Supplemental Digital Content is available in the text
Keywords: liver cancer, meta-analysis, prospective cohort studies, statins
Abstract
Previous studies have indicated that statins intake was associated with liver cancer risk, but presented controversial results.
Studies in PubMed and EMBASE were searched update to February 2017 to identify and quantify the potential dose–response association between statins intake and liver cancer.
Six eligible studies involving a total of 11,8961 participants with 9530 incident cases were included in this meta-analysis. Statistically significant association was observed between increasing statins intake and liver cancer risk reduction (OR = 0.46, 95%CI: 0.24–0.68, P <.001). Furthermore, the summary relative risk of liver cancer for an increase of 50 cumulative defined daily dose per year was 0.86 (95%CI: 0.81–0.90, P <.001). Evidence of a nonlinear dose–response relationship between statins intake and liver cancer risk was found (P for nonlinearity <.01). Subgroups analysis indicated that statins intake was associated with a significantly risk of liver cancer risk reduction in Asia (OR = 0.44, 95%CI: 0.11–0.77, P <.001) and Caucasian (OR = 0.49, 95%CI: 0.36–0.61, P <.001). Subgroup meta-analyses in study design, study quality, number of participants, and number of cases showed consistency with the primary findings.
Additional statins intake is associated with liver cancer risk reduction.
1. Introduction
Liver cancer is the fifth most common cancer worldwide in men and the sixth most common cancer worldwide in women, and costs on patients, caregivers, and society that remains the most common malignancy.[1] The etiology of liver cancer involves both genetic and environmental factors. According to the American Cancer Association statistics, liver cancer mortality gradually increased, the relative survival rate of liver cancer being 18%.[2] Based on cancer registry data available in China, the age-standardized 5-year relative survival for liver cancer is 10.1% in 2015.[3] These data reveal the poor prognosis of liver cancer, and thus to prevent the occurrence of liver cancer is essential. Previous studies investigating have showed that statins have a chemopreventive potential in the liver cancer.[4]
Statins are inhibitors of 3-hydroxy-3-methyl glutaryl coenzyme reductase A, which is a key enzyme in the rate-limiting step in cholesterol synthesis.[5] Statins are widely prescribed in the primary and secondary prevention of heart attack, stroke, and cardiovascular disease.[6] Recently, statin use has been reported to have a promising anticancer effect,[7] and statin monotherapy could potentially reduce any organ and colorectal cancer-related mortality.[8,9] Additionally, studies showing statin use has been found to be associated with decreased risks in hepatocellular carcinoma,[10] pancreatic cancer,[11] prostate cancer,[12] gastric cancer,[13] colorectal cancer,[14] and breast cancer.[15]
Several meta-analyses of randomized controlled trials have examined the relationship between statin use and risk of liver cancer and have found that statin use is significantly reduce liver cancer risk.[16–18] However, there is lack of study to quantitatively assess statin use in relation to liver cancer. Thus, we conducted a dose–response meta-analysis to clarify and quantitatively assess statin use and risk of liver cancer.
2. Methods
Our meta-analysis was conducted according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) checklist.[19] There are no ethical issues involved in our study for our data were based on published studies.
2.1. Search strategy
We included eligible studies to investigate the relationship between statins intake and liver cancer. To develop a flexible, nonlinear, r meta-regression model, we required that an eligible study should have been categorized into 3 or more levels. If multiple publications were available for a study, we included the longest follow-up study.
PubMed and EMBASE were searched for studies that were published update to February 2017, with keywords including “liver cancer” OR “hepatocellular” OR “hepatic” OR “intrahepatic” AND “statin.” We refer to the relevant original essays and commentary articles to determine further relevant research. Eligible study was also included through the reference lists of relevant review articles. The search strategy is shown in detail in the supplementary list S1.
2.2. Study selection
Two independent researchers (CY and ZS) investigated the information regarding the correlation between statin use and liver cancer: outcome was liver cancer; the relative risks (RR) at least 3 quantitative categories. Moreover, we precluded nonhuman studies, reviews, meta-analyses, editorials, and published letters. To ensure the correct identification of qualified research, the 2 researchers read the reports independently, and the disagreements were resolved through consensus by all of the researchers.
2.3. Data extraction
Each eligible article's information was extracted by 2 independent researchers (MW and YC). We extracted the following information: first author; publication year; mean value of age; country; study name; sex; cases and participants; the categories of statin use; and RR or odds ratio (OR). We collected the risk estimates with multivariable-adjusted.[20] Quality assessment was performed according to the Newcastle-Ottawa scale for nonrandomized studies.[21]
2.4. Statistical analysis
We pooled RR estimates as the common measure of association statin use and liver cancer risk; the hazard ratio was considered equivalent to the RR.[22] Any results stratified by different subgroups of statin use and liver cancer risk in any single article were treated as 2 separate reports.
Due to different cut-off points for categories in the included studies, we performed a RR with 95% confidence intervals (CI) by an increase of 50 cumulative defined daily dose per year using the method recommended by Greenland, Longnecker and Orsini and colleagues.[23] The dose of statin intake used the median stain intake. If the median stain intake category was not available, the midpoint of the upper and lower boundaries was considered as the dose of each category. In addition, using restricted cubic splines (RCS) to evaluate the nonlinear association between statin intake and liver cancer risk, with 3 knots at the 10th, 50th, and 90th percentiles of the distribution. A flexible meta-regression based on RCS function was used to fit the potential nonlinear trend, and generalized least-square method was used to estimate the parameters.[21] This procedure treats statin use (continuous data) as an independent variable and logRR of diseases as a dependent variable, with both tails of the curve restricted to linear. A P value is calculated for linear or nonlinear by testing the null hypothesis that the coefficient of the second spline is equal to zero.[23]
STATA software 12.0 (STATA Corp, College Station, TX) was used to evaluate the relationships between statin use and liver cancer risk. Q test and I2 statistic were used to assess heterogeneity among studies. The random-effect model was chosen if PQ <.10 or I2>50%, otherwise, the fixed-effect mode was applied. Begg and Egger tests were done to assess the publication bias of each study. P <.05 was considered significant for all tests.
3. Results literature search results
Figure 1 shows the results of literature research and selection. We identified 2601 articles from PubMed and 3723 articles from EMBASE. After exclusion of duplicates and studies that did not fulfill the inclusion criteria, 6 studies were chosen,[24–29] and the data were extracted, and a total of 6 reports datasets were included in the final meta-analysis. These studies were published update to February 2017.
Figure 1.
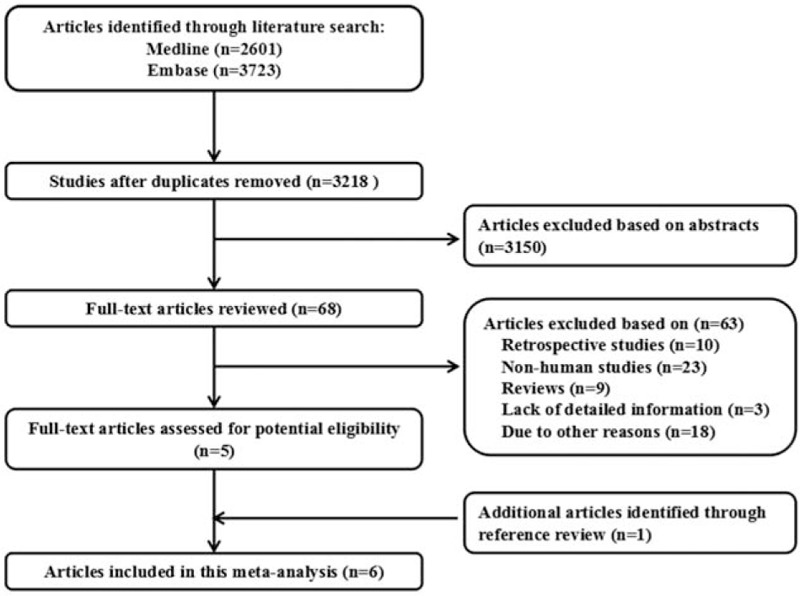
Flow diagram of the study selection process.
3.1. Study characteristics
The characteristics of the included studies are shown in the Tables 1 and 2. Among the selected studies, 6 eligible studies involving 4 cohort studies and 2 case–control studies, 2 studies are from Caucasia and 4 from Asia, a total of 11,8961 participants with 9530 incident cases were included in this meta-analysis.
Table 1.
Characteristics of participants in included studies of statins intake in relation to risk of Liver cancer.

Table 2.
Outcomes and covariates of included studies of statins intake in relation to risk of Liver cancer.

3.2. Overall meta-analysis
The results of statin use and the risk of liver cancer are shown in Table 3. The pooled results suggest that statin use is significantly associated with liver cancer risk, which was suggested both by the highest and lowest categories (RR = 0.46; 95% CI: 0.24–0.68; P <.001) (Table 3). We found evidence of between-study heterogeneity (I2 = 91.8%, P <.001) but we observed no evidence of publication bias (Egger asymmetry test, P = .063) (Table S1).
Table 3.
Stratified analyses of relative risk of liver cancer.

3.3. Dose–response meta-analyses between statins intake and liver cancer
Using RCS function, the test for a nonlinear dose–response relationship was significant (likelihood ratio test, P <.001), suggesting curvature in the relationship, with an increase of 50 cumulative defined daily dose per year was associated with a 14% decrement in the risk of liver cancer. The summary RR of liver cancer for an increase of 50 cumulative defined daily dose per year was 0.86 (95%CI: 0.81–0.90, P <.001) (Fig. 2).
Figure 2.

Dose–response relationship between statin intake and risk of liver cancer.
3.4. Subgroup analyses
Subgroup analysis was performed to check the stability of the primary outcome (Table 3). Subgroup analyses based on the study location found a similar risk reduction of liver cancer in Asia (OR = 0.44, 95%CI: 0.11–0.77, P <.001) and Caucasian (OR = 0.49, 95%CI: 0.36–0.61, P <.001) (Table 3). The relationship between statin use and liver cancer risk was similar in subgroup analyses, which were defined by study design, number of cases or participants, and study quality. An increment of 50 cumulative defined daily dose per year significantly decreased the liver cancer risk in any of the categories.
3.5. Publication bias
Each study in this meta-analysis was performed to evaluate the publication bias by both Begg funnel plot and Egger test. P >.05 was considered no publication bias. The results show that no obvious evidence of publication bias was found in the associations between statin use and liver cancer risk (supplementary Table S1). A funnel plot for publication bias assessment is illustrated in supplementary Figure S1.
4. Discussion
Statins are the most commonly used prescription drugs for the treatment of dyslipidemia. Recently, there has been an interest in a possible protective effect of statins on cancer risk,[30] and statin use has been reported to have a promising anticancer effect. Statins may also have cytostatic effects that extend the survival of cancer patients.[31]
Statins are inhibitors of 3-hydroxy-3-methyl glutaryl coenzyme reductase A (HMG-CoA), which can combine with HMG-CoA reductase activity sites to reverse HMG-CoA reductase activity, thus inhibiting hydroxyvaleric acid synthesis, thus inhibiting several downstream products of the mevalonate pathway.[7] The main substrate for statins is the protein of Ras and Rho family, plus some GTP-binding proteins such as Rab, Rac, and Ral. The main function of Rho family protein is to coordinate the movement of cells and regulate gene transcription.[32] Statins inhibit the proliferation and differentiation of tumor cells by inhibiting the isoprene of Ras and Rho protein, which cannot be activated. Studies have shown that bone morphogenetic protein (BMP) pathway also has certain relationship with the incidence of tumor; statins can activate the BMP and BMP gene to induce cell apoptosis.[33] Furthermore, statin inhibits the proteasome pathway activation, limits cell cycle-dependent kinase inhibitor p21, and p27 protein decomposition, so it plays a role of a growth inhibitor of these molecules.[34]
To our knowledge, several meta-analyses of observational studies and randomized controlled trials have examined the association between statin use and risk of liver cancer.[16–18] However, no study has been done to quantitatively assess statin use in relation to liver cancer. This is the first study to quantify the potential dose–response association between statin use and risk of liver cancer in a large cohort of both men and women. The primary finding in our meta-analysis is that statin use is significantly associated with liver cancer risk; an increase of 50 cumulative defined daily dose per year was associated with a 14% decrement in the risk of liver cancer. Subgroup analysis also proved the stability of the primary outcome. Previously it was hypothesized that the highest category of statins may have a greater chemoprotective effect in liver cancer, but in our hypothesis an increase of 50 cumulative defined daily dose per year was associated with a 14% decrement in the risk of liver cancer.
Although, we performed this meta-analysis very carefully, however, some limitations must be considered in the current meta-analysis. First, different sex of population should be included in this meta-analysis to explore the impact of different sex of population on statin use and liver cancer. Second, we only select literature that was written in English, which may have resulted in a language or cultural bias, other language should be chosen in further study. Third, there might be insufficient statistical power to check the association.
In conclusion, our meta-analysis suggests that statin use was independently associated with deleterious liver cancer risk reduction. However, large sample size, different ethnic, and different sex population of population are warranted to validate this association.
Supplementary Material
Footnotes
Abbreviations: CI = confidence intervals, RCS = restricted cubic splines, RRs = relevant risks.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- [3].Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer 2015;136:1921–30. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y, Tang W, Wang J, et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control 2014;25:237–49. [DOI] [PubMed] [Google Scholar]
- [5].Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet (London, England) 2005;366:1267–78. [DOI] [PubMed] [Google Scholar]
- [6].Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA 1996;275:1481–2. [PubMed] [Google Scholar]
- [7].Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer 2005;5:930–42. [DOI] [PubMed] [Google Scholar]
- [8].Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2013;368:576–7. [DOI] [PubMed] [Google Scholar]
- [9].Yokomichi H, Nagai A, Hirata M, et al. Statin use and all-cause and cancer mortality: BioBank Japan cohort. J Epidemiol 2017;27:S84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lonardo A, Loria P. Potential for statins in the chemoprevention and management of hepatocellular carcinoma. J Gastroenterol Hepatol 2012;27:1654–64. [DOI] [PubMed] [Google Scholar]
- [11].Cui X, Xie Y, Chen M, et al. Statin use and risk of pancreatic cancer: a meta-analysis. Cancer Causes Control 2012;23:1099–111. [DOI] [PubMed] [Google Scholar]
- [12].Bansal D, Undela K, D’Cruz S, et al. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PloS One 2012;7:e46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shimoyama S. Statins and gastric cancer risk. Hepatogastroenterology 2011;58:1057–61. [PubMed] [Google Scholar]
- [14].Bardou M, Barkun A, Martel M. Effect of statin therapy on colorectal cancer. Gut 2010;59:1572–85. [DOI] [PubMed] [Google Scholar]
- [15].Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat 2012;135:261–9. [DOI] [PubMed] [Google Scholar]
- [16].Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 2013;144:323–32. [DOI] [PubMed] [Google Scholar]
- [17].Shi M, Zheng H, Nie B, et al. Statin use and risk of liver cancer: an update meta-analysis. BMJ Open 2014;4:e005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pradelli D, Soranna D, Scotti L, et al. Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev 2013;22:229–34. [DOI] [PubMed] [Google Scholar]
- [19].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [20].Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- [21].Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Neglected Trop Dis 2013;7:e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu C, Zeng XT, Liu TZ, et al. Fruits and vegetables intake and risk of bladder cancer: a PRISMA-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine 2015;94:e759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tsan YT, Lee CH, Wang JD, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol 2012;30:623–30. [DOI] [PubMed] [Google Scholar]
- [25].Simon TG, Bonilla H, Yan P, et al. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology (Baltimore, MD) 2016;64:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peng YC, Lin CL, Hsu WY, et al. Statins are associated with a reduced risk of cholangiocarcinoma: a population-based case-control study. Brit J Clin Pharmacol 2015;80:755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chiu HF, Ho SC, Chen CC, et al. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol 2011;106:894–8. [DOI] [PubMed] [Google Scholar]
- [28].Chen CI, Kuan CF, Fang YA, et al. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine 2015;94:e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McGlynn KA, Hagberg K, Chen J, et al. Statin use and risk of primary liver cancer in the Clinical Practice Research Datalink. J Natl Cancer Inst 2015;107:djv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer (Oxford, England: 1990) 2008;44:2122–32. [DOI] [PubMed] [Google Scholar]
- [31].Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol 2013;10:625–42. [DOI] [PubMed] [Google Scholar]
- [32].Marcelli M, Cunningham GR, Haidacher SJ, et al. Caspase-7 is activated during lovastatin-induced apoptosis of the prostate cancer cell line LNCaP. Cancer Res 1998;58:76–83. [PubMed] [Google Scholar]
- [33].Wu J, Wong WW, Khosravi F, et al. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res 2004;64:6461–8. [DOI] [PubMed] [Google Scholar]
- [34].Rao S, Porter DC, Chen X, et al. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci USA 1999;96:7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


