Abstract
Rationale:
We reviewed 76 published cases of Doege–Potter syndrome, and non-islet cell tumor hypoglycemia (NICTH) secondary to a solitary fibrous tumor (SFT) between 1989 and 2016, to study disease pathogenesis, diagnosis, and treatment of this rare paraneoplastic disease. Further, we report 1 new case of a patient presenting with Doege–Potter syndrome.
Patients concerns:
The tumors originated from the pleural cavity, lung, pelvis, liver, retroperitoneum, kidney, mediastinal, the sella, uterus, bladder, intestine, mandibular, and the thigh. The most common location was the pleural cavity (left 12 cases and right 28 cases). Moreover, 28/71 (39.4%) were benign and 43/71 (60.6%) were malignant. SFTs with NICTH were more likely to be malignant and present at a higher rate than previously published (5%–10.4%). The malignancy rate of extrathoracic SFTs was higher than that of thoracic SFTs, 20 (66.7%) as compared with 23 (56.1%). Age of onset varied from 24 to 85 years (mean 59 years), with 47 males and 28 females, and gender unavailable for 1 case. When comparing clinical characteristics of patients with benign as compared malignant tumors, no significant differences in the age of onset, gender, or size of tumor were seen. Among 15/19 cases, the insulin-like growth factor II (IGF-II)/IGF-I ration was >10.0. Complete tumor resection remained the only definitive treatment.
Outcomes and lessens:
Glucocorticoids dose-dependently reduce the frequency and severity of hypoglycemic episodes. Low doses of prednisone were ineffective at relieving hypoglycemia. The effect of neoadjuvant treatment, consisting of chemoradiation, and consecutive selective embolization of vessels feeding the tumor were not identified.
Keywords: case report, Doege–Potter syndrome, literature review
1. Introduction
In 1930, Doege and Potter independently described non-islet cell tumor hypoglycemia (NICTH). Solitary fibrous tumor (SFT) that is associated with NICTH is referred to as Doege–Potter syndrome. Due to the rare incidence of this condition, no systemic studies have been published. Independent work by Meng et al and Kalebi et al reviewed pleural SFTs with Doege–Potter syndrome and summarized the clinical characteristics, treatments, and outcomes of both benign and malignant tumors. Peak age of disease incidence is in the sixth to eighth decade of life. Meng et al found that the incidence of pleura SFT with Doege–Potter syndrome was similar between genders.[1] By contrast, Kalebi AY et al[2] found a slight male preponderance. Hitherto, no previously published review has focused on extrathoracic SFT with Doege–Potter syndrome.
de Groot et al[3] reviewed tumors that presented with NICTH and the associated disease pathogenesis, diagnosis and treatment of this rare paraneoplastic condition. The mechanism of NICTH, especially the physiological role and pathology of the insulin-like growth factor (IGF) system, was described and summarized in detail. Moreover, the secretion of incompletely processed precursors of big-IGF-II was previously proposed to be a mechanism responsible for NICTH. New cases of NICTH were reported, and our understanding of the IGF system has evolved by now.
This review and the accompanying case report compare the clinical features of thoracic and extrathoracic SFT copresenting with Doege–Potter syndrome and discuss the details the of the associated disease pathogenesis, diagnosis, and treatment of NICTH, with a specific focus on the effect of the IGF system.
2. Case report
A 68-year-old male was admitted with episodic confusion of 2 months duration. He was delirious but improved after receiving a continuous intravenous infusion of 10% dextrose and an intermittent intravenous bolus administration of 50% dextrose. Clinical review revealed a 10-kg weight loss, without cough, dyspnea, or chest pain. The patient did not have a history of diabetes mellitus and was not taking any glucose lowering medications. He had a 20-pack-year history of smoking and a 40-year history of alcohol use. Physical examination revealed diminished breath sounds in the right middle and lower lung fields, dullness to percussion, and absent tactile fremitus, without any enlargement of the superficial lymph nodes. Cardiovascular and abdominal examinations were unremarkable. Routine clinical laboratories, including urine and peripheral blood examinations and tumor markers (i.e., carcinoembryonic antigen [CEA], alpha fetoprotein Carbohydrate antigen 125, Carbohydrate antigen 199, neuron specific enolase, and Cytokeratin 19 fragments), were all within normal limits. The serum level of growth hormone (GH) was normal. Serum cortisol secretion and adrenocorticotropic hormone were normal. Despite marked hypoglycemia (1.4 mmol/L), the serum insulin level was less than 0.2 μIU/mL (normal range: 2.6–24.9) and the C-peptide level was 0.41 nmol/L (normal range: 1.1–4.0 nmol/L). The serum IGF-II level was 1038.71 μg/dL, and the IGF-I level was less than 25 μg/L. Computed tomographic (CT) scan revealed a large heterogeneous mass with dimensions of 9.9 × 13.3 × 17.2 cm, taking up most of the right hemithorax, with coarse margins and ill-defined borders. The bronchus of the right middle lobe was compressed. Heterogeneous enhancement was found with an enlarged paraaortic lymph node; in addition, the mediastinum was compressed and shifted slightly to the left (Fig. 1). Bone emission CT and CT scanning of the liver and pancreas were unremarkable.
Figure 1.
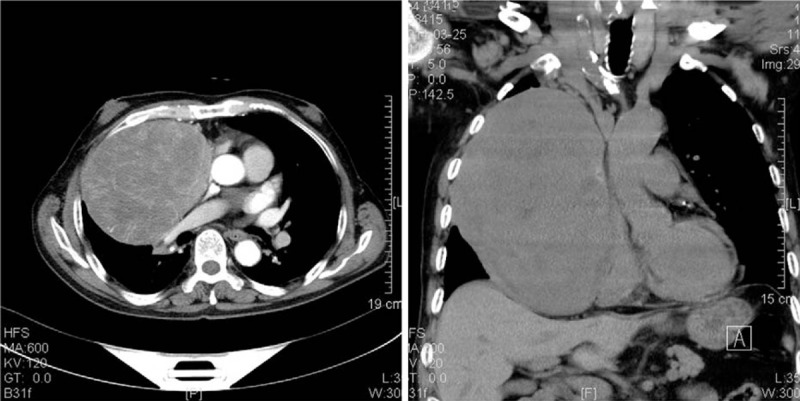
Computed tomographic scanning images of the thorax demonstrating a large heterogeneously enhanced mass seen in the right hemithorax, with dimensions of 20 × 16 × 12 cm.
The mass originated from the anterior mediastinum and was accompanied by right lung, pericardial and phrenic nerve invasion, and completely resected. The middle lobe of the right lung, part of the pericardium, and the phrenic nerve were resected. The tumor was a grayish-white solid, with dimensions of 20 × 16 × 12 cm and partially encapsulated with local necrosis found on the surface. Histological examination revealed interlacing fascicles of spindle tumor cells that were regular with minimal nuclear pleomorphism, and negligible mitotic activity. Immunohistochemical stains demonstrated strong positivity for CD34 expression, positive expression for IGF-II (Fig. 2), and negative S-100 protein expression, from which, a diagnosis of malignant SFT was established.
Figure 2.
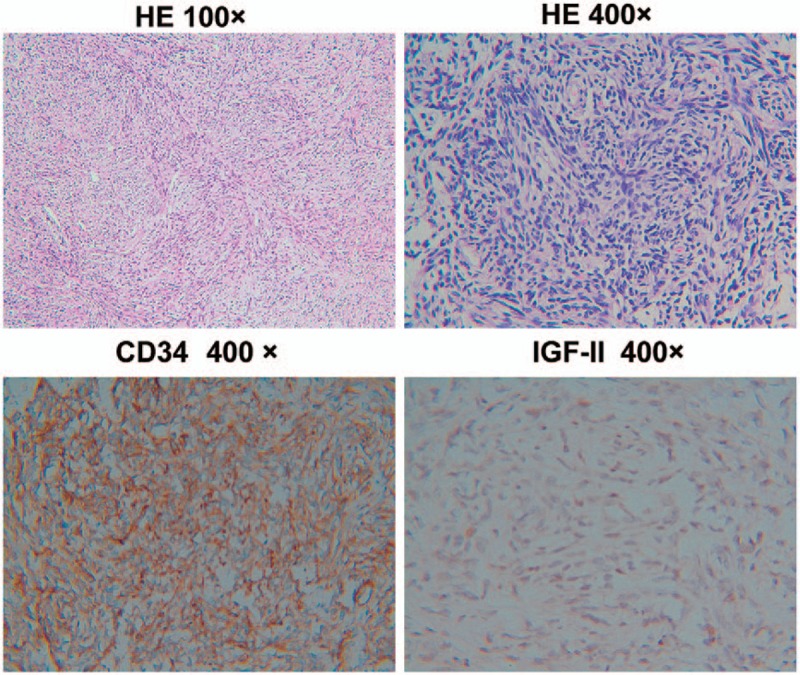
Histopathological findings showing spindle cells arranged in an organization lacking an obvious pattern. The tumor cells were immunohistochemically strongly reactive for CD34 and insulin-like growth factor-II.
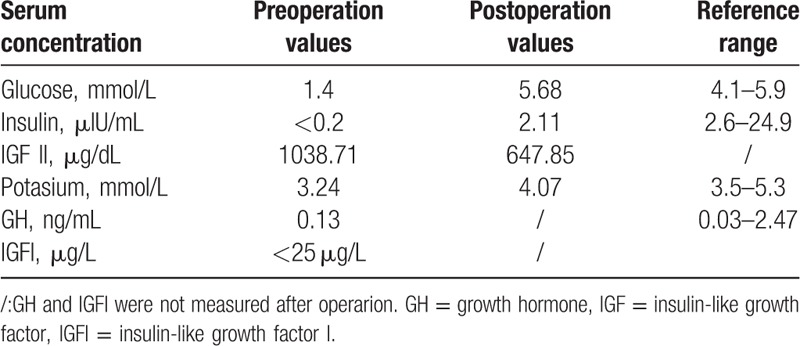
Postoperatively, serum glucose levels returned to normal, and episodes of hypoglycemia are resolved. A blood glucose and insulin level return to normal. In addition, serum IGF-II levels decreased from 1038.71 to 647.85 μg/dL (Table 1). At the 1.5-year follow-up period, no episodes of hypoglycemia occurred, and the patient remained tumor-free based on observations made from the most recent CT scan.
Table 1.
Summary of the laboratory tests results of the new case.
3. Discussion
In a literature search strategy, we searched the US National Library of Medicine's MeSH (Medical Subject Headings) controlled vocabulary thesaurus to reveal relevant indexed articles using the search terms “Solitary fibrous tumor” and “Hypoglycemia” in Medline. By employing this strategy, we accessed 76 cases of SFT with NICTH, which were reviewed in detail. All cases were published in English between 1989 and 2016.
4. Clinical features of Doege–Potter syndrome
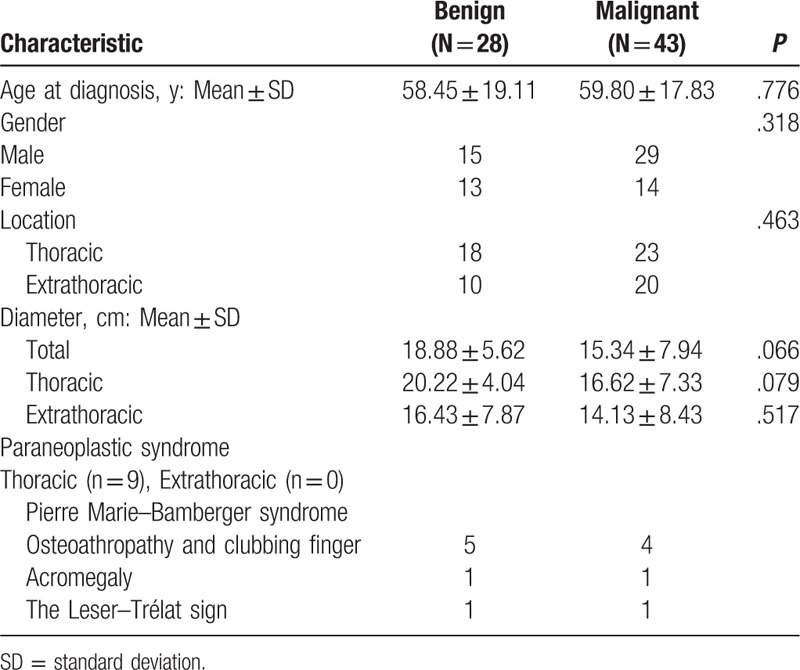
The common origins of SFT originate from the pleural cavity, pelvis, liver, retroperitoneum, kidney, mediastinum, sella, utera, bladder, intestinal tissue, mandibular, and thigh. We found that the most common location was the pleural cavity (i.e., left 12 and right 28), followed by the pelvis (n = 10). SFTs were classified as either benign or malignant according to the United Kingdom's pathologic criteria (hypercellularity, >4 mitotic figures/10 high-power fields (HPFs), pleomorphism/atypia, infiltrative growth pattern, necrosis, and hemorrhage).[4] Of the 71 cases with pathological analyses available, 28 (39.4%) were benign and 43 (60.6%) were malignant. Five cases did not provide any pathology results. SFTs with NICTH were more likely to be malignant and the malignancy rate was significantly higher than was previously noted in the literature (5%–10.4%). Among thoracic SFTs (i.e., of the pleural cavities, lungs, and mediastinum), 18 (43.9%) were benign, and 23 (56.1%) were malignant. Moreover, of the 30 extrathoracic SFTs, 10 (33.3%) were benign, and 20 (66.7%) were malignant. Extrathoracic SFTs were more likely to be malignant than were thoracic SFTs—an observation suggesting that SFTs with hypoglycemia were more likely to be malignant.
The previous study confirmed that overexpression of IGF-II endowed the neoplasm with the ability of limitless growth.[5] IGF-II is overexpressed ectopically in tumors and promotes mitogenesis, malignant transformation, and differentiation of tumor cells by binding the IGF-I receptor (IGF-1R).[3] In addition, IGF-II might exert biological effects in the context of tumor progression. The importance of IGF-1R signaling in malignant behavior of tumor cells is now well established. Insulin receptor isoform A (IR-A) was especially overexpressed in cancer, and its overexpression was also proposed to be a major mechanism responsible for overactivation of the IGF system in cancer following its binding to IGF-II.[6]
In a leiomyosarcoma cell-line model, insulin was more effective than IGF-II in activating the Insulin receptor substrate 1/Protein Kinase B (Akt) signaling pathway and protecting cells from apoptosis, while IGF-II was more effective in activating the Shc/Extracellular signal-regulated kinase (ERK) and stimulating cell invasion.[7] The previous studies demonstrated that hypoglycemia was also related to a poorer prognosis in cancer patients. An independent association between tumor recurrence/metastasis and hypoglycemia was also noted. Moreover, disease-free survival was significantly associated with hypoglycemia.[8]
Although variable, the mean age of onset of Doege–Potter syndrome was 59 years and was more common in males than in females (i.e., 47 vs 28 cases, and gender was not mentioned in one of the cases). When comparing the clinical characteristics of patients that presented with benign as compared malignant tumors, there were no significant differences in terms of the age of onset, gender, or size of the tumor. SFT diameter varied from 3.2 to 30 cm. In addition, malignant tumors were smaller than benign tumors, but the difference was not statistically significant (15.34 ± 7.94 vs 20.22 ± 4.04 cm, P = .066), (Table 2), with similar trends observed for both thoracic and extrathoracic SFTs.
Table 2.
Clinical characteristics of 71 cases with pathology analysis suffered from Doege–Potter syndrome, 1989–2016.
Previous studies reported that hypoglycemia occurs 3 times more frequently in females than in males, and that SFTs associated with hypoglycemia are typically located in the right hemithorax. Large SFTs (i.e., with dimensions greater than 20 cm), or SFTs with a high mitotic rate, are oftentimes accompanied by reactive hypoglycemia.[4] In the 76 SFT cases that presented with Doege–Potter syndrome, 29 cases were located in the right hemithorax, and 12 cases were located in the left hemithorax. Unlike previous reports, we found that male patients that presented with SFTs were more likely to exhibit hypoglycemia, although the differences were not statistically significant. The diameter of the tumor of 32 patients exceeded 20 cm. For thoracic SFTs, the diameter of the tumor derived from only 19 patients also exceeded 20 cm. Thus, we contend that tumor size (i.e., a tumor diameter >20 cm) was likely not an effective maker at predicting reactive hypoglycemia. For the tumor diameter, 77.6% (59/76) of cases showed a size that exceeded 15 cm, and in 96.1% (73/76) of cases, the tumor diameter exceeded 10 cm. In addition, when the tumor diameter exceeds 10 cm, we advise that the clinician should pay attention to the possibility of hypoglycemia being indicated as well.
Furthermore, IGF-II binds to IGF-IR in the pituitary gland. Increased levels of “big” IGF-II and total IGF-II in SFTs plays a negative feedback loop in the context of GH production by the anterior pituitary gland. Moreover, the synthesis of GH-dependent peptides such as IGF-I, IGF bingding protein (IGFBP-3), IGFBP-5, and acid-labile subunit (ALS) were all decreased. The IGF-II:IGF-I molar ratio is considered a surrogate marker of big IGF-II concentration, wherein a ratio of 3:1 is considered normal. In NICTH, the ratio was usually reported to be greater than 10.[9]
For the 76 cases of SFT presenting with NICTH, we determined GH levels in 8 cases, with only 1 case demonstrating decreased levels. Furthermore, 4 cases reported the presence of IGFBP3, in which 3 cases showed decreased levels, and 1 case did not have a reported value for the normal range of IGFBP3. For all cases, when hypoglycemia manifests, the levels of C peptide and insulin were found to have decreased somewhat. In addition, in 15/19 cases, the IGF-II/IGF-I ratio was more than 10, which was more than 3:1 when taking into account all 19 cases. In addition, 4 cases gave an IGF-II/IGF-I ratio less than 10, while total IGF-II levels were normal and showed significantly suppressed levels of IGF-I.[10] Western blot analysis of serum IGF-II revealed the presence of IGF-II species with increased molecular weights, and decreased levels of normal IGF-II.[11,12]
5. Symptoms characteristic of Doege–Potter syndrome
Hypoglycemia (diaphoresis, tremor, anxiety, and lost consciousness) was the predominant symptom of Doege–Potter syndrome. Hypoglycemia can be the initial indicator that leads to the diagnosis of SFTs. In other cases, hypoglycemia might develop after discovery of the tumor, or during occasions of tumor recurrence or when distance metastases have occurred. Other typical symptoms of the tumors arise from compression and mass effects. In addition, some patients may be asymptomatic, and paraneoplastic symptoms are usually found in thoracic SFTs with Doege–Potter syndrome.
With the exception of hypoglycemia, oestoarthorapathy was the most common paraneoplastic symptom. Hypertrophic osteoarthropathy and acromegaly of SFTs, which are also known as Pierre Marie–Bamberg syndrome, are induced by abnormal production of hyaluronic acid by tumor cells. Hypertrophic osteoarthropathy is a syndrome characterized by finger clubbing, hypertrophic skin changes, and periosteal bone changes.[13] Eleven of the 41 thoracic SFT presenting patients suffered from this condition. One thoracic SFT patient developed the Leser–Trélat sign, which is characterized by the sudden appearance and rapid increase in the size and number of seborrheic keratoses, including malignant acanthosis nigricans, florid cutaneous papillomatosis, and tylosis, which are associated with an increase in growth factor secretion, including that of transforming growth factor-α, epidermal growth factor, and IGF.[14,15] In addition, IGFs can reduce blood potassium levels in the short-term.[16,17] Two malignant SFT patients developed hypokalemia with potassium concentrations of 2.68 mmol/L and 2.3 mEq/L. After surgery, potassium levels returned to normal. Although the mechanism remains unclear, it has been postulated to be secondary to the insulin-like activity of IGF-II.
6. Diagnostic methods/markers
6.1. Image of the tumor
CT scanning and magnetic resonance imaging (MRI) are very effective tools in determining the invasion of the surrounding structures, including revealing information on distant metastasis and follow-up diagnosis of SFTs. CT imaging of SFTs typically shows a smooth, well circumscribed, and homogenous mass. Large heterogeneous masses can be seen with a necrotic area and probably with/without calcification. MRI demonstrated multinodular tumors of isointensity or low intensity on T1-weighted images and high signal intensity on T2-weighted images. Mature fibrous tissue has a low signal strength due to the high content of fibrous tissue or collagen, with intense enhancement on postcontrast dynamic MRI.[1] Also malignant fibrous tissue tends to show a high signal intensity.[18]
Fluorodeoxyglucose-positron emission tomography (FDG-PET) also benefits the diagnosis of malignant SFTs, which is usually false-negative on FDG-PET but can be benign positive on tyrosine-PET.[19] High levels of IGF-II induce liver, heart, and muscle uptake of FDG, leaving less available FDG for the tumor to take up. Using radiolabeled amino acid C-11-tyrosine as a tracer permits visualization of the tumor.[20] However, it should be noted that functional tumor imaging techniques like FDG-PET may lead to false-negative results.[19]
6.2. Pathology of the tumor
Most of the identified tumors were large, solid, and well circumscribed, with a lobular, firm, and gray-white cut surface. Microscopic examination demonstrated a bland spindle-cell proliferation that was arranged in fascicles, and hypo- and hypercellular areas that were separated by thick and thin collagen bundles. Most malignant tumors revealed hemorrhagic, high cellularity, pleomorphic, or necrotic changes. Atypical cells and mitotic cells were also found, and the number of mitotic cells was more than 4 mitotic figures as visualized under ×10 HPFs. The immunohistochemical analysis showed CD34+, B-cell lymphoma-2 (Bcl-2)+, Vimentin+, and S100− expression. In addition, 53 (72.6%) pathological reports used CD34, Bcl-2, vimentin, and S100 expression to determine the differential diagnosis. Furthermore, 52 (98%) cases expressed CD34+, and only 1 case had originated from the sella, while CD34 immunoreactivity was less intense.
Immunohistochemistry (IHC) could determine the probable tissue origin of SFTs. Thus, it could be an important differential diagnosis method. Strong positive reactions for the expression of CD34, CD99, Bcl2, and negative expression patterns for (pan-cytokeratin) AE1/AE3, synaptophysin, S-100, and calretinin were shown to be important markers related to the diagnosis of SFTs. These tumors were also negative for CEA and Factor VIII. Moreover, anti-melanoma antibody (HMB-45) Immunohistochemistry is helpful to distinguish SFT from angiomyolipoma. In some cases, negative IHC has been reported for c-kit, keratin, type II cytoskeletal 8, factor XIIIa, HMB-45, AE-1, smooth muscle actin, CD31, and Fli-1.[21,22] Overexpression of β-catenin and/or cyclin D1 occurs in some SFTs, and when present indicates an altered Wnt signaling pathway. In addition, dysregulation in cyclin D1 might be involved in a subset of SFT tumorigenic outcomes.[23,24]
In the setting of NICTH, this condition is confirmed by a blood sugar estimation <50 mg/dL, and low or negligible serum C-peptide and insulin levels, low serum IGF-I, as well as normal or elevated levels of IGF-II in the circulation. “Big”-IGF-II determination was also a useful laboratory evaluation. Identification of SFTs can also be based on characteristic histology when combined with the immunohistochemical profile. Specific CD34 positivity was very useful in differentiating SFTs from sarcomatous mesothelioma and from many other different mesenchymal tumors (e.g., hemangiopericytoma, monophasic synoviosarcoma, thymoma, and neurilemmoma). SFTs with NICTH were diagnosed as Doege–Potter syndrome.
6.3. Potential mechanism of non-islet cell tumor hypoglycemia
Doege–Potter syndrome is characterized by constant hypoglycemia, suppressed serum insulin, C peptide, GH and low serum IGF-I, against levels of IGF-II that are either normal, or elevated.
The IGF system is composed of 2 IGF ligands (i.e., IGF-I and IGF-II) and 2 IGF receptors (i.e., IGF-1R and IGF-II/mannose-6-phosphatereceptor) and circulating IGFBPs. IGF-I and IGF-II have an approximate 50% amino acid sequence similarity to insulin. In addition, IGF-I, IGF-II, and insulin bind to the IGF-IR and insulin receptor IR-A and activate its intrinsic tyrosine kinase activity to control crucial biological effects, including those of cellular growth, proliferation, differentiation, migration, and protection from apoptosis. IGF-IR and IGFBPs modulate the availability of IGF-I and IGF-II to bind to the receptors.[25] IGF-IR and the insulin receptor (i.e., isoform IR-A) had more than a 50% overall amino acid sequence homology and an 84% homology in the tyrosine kinase domains.[26]
The IGFs bound to IGFBPs. Six different affinity IGFBPs were identified as IGFBP-1 to -6, which are approximately 30-kDa proteins that share a common domain organization that consists of cysteine-rich N- and C-terminal domains that are connected by a flexible linker region. The interaction between IGF-I/II and IGF-IR is regulated by IGFBP-1 to -6.[25] In normal serum, IGFBP-3 is the most abundant IGFBP and binds more than 95% of the IGFs.[27] In addition, 20% to 30% of circulating IGFs bind IGFBPs to form a 50-kDa protein and 70% to 80% of the formed complex yields a 150-kDa protein by binding to IGFBPs. In addition, free IGFs form approximately 1% of the circulating pool with a 10-minute half-life. The half-life of the complex when combined with the 50-kDa protein was approximately 30 minutes, while the half-life of that combined with the 150-kDa protein was approximately 12 hours.[28]
In normal serum, IGF-II is present in a 150-kDa ternary complex with IGFBP3 and ALS. Due to its large molecular mass, the half-life of that combined with the 150-kDa protein is longer, and the ternary complex is unable to pass the capillary membrane. Normal O-linked glycosylation may be required for posttranslational processing of pre-pro-IGF-II. Lacking normal O-linked glycosylation, pro-IGF-II resulted in the higher molecular weight form, which we refer to as “big” IGF-II.[29] The “big” IGF-II that is derived from the tumor failed to form a complete ternary complex and was preferentially bound to IGFBP-2 in an abnormal binary complex.[30,31] This smaller complex has a lower affinity for serum binding proteins and could pass through the capillaries. Thus, “big” IGF-II could induce hypoglycemia by interacting with the insulin receptors in the target tissues.
Shapiro speculated that reduced affinity of IGF-II-related peptides for serum binding proteins may result from either the altered structure of big IGF-II, or from proteolytic cleavage at a site that is important for its interaction with binding proteins during metabolism to conversion intermediates. When binding to the insulin receptors (i.e., isoform IR-A), the “big” IGF-II serves to transport glucose into the muscles and inhibit gluconeogenesis in the liver and lipolysis in the adipose tissues, which represents the hypoglycemic mechanism in patients with tumors that secrete big IGF-II.[32]
The IGF-II gene is an imprinted gene, and normally only 1 parental allele is expressed.[33] IGF-II sits at the 11p15.5 locus adjacent to the H19 tumor suppressor and contains 9 exons and 4 promoter regions. Two regions that are known as the IGF-II differentially methylated region and the imprinting control region 1 were methylated in a parental-specific manner. IGF-II was imprinted maternally and H19 was imprinted paternally. That is, IGF-II is transcribed from only the paternal contribution to the genotype, whereas H19 is transcribed from the maternal copy.[3] Recent evidence showed that IGF-II overexpression in SFTs is independent of the anatomical location and is related to the loss of imprinting. In other words, the loss of imprinting resulting from reduced methylation of the maternal IGF-DMR induces increased expression of IGF-II.[34] The unprocessed high molecular weight form of IGF-II that is generated from the impaired processing of the IGF-II precursor by tumor-specific prohormone convertase 4 (PC4) expression.[35]
PC4 is a potential protease that is responsible for IGF-II processing. Moreover, steady-state messenger RNA (mRNA) levels of IGF-II and PC4 were about 14-fold greater and 5-fold less in the tumor tissue than those found in normal placental tissue, respectively. Promoter usage appears to regulate multiple transcripts that encode the same monomeric primary IGF-II translation product, referred to as pre-pro-IGF-II,[36] which consists of 180 amino acids that include an N-terminal signal peptide of 24 amino acid residues, the 67 amino acids long mature IGF-II (7.5 kDa) and an 89 residue extension at the C-terminus, which is designated the E-domain. After removal of the N-terminal signal, addition of sialic acid containing oligosaccharides through O-linking to 1 or more threonine residues of the E-domain, followed by sequential proteolysis of the latter extension into the mature protein, ultimately forms a relatively stable intermediate pro-IGF-IIE.[37]
IGF-II is expressed in 80% of SFTs (20/25).[38] The IGF-II mRNA is expressed higher in the SFT and fibrosarcoma of the liver at 193- and 312-fold, respectively, as compared to control liver samples. The expression of IGF-I mRNA is not significantly different from the control in the setting of SFT. In addition, the IGF-II receptor mRNA levels were approximately double that of controls in both SFT and in fibrosarcoma.[39]
Although these neoplasms often have elevated levels of IGF-II mRNA, they do not always produce elevated circulating levels of IGF-II. A previous study found that of 42 SFTs, 83.3% were positive for IGF-II mRNA, and 69% expressed IGF-II protein. In addition, tumor size was related to IGF-II production. Tumors less than 5 cm were usually negative for IGF-II mRNA, whereas 92.3% of tumors greater than 9 cm were positive for IGF-II mRNA. The intensity of the staining for IGF-II mRNA was variable and was not always more intense in patients that exhibited with clinical evidence of hypoglycemia.
Only 3 of 42 patients had associated hypoglycemia. The expression and production of IGF-II cannot accurately predict patients with clinical evidence of hypoglycemia.[40] The previous study showed that mRNA was detected in the original tumor 11 years before the patient displaying signs and symptoms of hypoglycemia.[41] Yuri et al reported a case of SFT in inguinal soft tissues. The diameter of the tumor was 2 × 3 cm, and immunoreactive IGF-II was detected by histology; however, hypoglycemia was not seen in this patient an observation that suggests only when the tumor increases in size will the levels of IGF-II increase at least to some degree, following which, the onset of hypoglycemia will be seen.[42]
7. Treatment
7.1. Tumor therapy
Complete tumor resection was reported to be the definitive treatment. Optimized tumor resection was completed in the cases with the notable exception of 4 cases in which tumors were unable to be completely resected.
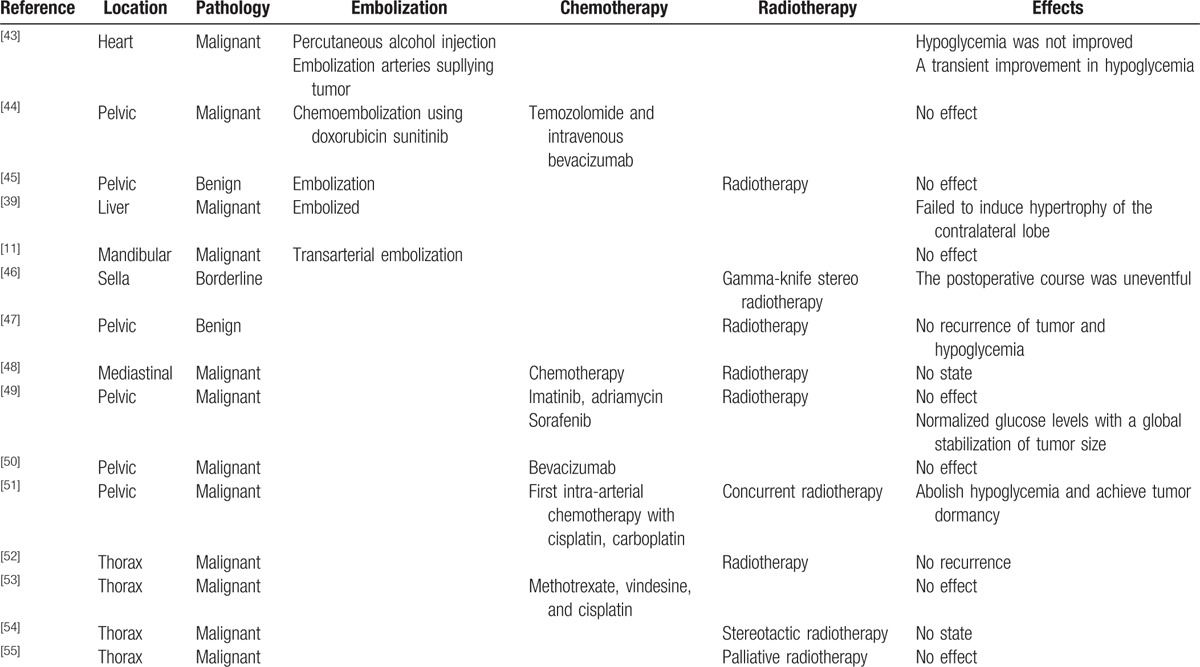
Neoadjuvant therapy, including chemoradiation and consecutive selective embolization of the feeding vessels of the tumor, were reported to be effective (Table 3). However, SFTs are considered relatively chemoresistant. Chemotherapy is used for metastatic or symptomatic nonresectable SFTs. However, there are still no standard chemotherapeutic indications or regimens. The effect of chemotherapy was also controversial. Radiation therapy is of some benefit, particularly when used as an adjuvant treatment with surgery and chemotherapy. Radiation was used in 9 sporadic SFTs. Preoperative percutaneous embolization has been attempted to reduce blood loss during surgery[56]; however, postoperative morbidity and mortality has been as high as 12%.[57] In our review, embolization was used in 4 sporadic cases of SFT, in which hypoglycemia was not improved and tumor atrophy was not found.
Table 3.
The adjunctive therapy, including embolization, chemotherapy, and radiotherapy, for Doege–Potter syndrome, 1898–2016.
The Akt/mammalian target of rapamycin (Akt/mTOR) pathway plays an essential role in modulating cellular functions in response to extracellular signals, such as growth factors and cytokines. The Akt/mTOR pathway was activated in about 50% of SFT cases, and activation of this pathway during tumorigenesis was related to malignancy-associated findings, such as tumor necrosis.[8] Thus, the Akt/mTOR pathway was considered a candidate therapeutic target for SFTs.
The rarity of SFT copresenting with hypoglycemia makes it very challenging to aggregate sufficient numbers of cases to adequately evaluate its prognosis. In the current review, 60 patients were followed up, from which, 6 cases died. One patient did not receive a surgical procedure. In addition, 5 patients did not survive the operation and died shortly thereafter. The survival rate at 1, 3, and 5 years postsurgery were 98.3%, 96.7%, and 95.0%, respectively.
7.2. Hypoglycemia therapy
Reducing the size of the tumor and diminishing the progressive and serious hypoglycemia represent 2 overarching goals of SFT therapy. Complete tumor resection remains the only definitive treatment, and recurrence-free survival generally exceeds 90% with complete resection.[58] Moreover, hypoglycemia would recover after the tumor was successfully resected.
7.2.1. Glucocorticoids
Glucagon,[59] somatostatin analog, recombinant human GH (rhGH), and glucocorticoids[60] were administrated to relieve hypoglycemia. Glucocorticoid treatment has been shown to be the most effective therapy for long-term remission of hypoglycemia, and does so by suppressing the production of “big” IGF-II and correcting the prevailing biochemical abnormalities that involve the GH–IGF axis. To abolish episodes of hypoglycemia, glucocorticoids were used in 13 cases (Table 4). Prednisolone at a dose of more than 25 mg/d (i.e., 0.5 mg/kg/d), dexamethesone at 2.0 mg/d, and methylprednisolone at 32 mg/d could effectively relieve hypoglycemia. When the dose was reduced to less than 20 mg/d, prednisolone was unable to abolish episodes of hypoglycemia.
Table 4.
Glucocorticoid therapy for NICTH in SFTs with Doege–Potter syndrome, 1989–2016.
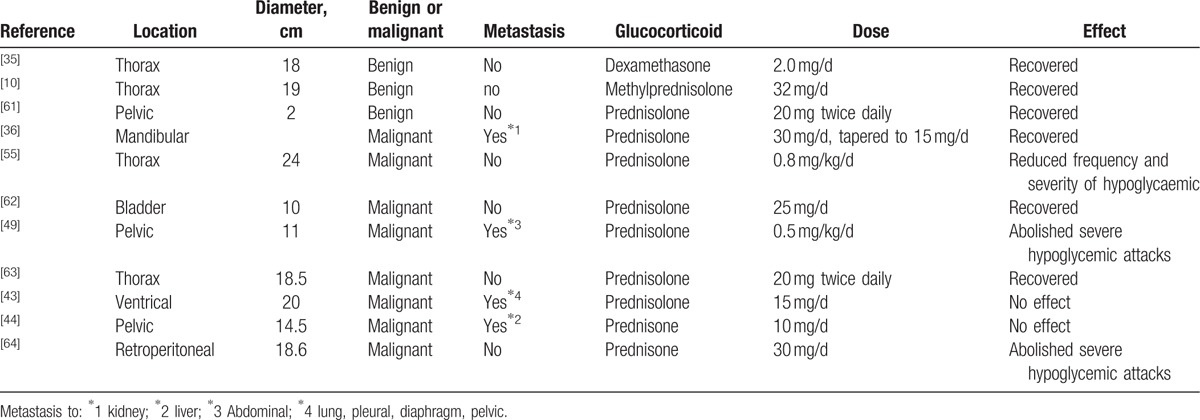
Glucocorticoids suppress tumor-specific secretion of “big” IGF-II, in which “big” IGF-II was decreased by as much as 75% in response to glucocorticoid therapy[43,44] Consequently, total IGF-II decreased and IGF-I increased, and the IGF-II-to-IGF-I ratio normalized. Serum levels of IGFBP-2 also had a tendency to decrease toward normal levels, and serum ALS concentrations increase significantly.[65] At the same time, insulin and C-peptide in the serum increased. Tsuro et al reviewed 20 Japanese cases of IGF-II-producing tumors, in which 5 of 6 cases were treated with glucocorticoid therapy and as a consequence, no longer suffered from hypoglycemic attacks in spite of receiving no antitumor therapy. Glucocorticoid therapy had an immediate beneficial influence on symptomatic hypoglycemia. In addition, only after a prolonged (5-month) period on a 30 mg/d dose did the IGF-II-to-IGF-I ratio value approach normality. When the dose was reduced to 20 mg/d and then to a dose of 15 mg/d, “big” IGF-II levels once again increased.[11] In the 73 SFT cases, we found that when the dose was lower than 20 mg/d, prednisolone could not abolish episodes of hypoglycemia. The effect of glucocorticoid therapy seems to be a dose-dependent phenomenon and is reversible if doses are administered below a critical level.
7.2.2. Growth hormone
GH can alleviate hypoglycemia by stimulating hepatic gluconeogenesis and glycogenolysis. Aside from their influence on serum insulin and glucose, in contrast to glucocorticoids, rhGH stimulates both total IGF-II and “big” IGF-II. For instance, total IGF-II was observed to increase on average by 50% and “big” IGF-II increased on average by 29% in hGH-treated patients.[9] Supraphysiological doses of rhGH increase IGFBP-3 and ALS levels, and in doing so, promote the formation of ternary complexes, and in turn, decrease the bioavailability of IGF-II. Previous studies have suggested that at daily doses of 4 to 12 U, rhGH markedly increased IGFBP-3 and alleviated hypoglycemia in 3 patients with NICTH.[66,67]
The diverse metabolic effects of GH (in protein sparing) and glucocorticosteroids (in tumor suppression) suggest that their combined use could be feasible in treating NICTH.[64,68] Decreasing the doses of prednisone (from 30 to 10 mg/d), followed by decreasing doses of rhGH (from 2.6 to 1.3 mg/d), followed by combination of the lowest doses of each, normalized fasting plasma glucose levels and enhanced IGF-I levels. Indeed, the combination was more effective than high-dose monotherapy with either drug alone in the context of reestablishing the IGF system. In addition, adverse effects were not found. In patient with inoperable NICTH, the combination of low doses of prednisone and rhGH was a successful long-term therapy for hypoglycemia.[51]
7.2.3. Glucagon
The effect of glucagon in recovering tumor hypoglycemia remains controversial. Seven patients with tumor hypoglycemia and liver metastasis had an acceptable glycemic response to long-term treatment with glucagon (i.e., at 0.06–0.3 mg/h, via intravenous infusion pump).[59] By contrast, for another SFT case, the infusion of glucagon and a lipid preparation did not abolish repeated episodes of hypoglycemic attacks. Among the 76 observed cases, glucagon and rhGH therapy were prescribed in 3 cases, although no significant measurable effect was found in that setting.
8. Conclusion
NICTH is the main clinical characteristic of Doege–Potter syndrome. The malignant rate of Doege–Potter syndrome was 60.3%, which was significantly higher than that in the absence of NICTH (5%–14.6%). The big IGF-II binding with insulin isoform A and IGF-1R, resulted in hypoglycemia, and promoted mitosis and malignant transformation of the tumor. Low serum insulin, higher big-IGF II and a ratio of IGF-II/IGF-I that exceeded 10 could be useful for the diagnosis of Doege–Potter syndrome. Also optimization of the resection procedure is recommended as the standard approach for surgical therapy, if indeed, surgery is possible. For treatment considerations, glucocorticoids can relieve hypoglycemia in a dose-dependent manner. However, the effect of adjunctive therapy, including chemotherapy, radiation, and embolization, was controversial.
Footnotes
Abbreviations: Akt/mTOR = Akt/mammalian target of rapamycin, ALS = acid-labile subunit, CT = computed tomographic, FDG-PET = fluorodeoxyglucose-positron emission tomography, GH = growth hormone, HPF = high-power field, IGF = insulin-like growth factor, IGF-1R = IGF-I receptor, IGFBP = IGF bingding protein, MRI = magnetic resonance imaging, NICTH = non-islet cell tumor hypoglycemia, PC4 = prohormone convertase 4, rhGH = recombinant human GH, SFT = solitary fibrous tumor.
Patient consent and ethics approval: The patient described as the case report in this study, provided consent to publish this work. The present study was approved by the Local Ethics Committee of the Affiliated Hospital of Chengde Medical University, China.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Kinoshita T, Ishii K, Higashiiwai H, et al. Malignant solitary fibrous tumour of the peritoneum. Clin Radiol 2000;55:157–60. [DOI] [PubMed] [Google Scholar]
- [2].Kalebi AY, Hale MJ, Wong ML, et al. Surgically cured hypoglycemia secondary to pleural solitary fibrous tumour: case report and update review on the Doege–Potter syndrome. J Cardiothorac Surg 2009;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].de Groot JW, Rikhof B, Van DJ, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer 2007;14:979–93. [DOI] [PubMed] [Google Scholar]
- [4].England DM, Hochholzer L, Mccarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640–58. [DOI] [PubMed] [Google Scholar]
- [5].Pavelic K, Spaventi S, Gluncic V, et al. The expression and role of insulin-like growth factor II in malignant hemangiopericytomas. J Mol Med (Berl) 1999;77:865–9. [DOI] [PubMed] [Google Scholar]
- [6].Cullen KJ, Lippman ME. Stromal-epithelial interactions in breast cancer. Cancer Treat Res 1991;61:413–31. [DOI] [PubMed] [Google Scholar]
- [7].Sciacca L, Mineo R, Pandini G, et al. In IGF-I receptor-deficient leiomyosarcoma cells autocrine IGF-II induces cell invasion and protection from apoptosis via the insulin receptor isoform A. Oncogene 2002;21:8240–50. [DOI] [PubMed] [Google Scholar]
- [8].Yamada Y, Kohashi K, Fushimi F, et al. Activation of the Akt-mTOR pathway and receptor tyrosine kinase in patients with solitary fibrous tumors. Cancer 2014;120:864–76. [DOI] [PubMed] [Google Scholar]
- [9].Teale JD, Marks V. Inappropriately elevated plasma insulin-like growth factor II in relation to suppressed insulin-like growth factor I in the diagnosis of non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf) 1990;33:87–98. [DOI] [PubMed] [Google Scholar]
- [10].Rosseel L, De Leu N, Van Hecke W, et al. A rare case of hypoglycemia in a patient with elevated right hemidiaphragm. BMJ Case Rep 2012;2012:bcr0320125972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tsuro K, Kojima H, Okamoto S, et al. Glucocorticoid therapy ameliorated hypoglycemia in insulin-like growth factor-II-producing solitary fibrous tumor. Intern Med 2006;45:525–9. [DOI] [PubMed] [Google Scholar]
- [12].Wakami K, Tateyama H, Kawashima H, et al. Solitary fibrous tumor of the uterus producing high-molecular-weight insulin-like growth factor II and associated with hypoglycemia. Int J Gynecol Pathol 2005;24:79–84. [PubMed] [Google Scholar]
- [13].Cardillo G, Lococo F, Carleo F, et al. Solitary fibrous tumors of the pleura. Curr Opin Pulm Med 2012;18:339–46. [DOI] [PubMed] [Google Scholar]
- [14].Fridlington J, Weaver J, Kelly B, et al. Secondary hypertrophic osteoarthropathy associated with solitary fibrous tumor of the lung. J Am Acad Dermatol 2007;57:S106–10. [DOI] [PubMed] [Google Scholar]
- [15].Schwartz RA. Sign of Leser-Trelat. J Am Acad Dermatol 1996;35:88–95. [DOI] [PubMed] [Google Scholar]
- [16].De PM, Fischer S, Bründler MA, et al. Solitary fibrous tumors of the pleura. Ann Thorac Surg 2002;74:285–93. [DOI] [PubMed] [Google Scholar]
- [17].Miell JP, Taylor AM, Jones J, et al. Administration of human recombinant insulin-like growth factor-I to patients following major gastrointestinal surgery. Clin Endocrinol (Oxf) 1992;37:542–51. [DOI] [PubMed] [Google Scholar]
- [18].Nagase T, Adachi I, Yamada T, et al. Solitary fibrous tumor in the pelvic cavity with hypoglycemia: report of a case. Surg Today 2005;35:181–4. [DOI] [PubMed] [Google Scholar]
- [19].Boer JD, Jager PL, Wiggers T, et al. The therapeutic challenge of a nonresectable solitary fibrous tumor in a hypoglycemic patient. Int J Clin Oncol 2006;11:478–81. [DOI] [PubMed] [Google Scholar]
- [20].Jager PL, Vaalburg W, Pruim J, et al. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 2001;42:432–45. [PubMed] [Google Scholar]
- [21].Hiraoka K, Morikawa T, Ohbuchi T, et al. Solitary fibrous tumors of the pleura: clinicopathological and immunohistochemical examination. Interact Cardiovasc Thorac Surg 2003;2:61–4. [DOI] [PubMed] [Google Scholar]
- [22].Schirosi L, Lantuejoul S, Cavazza A, et al. Pleuro-pulmonary solitary fibrous tumors: a clinicopathologic, immunohistochemical, and molecular study of 88 cases confirming the prognostic value of de Perrot staging system and p53 expression, and evaluating the role of c-kit, BRAF, PDGFRs (alpha/beta), c-met, and EGFR. Am J Surg Pathol 2008;32:1627–42. [DOI] [PubMed] [Google Scholar]
- [23].Andino L, Cagle PT, Murer B, et al. Pleuropulmonary desmoid tumors: immunohistochemical comparison with solitary fibrous tumors and assessment of beta-catenin and cyclin D1 expression. Arch Pathol Lab Med 2006;130:1503–9. [DOI] [PubMed] [Google Scholar]
- [24].Ng TL, Gown AM, Barry TS, et al. Nuclear beta-catenin in mesenchymal tumors. Mod Pathol 2005;18:68–74. [DOI] [PubMed] [Google Scholar]
- [25].Denley A, Cosgrove LJ, Booker GW, et al. Molecular interactions of the IGF system. Cytokine Growth Factor Rev 2005;16:421–39. [DOI] [PubMed] [Google Scholar]
- [26].Ullrich A, Gray A, Tam AW, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J 1986;5:2503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 2002;23:824–54. [DOI] [PubMed] [Google Scholar]
- [28].Empen K, Otto C, Müller-Höcker J, et al. Case 1: hypoglycemic syncope due to a peripheral neuroectodermal tumor. J Clin Oncol 2000;18:1594–6. [DOI] [PubMed] [Google Scholar]
- [29].Daughaday WH, Trivedi B, Baxter RC. Serum “big insulin-like growth factor II” from patients with tumor hypoglycemia lacks normal E-domain O-linked glycosylation, a possible determinant of normal propeptide processing. Proc Natl Acad Sci U S A 1993;90:5823–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shapiro ET, Bell GI, Polonsky KS, et al. Tumor hypoglycemia: relationship to high molecular weight insulin-like growth factor-II. J Clin Invest 1990;85:1672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zapf J. Role of insulin-like growth factor II and IGF binding proteins in extrapancreatic tumor hypoglycemia. Horm Res 1994;42:20–6. [DOI] [PubMed] [Google Scholar]
- [32].Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med 1997;336:633–40. [DOI] [PubMed] [Google Scholar]
- [33].Lewis A, Reik W. How imprinting centres work. Cytogenet Genome Res 2006;113:81–9. [DOI] [PubMed] [Google Scholar]
- [34].Lawson EA, Zhang X, Crocker JT, et al. Hypoglycemia from IGF2 overexpression associated with activation of fetal promoters and loss of imprinting in a metastatic hemangiopericytoma. J Clin Endocrinol Metab 2009;94:2226–31. [DOI] [PubMed] [Google Scholar]
- [35].Tani Y, Tateno T, Izumiyama H, et al. Defective expression of prohormone convertase 4 and enhanced expression of insulin-like growth factor II by pleural solitary fibrous tumor causing hypoglycemia. Endocr J 2008;55:905–11. [DOI] [PubMed] [Google Scholar]
- [36].Sussenbach JS, Rodenburg RJ, Scheper W, et al. Transcriptional and post-transcriptional regulation of the human IGF-II gene expression. Adv Exp Med Biol 1993;343:63–71. [DOI] [PubMed] [Google Scholar]
- [37].Daughaday WH, Trivedi B. Heterogeneity of serum peptides with immunoactivity detected by a radioimmunoassay for proinsulin-like growth factor-II E domain: description of a free E domain peptide in serum. J Clin Endocrinol Metab 1992;75:641–5. [DOI] [PubMed] [Google Scholar]
- [38].Steigen SE, Schaeffer DF, West RB, et al. Expression of insulin-like growth factor 2 in mesenchymal neoplasms. Mod Pathol 2009;22:914–21. [DOI] [PubMed] [Google Scholar]
- [39].Chan G, Horton PJ, Thyssen S, et al. Malignant transformation of a solitary fibrous tumor of the liver and intractable hypoglycemia. J Hepatobiliary Pancreat Surg 2007;14:595–9. [DOI] [PubMed] [Google Scholar]
- [40].Lloyd RV, Erickson LA, Nascimento AG, et al. Neoplasms causing nonhyperinsulinemic hypoglycemia. Endocr Pathol 1999;10:291–7. [DOI] [PubMed] [Google Scholar]
- [41].Hoog A, Sandberg Nordqvist AC, Hulting AL, et al. High-molecular weight IGF-2 expression in a haemangiopericytoma associated with hypoglycaemia. APMIS 1997;105:469–82. [DOI] [PubMed] [Google Scholar]
- [42].Yuri T, Kanematsu S, Lei YC, et al. Solitary fibrous tumor of soft tissue: a case report and immunohistochemical study. Med Mol Morphol 2010;43:60–4. [DOI] [PubMed] [Google Scholar]
- [43].Weiss B, Horton DA. Preoperative embolization of a massive solitary fibrous tumor of the pleura. Ann Thorac Surg 2002;73:983–5. [DOI] [PubMed] [Google Scholar]
- [44].Santos RS, Haddad R, Lima CE, et al. Patterns of recurrence and long-term survival after curative resection of localized fibrous tumors of the pleura. Clin Lung Cancer 2005;7:197–201. [DOI] [PubMed] [Google Scholar]
- [45].Cardillo G, Carbone L, Carleo F, et al. Solitary fibrous tumors of the pleura: an analysis of 110 patients treated in a single institution. Ann Thorac Surg 2009;88:1632–7. [DOI] [PubMed] [Google Scholar]
- [46].Hoff AO, Vassilopoulou-Sellin R. The role of glucagon administration in the diagnosis and treatment of patients with tumor hypoglycemia. Cancer 1998;82:1585–92. [PubMed] [Google Scholar]
- [47].Tominaga N, Kawarasaki C, Kanemoto K, et al. Recurrent solitary fibrous tumor of the pleura with malignant transformation and non-islet cell tumor-induced hypoglycemia due to paraneoplastic overexpression and secretion of high-molecular-weight insulin-like growth factor II. Intern Med 2012;51:3267–72. [DOI] [PubMed] [Google Scholar]
- [48].Teale JD, Wark G. The effectiveness of different treatment options for non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf) 2004;60:457–60. [DOI] [PubMed] [Google Scholar]
- [49].Holt RI, Simpson HL, Sonksen PH. The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet Med 2003;20:3–15. [DOI] [PubMed] [Google Scholar]
- [50].Teale JD, Blum WF, Marks V. Alleviation of non-islet cell tumour hypoglycaemia by growth hormone therapy is associated with changes in IGF binding protein-3. Ann Clin Biochem 1992;29(Pt 3):314–23. [DOI] [PubMed] [Google Scholar]
- [51].Bourcigaux N, Arnault-Ouary G, Christol R, et al. Treatment of hypoglycemia using combined glucocorticoid and recombinant human growth hormone in a patient with a metastatic non-islet cell tumor hypoglycemia. Clin Ther 2005;27:246–51. [DOI] [PubMed] [Google Scholar]
- [52].Rose MG, Tallini G, Pollak J, et al. Malignant hypoglycemia associated with a large mesenchymal tumor: case report and review of the literature. Cancer J Sci Am 1999;5:48–51. [PubMed] [Google Scholar]
- [53].Schutt RC, Gordon TA, Bhabhra R, et al. Doege–Potter syndrome presenting with hypoinsulinemic hypoglycemia in a patient with a malignant extrapleural solitary fibrous tumor: a case report. J Med Case Rep 2013;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mahboob A, Eckford SD. Solitary fibrous tumour: an unusual cause of hypoglycemia. Internet J Endocrinol 2009;7. [Google Scholar]
- [55].Yin W, Ma C, Wu J, et al. A primary atypical solitary fibrous tumor of the sella mimicking nonfunctional pituitary adenoma: a case report. Acta Neurochir (Wien) 2010;152:519–22. [DOI] [PubMed] [Google Scholar]
- [56].Dahiya D, Bhadada S, Nahar U, et al. IGF-II-secreting pelvic tumor presenting with neuropsychiatric symptoms. J Surg Case Rep 2013;2013:rjt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Witkin GB, Rosai J. Solitary fibrous tumor of the mediastinum. A report of 14 cases. Am J Surg Pathol 1989;13:547–57. [DOI] [PubMed] [Google Scholar]
- [58].Le Jeune S, Des Guetz G, Bihan H, et al. Refractory hypoglycemia controlled by sorafenib in solitary fibrous tumor. J Clin Oncol 2013;31:e118–21. [DOI] [PubMed] [Google Scholar]
- [59].Wagner S, Greco F, Hamza A, et al. Retroperitoneal malignant solitary fibrous tumor of the small pelvis causing recurrent hypoglycemia by secretion of insulin-like growth factor 2. Eur Urol 2009;55:739–42. [DOI] [PubMed] [Google Scholar]
- [60].Hosaka S, Katagiri H, Wasa J, et al. Solitary fibrous tumor in the pelvis: induced hypoglycemia associated with insulin-like growth factor II. J Orthop Sci 2015;20:439–43. [DOI] [PubMed] [Google Scholar]
- [61].Hu Y, Mahar TJ, Hicks DG, et al. Malignant solitary fibrous tumor: report of 3 cases with unusual features. Appl Immunohistochem Mol Morphol 2009;17:451–7. [DOI] [PubMed] [Google Scholar]
- [62].Kishi K, Homma S, Tanimura S, et al. Hypoglycemia induced by secretion of high molecular weight insulin-like growth factor-II from a malignant solitary fibrous tumor of the pleura. Intern Med 2001;40:341–4. [DOI] [PubMed] [Google Scholar]
- [63].Fritzsche C, Reisinger EC, Oerter R. Hypoglycemia and finger clubbing-consider Doege–Potter. Am J Med 2013;126:e35–6. [DOI] [PubMed] [Google Scholar]
- [64].Lee CE, Zanariah H, Masni M, et al. Solitary fibrous tumour of the pleura presenting with refractory non-insulin mediated hypoglycaemia (the Doege–Potter syndrome). Med J Malaysia 2010;65:72–4. [PubMed] [Google Scholar]
- [65].Krishnan L, Clark J. Non-islet cell tumour hypoglycaemia. BMJ Case Rep 2011;2011:bcr0220113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mohammedi K, Abi Khalil C, Olivier S, et al. Paraneoplastic hypoglycemia in a patient with a malignant solitary fibrous tumor. Endocrinol Diabetes Metab Case Rep 2014;2014:140026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jang JG, Chung JH, Hong KS, et al. A case of solitary fibrous pleura tumor associated with severe hypoglycemia: Doege–Potter syndrome. Tuberc Respir Dis (Seoul) 2015;78:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mathez ALG, Moroto D, Dib SA, et al. Seborrheic keratoses and severe hypoinsulinemic hypoglycemia associated with insulin grow factor 2 secretion by a malignant solitary fibrous tumor. Diabetol Metab Syndr 2016;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]


