Abstract
Over the years, with the advancement in hematology analyzer technology, the use of fluid analysis method has seen a drastic increase in clinical examinations. Cell counting and classification in independent body fluid analysis method are conducted by semiconductor laser flow cytometry and nucleic acid fluorescence staining techniques. This study is to evaluate the efficacy of Sysmex XN-1000 hematology analyzer in cell counting and to screen malignant cells with serous cavity effusion. Specimens (N = 206) with serous cavity effusion from our hospital were included in this study. Manual and instrumental methods for cell counting, nucleated cell classification, and high-fluorescent cells (HFC) were used in this study. The correlation between RBC, nucleated cell count (NUC), the percentages of polymorphonuclear cell (PMN%), and mononuclear cells (MN%) was statistically analyzed using manual and instrumental methods. The regression equations of RBC, NUC, PMN%, and MN% in the manual and instrumental methods were RBC y = 0.88x + 426.4; NUC y = 0.85x + 33.4; PMN% y = 0.91x + 4.2; and MN% y = 0.91x + 5.1. Correlation coefficient R2 was 0.99, 0.98, 0.90, and 0.90 (P < .001). ROC curve analysis showed that when the cut-off value of HFC% was 4.4% and HFC# was 24.5/μL, area under curve (AUC), sensitivity, specificity, and 95% confidence interval were 0.707, 0.792, 0.558, 0.637–0.777; 0.708, 0.753, 0.550, 0.635–0.780, respectively. XN-1000 hematology analyzer body fluid method can accurately and rapidly count cell and nucleated cell classification with serous cavity effusion. HFC can indicate the possible existence of malignant cells; however, further investigations are required to validate its efficacy.
Keywords: HFC, malignant cells, serous cavity effusion, XN-1000 hematology analyzer
1. Introduction
Cell count, nucleated cell classification, and exfoliative cytology of serous cavity effusion are critical in clinical judgment of the fluid nature.[1] These methods are not only convenient and rapid, but also provide cytological evidence for the clinical examinations. Until now, manual Neubauer hemocytometer and nucleated cell classification after slide-making and staining are still the “gold standard.”[2] However, these methods are time-consuming, laborious, and demand stringent specifications for technical personnel, with poor reproducibility.[3,4] Over the years, with the advancement in hematology analyzer technology, the use of fluid analysis method has been found to be effective for clinical examinations. Cell counting and classification in independent body fluid analysis method in Sysmex XE-5000 and XN-1000 hematology analyzer (Sysmex Corporation, Kobe, Japan) are carried using semiconductor laser flow cytometry and nucleic acid fluorescence staining techniques. There have been a few reports on the performance evaluation of body fluid mode and malignant cell screening,[4–7] the evidence to validate the efficacy of automatic nucleated cell counting, nucleated classification, and malignant cell screening of serous cavity effusion is still very rare.
2. Specimen sources
Two hundred six specimens with serous cavity effusion were collected from inpatients in the First Affiliated Hospital of Zhejiang University from October 2015 to May 2017. Among them, 146 cases were male, with an average age of 59 years old, and 60 cases were female, with an average age of 55 years old. Ninety-five cases were associated with pleural effusion, while 111 cases were associated with ascites. Based on the existence of tumor cells in the effusion cytology, these cases were divided into malignant effusion group of 77 cases and nonmalignant effusion group of 129 cases. Specimens with more than 10% of denatured cells or viscous specimens were excluded from the test. This project was approved by the ethics committee of the First Affiliated Hospital of Zhejiang University Medical College. Informed consents have obtained.
3. Specimen detection
Specimens were collected, stored, transported, and detected according to the requirements of CLSI H56-A document.[2] Specimens with EDTA-K2 anticoagulation were collected and transported immediately after collection. Cell detection was performed by instrumental method and manual cell counting, centrifuged for 5 min at 400g, and sediments were kept on slides for nucleated cell classification using Wright–Giemsa staining and pathologic examination was performed using hematoxylin–eosin (HE) stained, followed by immunocytochemistry if applicable. Manual cell count and nucleated cell classification were completed using 2 experienced microscope operators independently, and the count results required CV <10%. Nucleated cells in each specimen were classified by identifying 200 nucleated cells, and identifications from cytology experts were taken for dissents. The neutrophils, eosinophils, and basophils were classified as PMN cells, and lymphocytes, plasma cells, mesothelial cells, macrophages, and malignant cells were classified as MN cells. Cell counting and classification of the samples were manually detected by XN-1000 hematology analyzer in the body fluid mode through the instrument electrical impedance, flow cytometry, and nucleic acid fluorescence staining and other techniques. Main detecting parameters were RBC, WBC, PMN#, PMN%, MN#, MN%, HFC#, HFC%, and WBC and HFC# were classified as NUC. Cell counting, slides making and staining, and instrumental analysis were completed within 2 h after receiving the samples.
4. Instruments and reagents
Cells were stained by Wright–Giemsa stain (BASO) and manually counted by Neubauer hemocytometer. Exfoliative cells were detected by HE stain. RBC, NUC, and NUC differential counts were measured in duplicate on the Sysmex XN-1000 in body fluid open mode. Two levels (low and high) of body fluid XN-check were measured before sample analysis.
5. Statistical method
SPSS17.0 statistical software and linear regression analysis were used to compare the results of the 2 methods, whereas Mann–Whitney U test was used for comparison between the groups. ROC curve was used to analyze the cut-off value, AUC, sensitivity, and specificity of HFC% and HFC# in malignant effusion screening, and the difference of P < .05 was considered statistically significant.
6. Results
The linear regression analysis of the cell count and nucleated cell classification by manual method and instrumental method were red blood cell count y = 0.88x + 426.4, R2 = 0.99, P < .001; nucleated cell count y = 0.85x + 33.4, R2 = 0.98, P < .001; nucleated cell classification PMN% y = 0.91x + 4.2, R2 = 0.90, P < .001; and MN% y = 0.91x + 5.1, R2 = 0.90, P < .001, respectively. The correlation scatter plots of each parameter in 2 methods are shown in Fig. 1.
Figure 1.
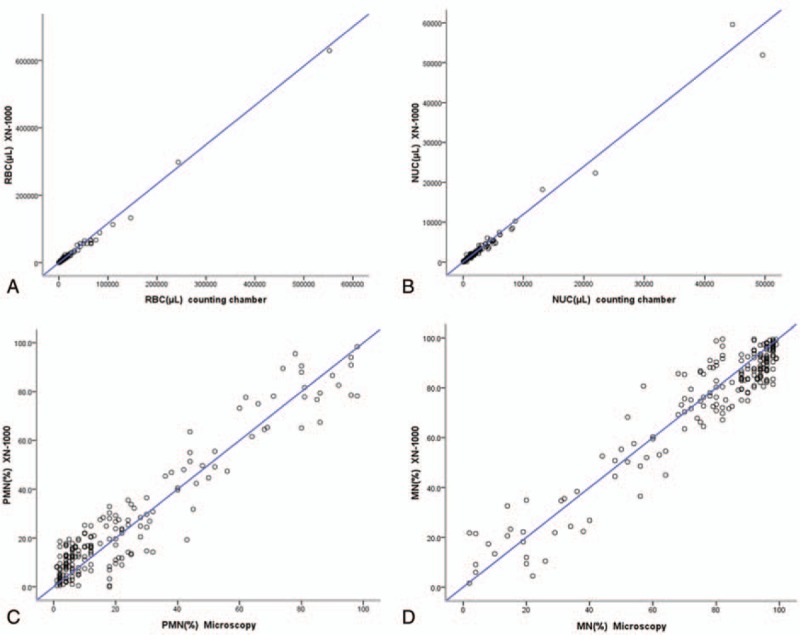
Correlation of cell counts from body fluids between automated and manual counting methods. (A) RBC count; (B) NUC count; (C) PMN cell count; (D) MN cell count. MN = mononuclear cells, NUC = nucleated cell count, PMN = polymorphonuclear cell, RBC = red blood cell.
ROC curve was used to analyze the ability of HFC% and HFC# in malignant cell screening. When the cut-off values of HFC% and HFC# were 4.4% and 24.5/μL, AUC, sensitivity, specificity, and 95% confidence interval were 0.707, 0.792, 0.558, 0.637–0.777 and 0.708, 0.753, 0.550, 0.635–0.780, respectively (Fig. 2).
Figure 2.
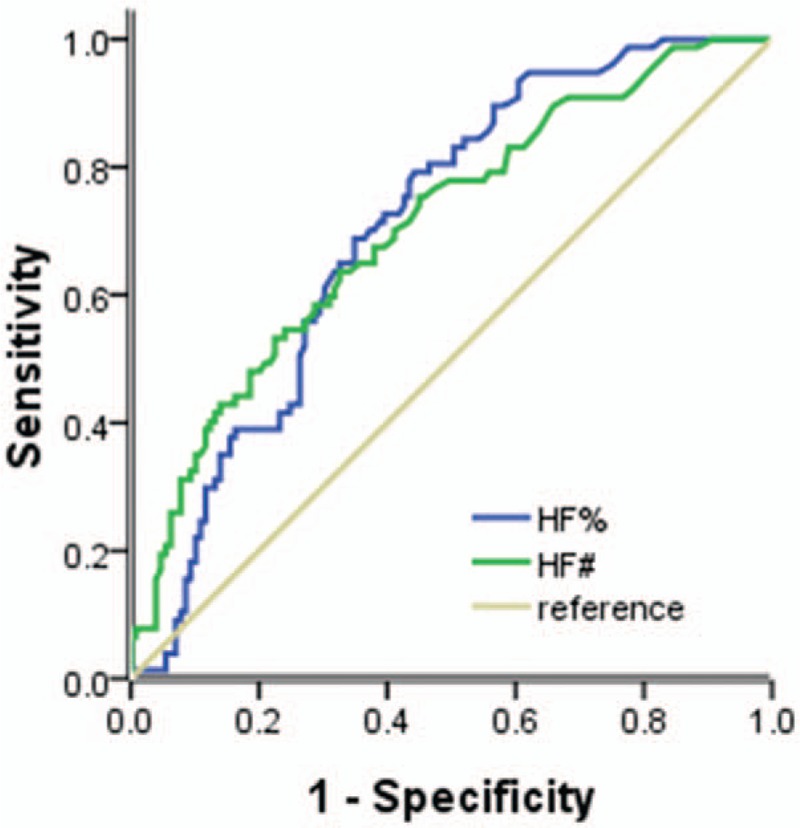
HFC% and HFC# receiver operating characteristic curves for predicting fluids containing malignant cells. HFC = high-fluorescent cells.
Cell count, nucleated cell classification, and high fluorescent cell count in malignant effusion group and nonmalignant effusion group were compared using XN-1000 hematology analyzer body fluid mode (Table 1). There were no significant differences in RBC, NUC, PMN%, or MN% between the 2 groups. However, HFC# and HFC% were significantly different between the 2 groups (P < .001; Fig. 3).
Table 1.
Comparison of cell counts and classification results by XN-1000 hematology analyzer between the 2 groups.

Figure 3.
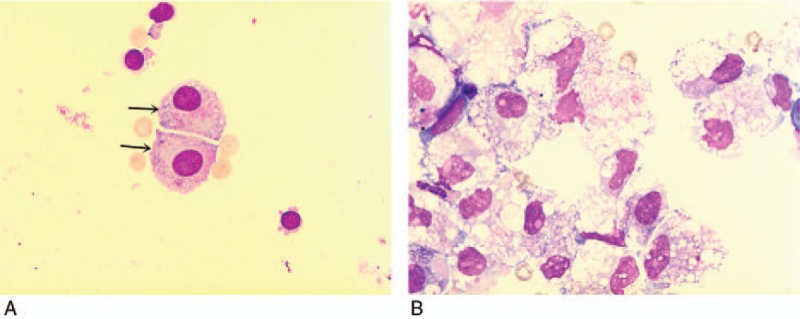
High proportion of macrophages and/or mesothelial cells results in the high values of HFC% and HFC#, causing false positive. (A) The arrow signified is mesothelial cells; (B) a lot of macrophages. Magnification A and B (×1000). HFC = high-fluorescent cells.
7. Discussion
It is well known that cell count and nucleated cell classification of serous cavity effusion are effective in identifying the damage or infection to the organs and provide useful laboratory evidence for the diagnosis and treatment of diseases.[8,9] The traditional method of manual cell counting by Neubauer hemocytometer is time-consuming and is usually associated with poor reproducibility. In order to develop automated testing, blood mode of hematology analyzer is used in the analysis of body fluid cells. However, blood mode detected body fluid and blood components with the same stroma, which could not overcome the matrix effect caused by the different components of the blood and body fluid. Also, the interference from mesothelial cells, macrophages, and tumor cells may contribute to the error of cell classification results.[10–12] Reports on the cell count of body fluid by urine flow cytometry analyzer are also available.[13] This method has the advantage of rapid and accurate cell count, but it cannot classify nucleated cells. In addition, Walker et al[14] detected body fluid cells using iQ200 urine sediment analyzer. The study showed good correlation with manual cell counts, but could not compare the 2 methods for efficacy in nucleated cell classification. In addition, the requirement for sample volume of this method is relatively high.
These findings showed that XN-1000 hematology analyzer body fluid mode could be used for the cell count of serous cavity effusion. Also, the method exhibits a good correlation with manual method. The correlation coefficients of RBC and NUC were 0.99 and 0.98, respectively, which were consistent with the reported literature.[5,7] XN-1000 hematology analyzer body fluid mode is capable of classifying and counting nucleated cells, including neutrophils, lymphocytes, monocytes, eosinophils, and HFC. The neutrophils, eosinophils, and basophils were classified as polymorphocytes (PMN), and lymphocytes, macrophages, mesothelial cells, and malignant cells were classified as monocytes (MN). HFC was able to bind more nucleic acid fluorescent dyes, thereby resulting in a high-fluorescent reading of the cells, including tumor cells, mesothelial cells, and macrophages.[15] The cell classification by instrumental method and manual staining microscopy method had a high correlation, PMN% was similar to MN%, both were 0.90, respectively. This finding was consistent with the report of Cho et al,[7] but slightly weaker than what was reported by Fleming et al. The main reason for this was that the testing specimens had a high proportion of peritoneal dialysis fluid, and the test was not focused on malignant effusions, which resulted in a great improvement in the correctness of the instrument. The cut-off values of HFC% and HFC# were 4.4% and 24.5/μL, respectively, whereas the values for AUC and sensitivity and specificity were 0.707, 0.792, 0.558 and 0.708, 0.753, 0.550, respectively, which were slightly lower than what was reported in the literature.[6,7] During chronic inflammation, the number of mesothelial cells and macrophages were significantly increased in the effusion, and their nuclei contained more nucleic acids, which can be combined with more nucleic acid fluorescent dyes, and thus can be classified as high-fluorescent nucleated cells. In this experiment, we observed that liver cirrhosis ascites specimens contained many high-fluorescence nucleated cells, which were mainly classified as mesothelial cells and/or macrophages by microscopic examination Fig. 3. In addition, false negative results may appear in the specimens, with little tumor cells.
In conclusion, XN-1000 hematology analyzer fluid mode is capable of rapid cell count and nucleated cell classification rapidly and accurately and can be used as a rapid screening tool for laboratory analysis of humoral cells. However, the composition of body fluid samples is complex. Therefore, when the scatter plot is abnormal, HFC exceeds the threshold value, or there are clinical suspicions, the nucleated cell classification of specimens should be confirmed to improve the quality of the analysis. The clinical operations should develop internal quality control for automated body fluid analysis and establish the corresponding standard operating procedures, quality control procedures, and inspection procedures to guide daily works.[16,17]
Footnotes
Abbreviations: HE = hematoxylin–eosin, HFC = high-fluorescent cells, MN = mononuclear cells, NUC = nucleated cell count, PMN = polymorphonuclear cell.
This project was approved by the ethics committee of the First Affiliated Hospital of Zhejiang University Medical College. Informed consents have obtained.
The study was supported by the Health and Family Planning Commission of Zhejiang Province (grant number: 2014KYB097) and the Foundation of Zhejiang University (grant number: 15-491010-026).
The authors have no conflicts of interest to disclose.
References
- [1].Karcher DS, McPherson RA. McPherson RA, Pincus MR. Cerebrospinal, synovial, serous body fluids, and alternative specimens. Henry's Clinical Diagnosis and Management by Laboratory Methods 22nd edn.Philadelphia, PA: Elsevier Saunders; 2011. 480–506. [Google Scholar]
- [2].Clinical and Laboratory Standards Institution (CLSI). Body Fluid Analysis for Cellular Composition; Approved Guideline CLSI Document H56-A. Waye, PA: CLSI; 2006. [Google Scholar]
- [3].Zimmermann M, Ruprecht K, Kainzinger F, et al. Automated vs. manual cerebrospinal fluid cell counts: a work and cost analysis comparing the Sysmex XE-5000 and the Fuchs-Rosenthal manual counting chamber. Int J Lab Hematol 2011;33:629–37. [DOI] [PubMed] [Google Scholar]
- [4].Danise P, Maconi M, Rovetti A, et al. Cell counting of body fluids: comparison between three automated haematology analysers and the manual microscope method. Int J Lab Hematol 2013;35:608–13. [DOI] [PubMed] [Google Scholar]
- [5].Fleming C, Brouwer R, Lindemans J, et al. Validation of the body fluid module on the new Sysmex XN-1000 for counting blood cells in cerebrospinal fluid and other body fluids. Clin Chem Lab Med 2012;50:1791–8. [DOI] [PubMed] [Google Scholar]
- [6].Labaere D, Boeckx N, Geerts I, et al. Detection of malignant cells in serous body fluids by counting high-fluorescent cells on the Sysmex XN-2000 hematology analyzer. Int J Lab Hematol 2015;37:715–22. [DOI] [PubMed] [Google Scholar]
- [7].Cho YU, Chi HS, Park SH, et al. Body fluid cellular analysis using the Sysmex XN-2000 automatic hematology analyzer: focusing on malignant samples. Int J Lab Hematol 2015;37:346–56. [DOI] [PubMed] [Google Scholar]
- [8].Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507–13. [DOI] [PubMed] [Google Scholar]
- [9].Joseph J, Badrinath P, Basran GS, et al. Is the pleural fluid transudate or exudate? A revisit of the diagnostic criteria. Thorax 2001;56:867–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Andrews J, Setran E, McDonnel L, et al. An evaluation of the cell-dyn 3200 for counting cells in cerebrospinal and other body fluids. Lab Hematol 2005;11:98–106. [PubMed] [Google Scholar]
- [11].Brown W, Keeney M, Chin-Yee I, et al. Validation of body fluid analysis on the Coulter LH 750. Lab Hematol 2003;9:155–9. [PubMed] [Google Scholar]
- [12].Kresie L, Benavides D, Bollinger P, et al. Performance evaluation of the application of body fluids on the Sysmex XE-2100 series automated hematology analyzer. Lab Hematol 2005;11:24–30. [PubMed] [Google Scholar]
- [13].Fleming C, Brouwer R, van Alphen A, et al. UF-1000i: validation of the body fluid mode for counting cells in body fluids. Clin Chem Lab Med 2014;52:1781–90. [DOI] [PubMed] [Google Scholar]
- [14].Walker TJ, Nelson LD, Dunphy BW, et al. Comparative evaluation of the Iris iQ200 body fluid module with manual hemacytometer count. Am J Clin Pathol 2009;131:333–8. [DOI] [PubMed] [Google Scholar]
- [15].Seo JY, Lee ST, Kim SH. Performance evaluation of the new hematology analyzer Sysmex XN-series. Int J Lab Hematol 2015;37:155–64. [DOI] [PubMed] [Google Scholar]
- [16].Cui W, Du J. Pay attention to the automated body fluid cytology analysis and quality control. Chin J Lab Med 2013;36:1057–9. [Google Scholar]
- [17].Buoro S, Mecca T, Azzara G, et al. Cell population data and reflex testing rules of cell analysis in pleural and ascitic fluids using body fluid mode on Sysmex XN-9000. Clin Chim Acta 2016;452:92–8. [DOI] [PubMed] [Google Scholar]


