Supplemental Digital Content is available in the text
Keywords: induction therapy, lupus nephritis, monotherapy, pure class V lupus nephritis, rituximab, systemic lupus erythematosus
Abstract
The optimal treatment for pure membranous lupus nephritis (MLN) remains undetermined. Rituximab constitutes a promising therapeutic option for lupus nephritis and is currently being evaluated for use in idiopathic membranous nephritis. We retrospectively analysed the efficacy and tolerance of rituximab as a monotherapy in the induction treatment of pure MLN.
We retrospectively investigated SLE patients with biopsy-proven pure class V lupus nephritis presenting with a protein-to-creatinine ratio of at least 2 g/g and treated with rituximab as monotherapy. A background low dose of corticosteroids (≤20 mg/day) was allowed, as was hydroxychloroquine; higher doses of steroids and/or immunosuppressive drugs fell under the exclusion criteria. Remission status was evaluated at baseline and 6, 12, and 24 months after rituximab.
The study included 15 patients (13 women, median age 37 years, 27% with extra-renal manifestations, median SLE duration 1.5 years). The median protein-to-creatinine ratio was 4.9 g/g, 80% of the patients had nephrotic-range proteinuria, the median serum albumin was 24 g/L, the median serum creatinine was 0.7 mg/dL, and the median eGFR was 122 mL/min/1.73 m2. The median follow-up was 29 months (6–112 months). Treatment failure occurred in 2 patients. However, remission was recorded in the remaining 13 (87%, complete remission in 8 patients) with a median time to remission of 5 months. Median proteinuria decreased from 4.9 g/g to 0.16 g/g at month 12 and to 0.11 g/g at month 24. Median serum albumin increased to 36.5 g/L at month 24, and all patients had serum albumin levels greater than 30 g/L at month 12. Renal function remained stable in all patients. Relapse of proteinuria was recorded in 3 patients (at 12, 29, and 34 months). No patients experienced serious adverse events.
Rituximab as monotherapy may represent an effective treatment for pure MLN with an excellent tolerance profile.
1. Introduction
Lupus nephritis is a severe complication of systemic lupus erythaematosus (SLE). Lupus nephritis affects between 20% and 60% of patients[1,2] and is associated with a poor renal and cardiovascular prognosis. Progression to end-stage kidney disease (ESRD) occurs in 10% to 30% of patients despite treatment.[2]
Class V or pure membranous lupus nephritis (MLN) occurs in 7% to 20% of patients with lupus nephritis.[3,4] The prognosis of MLN is generally considered better than proliferative forms (class III or class IV A +/− C +/− V lupus nephritis). However, many observations support the rationale for specific treatment in some patients with MLN. First, the risk of progression to ESRD remains significant. Indeed, despite heterogeneous data and few series, up to 25% of patients progress to ESRD at 10 years of follow-up. High proteinuria is a risk factor for this poor renal outcome.[5,6] Additionally, the clinical presentation of MLN is dominated by nephrotic syndrome, exposing patients to morbidities such as hypercoagulability, thrombosis, hyperlipidaemia, and increased cardiovascular risk.[5,7] Therefore, a specific therapy is recommended for patients presenting with subnephrotic or nephrotic proteinuria.[8,9]
There are no evidence-based guidelines regarding treatment for MLN. Based on observational studies or sporadic reports, most authors suggest a treatment including glucocorticoids combined with immunosuppressive agents such as cyclophosphamide,[10] mycophenolate mofetil,[11] azathioprine,[12] or cyclosporine,[10,13] considering that steroids alone are ineffective.[10,14,15] However, these drugs are associated with many adverse effects, and their long-term efficiency remains unclear.
Rituximab is a chimeric anti-CD20 antibody that induces a long-lasting B cell depletion. Rituximab monotherapy is effective and well tolerated in idiopathic membranous nephropathy[16,17] and represents a promising treatment option when associated with steroids and/or immunosuppressive drugs in lupus nephritis,[18] including patients with pure MLN.[19–21] Thus, we questioned whether rituximab as monotherapy could be of interest in pure MLN.
We conducted this retrospective study to provide data on the efficacy and safety of rituximab alone in SLE patients with pure MLN.
2. Patients and methods
2.1. Patients
This retrospective study was conducted according to the principles expressed in the Declaration of Helsinki. The ethics committee of the Groupe Coopératif sur le Lupus Rénal approved the study. The study registry was declared to the Commission Nationale de l’Informatique et des Libertés with the registration number 2070868.
We retrospectively identified all patients who received rituximab as monotherapy for pure MLN in 27 French medical centers during the past 10 years. Eligible patients fulfilled the following inclusion criteria: (1) diagnosis of SLE based on 4 or more of the 11 revised criteria of the American College of Rheumatology (ACR) for the classification of SLE;[22] (2) biopsy-proven pure membranous lupus nephritis (class V but not classes IIIA+V or IVA+V) according to the International Society of Nephrology/Renal Pathology Society 2003 (ISN/RPS) classification,[23] adequate kidney biopsy containing at least 10 glomeruli and performed less than 12 months before initiation of rituximab; (3) protein-to-creatinine ratio of at least 2 g/g; (4) induction treatment for pure MLN with rituximab alone. We excluded patients with membranous nephropathy who did not have a clear clinical or histological diagnosis of SLE and patients who received concomitantly high-dose corticosteroids (>20 mg per day) and/or immunosuppressive therapy for MLN (e.g., cyclophosphamide, mycophenolate mofetil, azathioprine, cyclosporine, or tacrolimus) with the exception of corticosteroids infused at low doses with rituximab for its tolerability. Patients treated with background steroids ≤20 mg per day with hydroxychloroquine, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers in addition to rituximab could be included.
2.2. Evaluation
Response was evaluated by assessing clinical activity and renal response at baseline (initiation of rituximab therapy) and after 6, 12, and 24 months of treatment. Serial evaluation of CD19-positive blood cells count was not analyzed due to missing data.
2.3. Efficacy assessment
2.3.1. Renal response
We evaluated renal response according to definitions proposed by KDIGO guidelines and the Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) consensus statement.[8,9]
Complete renal remission (CR) was defined as a urine protein to creatinine ratio (UPCR) <0.5 g/g and normal or near-normal (within 10% of normal GFR if previously abnormal) GFR.
Partial renal response (PR) was defined as a ≥50% reduction in proteinuria to subnephrotic levels and normal or near-normal GFR.
Early failure was considered for patients in whom the physician added an immunosuppressive therapy and/or increased the steroids dosage to greater than 20 mg per day during the 12 months after the first rituximab infusion. Patients who were reinfused with rituximab were not included in this category.
Nonresponders (NR) were patients with no CR or PR AND with no early failure.
Rituximab failure was considered for nonresponders after 12 months postrituximab AND for patients with early failure.
Proteinuric flares (relapse) include reproducible doubling of UPCR to >1 g/g after complete response or reproducible doubling of UPCR to >2 g/g after partial response.
2.3.2. Nonrenal manifestations of SLE
Disease activity was assessed for each patient using the SLEDAI score. We also reported all clinical or biological manifestations related to lupus activity.
2.4. Statistical analysis
Descriptive statistical analyses were performed. Data were represented as the medians (range) for continuous variables and frequencies for categorical variables.
Kaplan–Meier plots were constructed for the probability of remission and time to flare. For these analyses, the Prism (GraphPad Software Inc., La Jolla, CA) software program was used. Results with P values less than .05 were considered significant.
3. Results
3.1. Baseline characteristics
The study included 15 patients (13 females; 87%). Patient characteristics are detailed in Table 1. The median age at inclusion was 37 years, and the median SLE duration before inclusion was 1.5 years. Previous renal flares were recorded in 6 patients, as indicated in Table 1, and 5 patients had received prior immunosuppressive therapy. At MLN diagnosis, 9 were treated with HCQ and 10 with steroids. Concurrent nonrenal manifestations of SLE (articular in 3 patients, cutaneous in 3 patients, and a pericarditis in 1 patient) were recorded in 4 patients (27%). The median SELENA-SLEDAI score was 10. Anti-dsDNA antibodies and/or hypocomplementemia were detected in eleven patients (73%). The median proteinuria was 4.9 g/g (2–28 g/g); the nephrotic range proteinuria was present in 12 patients (80%), nephrotic syndrome in 9 patients (60%), and microscopic haematuria in 9 patients (60%). The median albuminemia was 24 g/L (9.5–35 g/L). The median serum creatinine was 0.69 mg/dL and median eGFR was 122 mL/min/1.73 m2 according to the CKD-EPI formula.
Table 1.
Individual characteristics of patients at baseline and during follow-up.

Thrombotic events at diagnosis of MLN occurred in 3 patients (20%) with nephrotic syndrome (pulmonary embolism in 2, renal vein thrombosis in 2). None were diagnosed with antiphospholipid syndrome.
Rituximab was initiated for the current MLN as a second-line therapy in 3 patients with a persistent proteinuria after a first treatment combining mycophenolate mofetil in 2 of them or azathioprine with steroids in 1.
3.2. Rituximab therapy
In all patients, rituximab was initiated after 3 to 6 months of nonresolving proteinuria. Rituximab was administered as 2 infusions of 1 g at day 0 and day 14 in 9 patients (60%) and 375 mg/m2 once per week for 4 weeks in 6 patients (40%). Premedication with 60 mg of methylprednisolone was given to all patients prior to each infusion of rituximab. A concurrent treatment with low-dose oral steroids (median dose = 7 mg per day; 2.5 mg to 20 mg) was given in 13 patients at rituximab initiation. All patients but one (93%) were treated with hydroxychloroquine at inclusion (initiated in 5 and previously given in 9).
A re-administration of rituximab was performed in 4 patients during the first 12 months, 2 for NR at months 8 and 9, 2 for partial response at month 6 (Table 1).
3.3. Follow-up and renal outcome
The median follow-up was 29 months (6–112 months). Individual patient statuses during follow-up are given in Table 1, and whole-cohort characteristics are shown in Table 2. Early failure was recorded in 2 patients due to MMF introduction in the first 12 months: at month 4 in patient 6 in a context of persistent proteinuria and in patient 14 at month 9, despite a recorded PR with a 60% decrease in proteinuria. The remaining 13 patients (87% of the cohort) experienced remission in the 12 months following rituximab, CR in 9 (60% of the entire cohort) or PR in the remaining patients. The median time to PR or CR was 5 months (10 months for CR). The cumulative probability of remission was of 73% at month 6 and 87% at month 12 (Fig. 1A).
Table 2.
Patient characteristics at baseline and follow-up.

Figure 1.
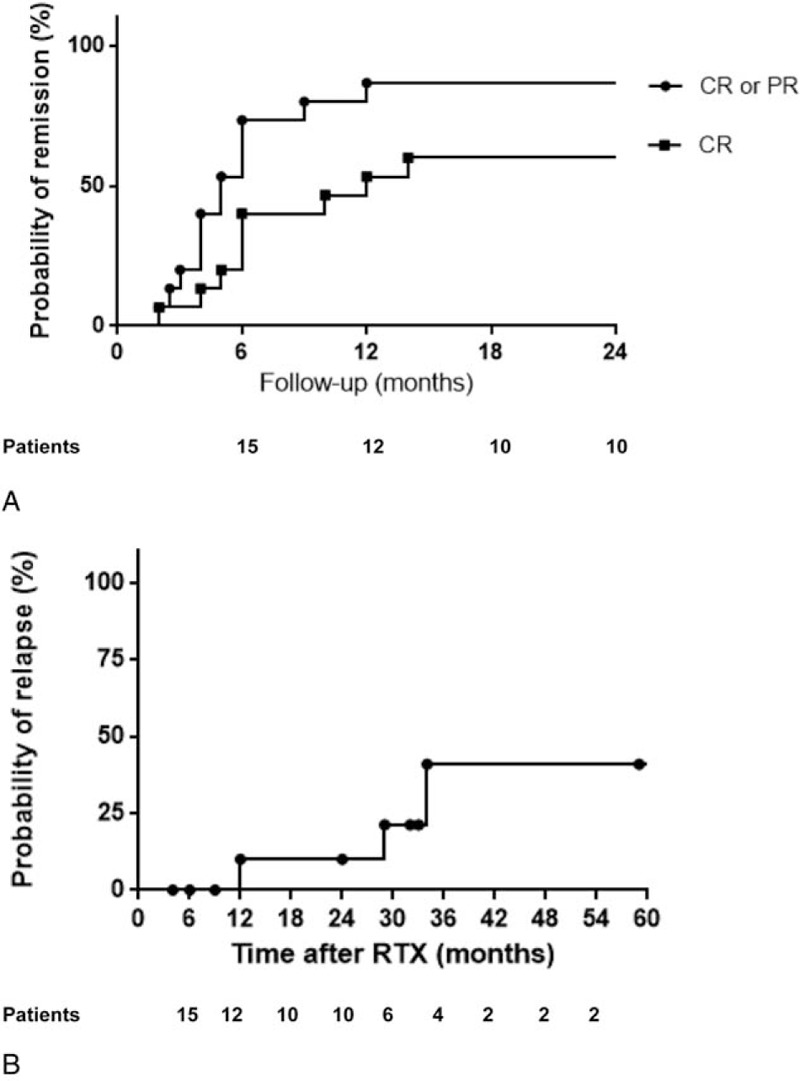
(A) Probability of remission and the absence of rituximab failure from the first rituximab infusion. (B) Probability of relapse from the first rituximab infusion. (Kaplan-Meier plots were constructed for the probability of remission and time to flare).
Median proteinuria improved from 4.9 g/g at presentation to 0.2 g/g at month 12 and 0.1 g/g at month 24. There was a significant increase in serum albumin with a median value rising from 25.5 g/L at diagnosis to 37.5 g/L at month 12 and 36.5 g/L at month 24. Notably, all patients had an albumin greater than 30 g/L at month 12. Renal function remained stable in all patients during all the follow-up.
Proteinuria relapsed in 3 patients (20%) in whom CR had been obtained (at month 12, at month 34, between months 12 and 29). The cumulative risk of relapse was of 10% at month 12 and 41% at month 36 (Fig. 1B). Renal biopsy at relapse showed a pure MLN (patient 2) and class III+V LN (patient 5) (no renal biopsy in patient 10; her clinician assumed that proteinuria relapse was related to pure MLN).
In other patients, persistent remission was recorded after month 24 (in 7 of the 9 patients [78%] with a long follow-up). During follow-up, 2 patients became pregnant, and no relapse occurred during or after pregnancy (See Supplemental Fig. 1 that shows the flow chart of patients treated with rituximab).
Patient 7 was re-treated with rituximab after month 12: at month 17 and month 29 for PR (Table 1).
CD19-positive blood cell counts could not be analyzed regarding relapse or remission status during follow-up due to missing data.
3.4. Nonrenal lupus activity
Rituximab was efficient for extra-renal manifestations in all 4 patients who presented with SLE nonrenal manifestations at inclusion. Patient 12, who was in sustained CR since month 4, was treated with methotrexate at month 16 for a cutaneo-articular flare of SLE. Among the remaining patients, none required increased corticosteroids doses or the initiation of an additional IS therapy for extra-renal manifestations of SLE. The median SELENA-SLEDAI score decreased from 10 at baseline to 4 at 24 months (Table 2).
3.5. Adverse events
Rituximab infusions were well tolerated. One patient complained of sore throat during rituximab infusion that resolved after slowing the infusion rate. During follow-up, only 2 adverse events were reported in 2 different patients: a nonsevere rhino-bronchitis 6 months after rituximab that resolved without antibiotics, and a cutaneous herpes zoster virus infection 26 months after rituximab.
4. Discussion
In this multicenter retrospective study, we show that the use of rituximab as monotherapy for pure MLN with high-level proteinuria is associated with a subsequent high rate of remission (87%) and an excellent tolerance profile. No other study has investigated the effects of rituximab in SLE without any other associated therapy, immunosuppressive agents, or steroids background.
Combinations of steroids or other immunosuppressive agents and B-cell depletion therapy with rituximab have recently been added to treatment options in MLN.[8,9,24] In addition to its well-demonstrated efficacy and safety for non-Hodgkin lymphomas,[25] rituximab has been shown to be effective in several autoimmune diseases including SLE.[18,26] Although the most influential clinical trial investigating rituximab for proliferative LN failed to demonstrate a higher efficacy when added to steroids and mycophenolate,[27] successful or promising experiences have been recorded using rituximab with other drug combinations in other clinical contexts including MLN.[19,28] Interestingly, rituximab in association with mycophenolate but no steroids (the rituxilup regimen) has been shown to be of potential interest in treating LN including MLN.[18] Lightstone and colleagues are currently evaluating this strategy in an ongoing prospective controlled trial. In this context, and as proposed for severe idiopathic membranous nephropathy,[16,17] we proposed rituximab monotherapy for MLN with high-level proteinuria, a strategy that we preliminarily evaluated in this multicenter retrospective cohort study. Our primary conclusion is that rituximab as monotherapy may be effective and safe in patients with pure MLN. Indeed, 13 of the 15 patients (87%) experienced remission of severe proteinuria in a median time of 5 months in the absence of serious adverse events. Long-lasting CR (more than 2 years after rituximab infusion) were observed in 7 of 9 patients with a follow-up of more than 2 years. Conversely, only 3 patients experienced proteinuria relapse.
Several points support the underlying role of rituximab instead of a spontaneous remission of proteinuria: (i) We observed higher remission rates than previous series of untreated or steroids-only treated patients in which remission occurred in 7 to 50% of patients.[3,5,14,15] (ii) Most of the patients in the study (80%) presented with a nephrotic-range proteinuria, a condition that is rarely associated with spontaneous remission.[3]
HCQ was shown to increase the remission rate in pure MLN. In the present study, we feel that remission of proteinuria was unlikely to be related to HCQ instead of rituximab because most of patients were treated by HCQ for years when MLN-related proteinuria occurred. Nonetheless, we cannot exclude a synergistic effect of HCQ when associated with rituximab, as previously observed when associated with azathioprine plus steroids or mycophenolate plus steroids.[29]
Rituximab alone in our series appears to induce a success rate similar to that in typical therapies. Previous reports assessed CR and PR in 67% and 22% of patients, respectively, under steroids plus azathioprine at 12 months with a relapse rate of 19%;[12] in 55% and 35% of patients, respectively, under steroids plus cyclophosphamide; and in 40% to 62% and 15 to 20% of patients, respectively, treated with steroids plus mycophenolate mofetil.[11,30] In the only randomized controlled trial dedicated to MLN by Austin et al,[10] the cumulative probability of remission was 83% in patients treated with steroids plus ciclosporin and 60% in those treated with steroids plus cyclophosphamide. In light of these data, the present study suggests that rituximab may be as effective as these drugs in inducing remission with a better safety profile. Furthermore, as previously suggested,[21] our data indicate that rituximab may also be effective in extra-renal manifestations of SLE and in reducing disease activity.
Our study's limitations include the small number of patients in and its retrospective nature, which introduces a risk of the overrepresentation of successful cases and an overestimation of rituximab monotherapy benefits.
In conclusion, although retrospective, this study is a first attempt to assess the efficacy of rituximab monotherapy in pure MLN associated with high-level proteinuria. This study demonstrates the potential efficacy of rituximab in the management of pure MLN by producing long-term proteinuria remission and an excellent tolerance profile. Double-blind controlled trials are now required to evaluate the benefit of rituximab as monotherapy in pure MLN.
Acknowledgments
The authors thank the HUPNVS of Assistance Publique - Hôpitaux de Paris for funding publication fees.
Supplementary Material
Footnotes
Abbreviations: ACR = American College of Rheumatology, CKD = chronic kidney disease, CR = complete remission, ESRD = end-stage kidney disease, EULAR/ERA-EDTA = European Renal Association–European Dialysis and Transplant Association, HCQ = hydroxychloroquine, MLN = pure membranous lupus nephritis, NRs = nonresponders, PR = partial response, SLE = systemic lupus erythematosus, UPCR = urine protein to creatinine ratio.
Authorship: NC contributed to the data collection and data interpretation, performed the statistical analysis, designed the tables and figures, and participated in drafting and editing further drafts of the manuscript. ED conceived and designed the study, participated in data interpretation and the writing of the manuscript, and provided critical comments on the manuscript. All authors were involved in providing study materials and patient care and in reviewing the manuscript. All authors have approved the final version of the manuscript for submission.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Cameron JS. Lupus nephritis. J Am Soc Nephrol 1999;10:413–24. [DOI] [PubMed] [Google Scholar]
- [2].Hanly JG, O’Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016;55:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baldwin DS, Gluck MC, Lowenstein J, et al. Lupus nephritis. Clinical course as related to morphologic forms and their transitions. Am J Med 1977;62:12–30. [DOI] [PubMed] [Google Scholar]
- [4].Mok CC. Membranous nephropathy in systemic lupus erythematosus: a therapeutic enigma. Nat Rev Nephrol 2009;5:212–20. [DOI] [PubMed] [Google Scholar]
- [5].Mercadal L, Montcel ST, Nochy D, et al. Factors affecting outcome and prognosis in membranous lupus nephropathy. Nephrol Dial Transplant 2002;17:1771–8. [DOI] [PubMed] [Google Scholar]
- [6].Bono L, Cameron JS, Hicks JA. The very long-term prognosis and complications of lupus nephritis and its treatment. QJM 1999;92:211–8. [DOI] [PubMed] [Google Scholar]
- [7].Pasquali S, Banfi G, Zucchelli A, et al. Lupus membranous nephropathy: long-term outcome. Clin Nephrol 1993;39:175–82. [PubMed] [Google Scholar]
- [8].Bertsias GK, Tektonidou M, Amoura Z, et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 2012;71:1771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient. Kidney Int 2012;82:840–56. [DOI] [PubMed] [Google Scholar]
- [10].Austin HA, 3rd, Illei GG, Braun MJ, et al. Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol 2009;20:901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Radhakrishnan J, Moutzouris DA, Ginzler EM, et al. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int 2010;77:152–60. [DOI] [PubMed] [Google Scholar]
- [12].Mok CC, Ying KY, Yim CW, et al. Very long-term outcome of pure lupus membranous nephropathy treated with glucocorticoid and azathioprine. Lupus 2009;18:1091–5. [DOI] [PubMed] [Google Scholar]
- [13].Radhakrishnan J, Kunis CL, D’Agati V, et al. Cyclosporine treatment of lupus membranous nephropathy. Clin Nephrol 1994;42:147–54. [PubMed] [Google Scholar]
- [14].Donadio JV, Jr, Burgess JH, Holley KE. Membranous lupus nephropathy: a clinicopathologic study. Medicine (Baltimore) 1977;56:527–36. [DOI] [PubMed] [Google Scholar]
- [15].Gonzalez-Dettoni H, Tron F. Membranous glomerulopathy in systemic lupus erythematosus. Adv Nephrol Necker Hosp 1985;14:347–64. [PubMed] [Google Scholar]
- [16].Remuzzi G, Chiurchiu C, Abbate M, et al. Rituximab for idiopathic membranous nephropathy. Lancet 2002;360:923–4. [DOI] [PubMed] [Google Scholar]
- [17].Dahan K, Debiec H, Plaisier E, et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol 2017;28:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis 2013;72:1280–6. [DOI] [PubMed] [Google Scholar]
- [19].Melander C, Sallee M, Trolliet P, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol 2009;4:579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Catapano F, Chaudhry AN, Jones RB, et al. Long-term efficacy and safety of rituximab in refractory and relapsing systemic lupus erythematosus. Nephrol Dial Transplant 2010;25:3586–92. [DOI] [PubMed] [Google Scholar]
- [21].Terrier B, Amoura Z, Ravaud P, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010;62:2458–66. [DOI] [PubMed] [Google Scholar]
- [22].Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- [23].Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004;65:521–30. [DOI] [PubMed] [Google Scholar]
- [24].Gregersen JW, Jayne DR. B-cell depletion in the treatment of lupus nephritis. Nat Rev Nephrol 2012;8:505–14. [DOI] [PubMed] [Google Scholar]
- [25].Grillo-Lopez AJ, White CA, Varns C, et al. Overview of the clinical development of rituximab: first monoclonal antibody approved for the treatment of lymphoma. Semin Oncol 1999;26(5 suppl 14):66–73. [PubMed] [Google Scholar]
- [26].Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572–81. [DOI] [PubMed] [Google Scholar]
- [27].Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012;64:1215–26. [DOI] [PubMed] [Google Scholar]
- [28].Diaz-Lagares C, Croca S, Sangle S, et al. Efficacy of rituximab in 164 patients with biopsy-proven lupus nephritis: pooled data from European cohorts. Autoimmun Rev 2012;11:357–64. [DOI] [PubMed] [Google Scholar]
- [29].Kasitanon N, Fine DM, Haas M, et al. Hydroxychloroquine use predicts complete renal remission within 12 months among patients treated with mycophenolate mofetil therapy for membranous lupus nephritis. Lupus 2006;15:366–70. [DOI] [PubMed] [Google Scholar]
- [30].Kasitanon N, Petri M, Haas M, et al. Mycophenolate mofetil as the primary treatment of membranous lupus nephritis with and without concurrent proliferative disease: a retrospective study of 29 cases. Lupus 2008;17:40–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


