. [Google Scholar]
- [5].Caruso M, Colombo AL, Fedeli L, et al. Isolation of endophytic fungi and actinomycetes taxane producers. Ann Microbiol 2000;50:3–13. [Google Scholar]
- [6].Gangadevi V, Muthumary J. Isolation of Colletotrichum gloeosporioides, a novel endophytic taxol-producing fungus from the leaves of a medicinal plant, Justicia gendarussa. Mycol Balcanica 2008;5:1–4. [Google Scholar]
- [7].Jiayao L, Strobel G, Sidhu R, et al. Endophytic taxol-producing fungi from bald cypress, Taxodium distichurn. Microbiology 1996;142:2223–6. [DOI] [PubMed] [Google Scholar]
- [8].Kaihui L, Xiaowei D, Baiwan D, et al. Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis. J Ind Microbiol Biotechnol 2009;36:1171–7. [DOI] [PubMed] [Google Scholar]
- [9].Stierl A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993;260:214–6. [DOI] [PubMed] [Google Scholar]
- [10].Strobel G, Yang X, Sears J, et al. Taxol from Pestalotiopsis microspora, and endophytic fungus from Taxus wallachiana. Microbiology 1996;142:435–40. [DOI] [PubMed] [Google Scholar]
- [11].Tayung K, Jha DK, Deka DC. Isolation and identification of antimicrobial agent-producing bacterium from Taxus baccata rhizosphere antagonistic against clinically significant microbes. Indian J Microbiol 2007;47:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wiyakrutta S, Sriubolmas N, Panphut W, et al. Endophytic fungi with anti-microbial, anti-cancer and anti-malarial activities isolated from Thai medicinal plants. World J Microbiol Biotechnol 2004;20:265–72. [Google Scholar]
- [13].Xuanwei Z, Huifang Z, Lu L, et al. A review: recent advances and future prospects of taxol-producing endophytic fungi. Appl Microbiol Biotechnol 2010;86:1707–17. [DOI] [PubMed] [Google Scholar]
- [14].Flores Bustamante FZ, Rivera Orduna FN, Martinez Cádenas A, et al. Microbial paclitaxel: advances and perspectives. J Antibiot 2010;63:460–7. [DOI] [PubMed] [Google Scholar]
- [15].Nicoletti R, De Filippis A, Buommino E. Antagonistic aptitude and antiproliferative properties on tumor cells of fungal endophytes from the Astroni Nature Reserve, Italy. Afr J Microbiol Res 2013;7:4073–83. [Google Scholar]
- [16].Giusiano G, Rodolfi M, Mangiaterra M, et al. Endophytic fungi in medicinal plants of northeast of Argentina. I: Morphotaxonomic approach of their foliar community. Bol Micológ 2010;25:15–27. [Google Scholar]
- [17].Specian V, Orlandelli RC, Felber AC, et al. Secondary Metabolites Produced by Endophytic Fungi of Pharmaceutical Interest. UNOPAR Cient Ciênc Biol Saúde 2014;16:345–51. [Google Scholar]
- [18].Wijeratne EM, He H, Franzblau SG, et al. Phomapyrrolidones A–C, antitubercular alkaloids from the endophytic fungus phoma sp. NRRL 46751. Nat Prod 2013;76:1860–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Weber RW, Stenger E, Meffert A, et al. Brefeldin A production by Phoma medicaginis in dead pre-colonized plant tissue: a strategy for habitat conquest. Mycol Res 2004;108:662–71. [DOI] [PubMed] [Google Scholar]
- [20].Liermann JC, Kolshorn H, Opatz T, et al. Xanthepinone, an antimicrobial polyketide from a soil fungus closely related to Phoma medicaginis. J Nat Prod 2009;72:1905–7. [DOI] [PubMed] [Google Scholar]
- [21].Porras-Alfaro A, Bayman P. Hidden fungi, emergent properties: endophytes and microbiomes. Annu Rev Phytopathol 2011;49:291–315. [DOI] [PubMed] [Google Scholar]
- [22].Rivera-Orduna FN, Suarez-Sanchez RA, Flores-Bustamante ZR, et al. Diversity of endophytic fungi of Taxus globosa (Mexican yew). Fungal Divers 2011;47:65–74. [Google Scholar]
- [23].Yijian C, Zhuo Z, Yan W, et al. Screening endophytic fungus to produce taxol from Taxus yunnanensis. Biotechnology 2003;13:10–1. [Google Scholar]
- [24].Zaiyou J, Hongsheng W, Ning W, et al. Isolation and identification of an endophytic fungus producing paclitaxel from Taxus wallichiana var mairei. Nutr Hosp 2015;32:2932–7. [DOI] [PubMed] [Google Scholar]


