Abstract
Background:
The effect of low serum vitamin E levels on the risk of colorectal cancer (CRC) remains inconclusive. This meta-analysis aims to synthesize relevant studies to evaluate the association between serum vitamin E and the risk of CRC based on case–control studies.
Methods:
Potentially relevant studies were selected by searching PubMed, EMBASE, and China National Knowledge Infrastructure databases according to inclusion and exclusion criteria. The association between serum vitamin E levels and CRC was estimated by the weighted mean difference (WMD) and 95% confidence interval (CI) using a random-effects model. Heterogeneity was evaluated using Q test and I2 statistic. Subgroup analysis was conducted to explore sources of heterogeneity. Sensitivity analysis was performed to reveal stability and reliability.
Results:
A total of 10 papers with 11 studies, including 6431 subjects with 520 CRC patients and 5981 controls, were included in this present meta-analysis. The results indicated that compared with healthy controls, patients with CRC showed lower concentrations of serum vitamin E (WMD = −2.994 μmol/L, 95% CI = −4.395 to −1.593). Ethnicity subgroup analysis indicated that the serum vitamin E levels were lower in European (WMD = −1.82 μmol/L, 95% CI = −3.00 to −0.65), but not in Asian. Control-source subgroup analysis revealed that a significant association was observed in subgroup with hospital-based controls (WMD = −3.43 μmol/L, 95% CI = −6.27 to −0.59), but not in those with population-based controls. Sensitivity analysis suggested no significant difference in the pooled estimates, indicating stable results.
Conclusions:
CRC is associated with a lower concentration of serum vitamin E. However, necessary prospective cohort studies should be conducted to assess the effect of serum vitamin E on the risk of CRC in the future.
Keywords: α-tocopherol, colorectal cancer, meta-analysis, serum, vitamin E
1. Introduction
Colorectal cancer (CRC) is the fourth leading cause of cancer death worldwide, the second most common cancer in women, and the third most commonly occurring cancer in men.[1,2] In the United States, an estimated 134,490 new cases of CRC and an estimated 49,190 individuals are predicted to die of CRC in 2016.[3] Despite advances in surgery, the 5-year survival rate of CRC was 65.1% between 2006 and 2012 in the United States.[4] The 5-year survival rate of CRC is much lower in developing countries than in developed countries. The burden caused by CRC has drawn attention worldwide.
To date, the causes of CRC remain incompletely understood. Recently, 2 studies[5,6] systematically reviewed the relationship between dietary vitamin E and risk of CRC; however, both found no significant association. In despite of these, findings suggest that dietary vitamin E could not prevent the development of CRC in humans, a hypothesis whether serum vitamin E concentration could influence the progress of CRC has aroused our great interest. After reviewing these studies further, we found that several studies had evaluated the relationship between serum vitamin E levels and the risk of CRC. In a case–control study with 12 newly diagnosed patients with CRC and 12 age-matched healthy individuals, Kocer and Naziroglu[7] indicated that serum vitamin E concentration was significantly lower in patients with CRC than in healthy controls. In the USA, Kabat et al[8] assessed the association between serum vitamin E levels and the risk of CRC by using the data on a subsample of women in the Women's Health Initiative with repeated measurements and no association was found. Longnecker et al[9] pooled data from 5 cohorts to explore the serum vitamin E concentration in relation to subsequent CRC and no clear association was indicated. Other studies attempted to explore the relationship between serum vitamin E levels and the risk of CRC,[10–20] however, these studies found contradictory results.
To the best of our knowledge, no comprehensive study assessed the association of serum vitamin E concentration with the risk of CRC to date. Thus, we conducted the first meta-analysis to fill this research gap. This meta-analysis primarily aims to evaluate the relationship between serum vitamin E levels and the risk of CRC.
2. Methods
2.1. Literature search
This meta-analysis, which evaluated the association between serum vitamin E levels and CRC was designed using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist. We conducted a comprehensive literature search in PubMed (1966 to September 2016), EMBASE (1950 to September 2016), and China National Knowledge Infrastructure (1994 to September 2016). The search terms included the following: colorectal/ colon/ bowel/ rectal/ rectum/ sigmoid/ anal/ anus AND cancer/ neoplasm∗/ tumor∗/ carcinoma∗/ sarcoma∗/ adenocarcinoma∗/ adenoma∗/ lesion∗ AND serum/ plasma/ cerebrospinal fluid AND vitamin E/tocopherol. In addition, we searched the reference lists of the identified articles to determine the relevant studies.
2.2. Study selection
Two reviewers independently selected the potential articles in accordance with the predetermined inclusion and exclusion criteria. In the process of retrieval, if divergences of opinion on the articles arose, a third reviewer evaluated the eligibility of the article in question.
In this review, all selected studies were required to meet the following inclusion criteria: the study adopted a case–control study design based on human population; the study evaluated the relationship between serum/plasma vitamin E and CRC; the study provided sufficient information to calculate the magnitude of the effect; all CRC patients and controls did not take any vitamin E pills; and language was limited in English and Chinese. If a study did not meet the aforementioned criteria, it would be excluded.
In this meta-analysis, ethical approval was not necessary as all the data were based on the previous published studies.
2.3. Data extraction
Basic information was extracted independently by 2 researchers with a standardized form for each study. The information included the first author's name, year of publication, mean age of participants, country, ethnicity, mean and standard deviation of vitamin E, and sample size. If required, information that had previously been omitted was retrieved by communicating with the authors of the studies.
2.4. Quality assessment
The Newcastle–Ottawa Scale (NOS),[21] which was in accordance with the Cochrane Collaboration, was used to evaluate the quality studies included in this review. This scale referred to 3 broad perspectives, which included the selection of study objective, comparability of study groups, and measurement of exposure. If a study scored <5 stars, it was considered of low quality; if a study scored 5–7 stars, it was considered of moderate quality.; and if a study scored >7 stars, it was considered of high quality.
2.5. Meta-analysis
Stata version 11.0 (Stata Corporation College Station, TX) was used to calculate the pooled estimation. In this meta-analysis, we used the random effects model to complete the analyses. Cochran's Q-statistics was applied to evaluate the heterogeneity among studies. The I2 was determined to evaluate the amount of variation across studies attributed to heterogeneity.[22] If P >.10 by the Q test and I2 <50%, the study showed no obvious heterogeneity. The results were expressed as weighted mean difference (WMD) and 95% confidence intervals (CI). Forest plots were used to describe results graphically. A funnel plot and the Egger test were applied to assess publication bias.[23]
To identify the source of potential heterogeneity, subgroup analysis was conducted in this meta-analysis. Subgroup analysis was performed by different characteristics of the studies, such as ethnicity (Caucasian vs Asian), source of control subjects (hospital-based vs population-based), and sex (men vs women vs mixed). Sensitivity analysis was performed to estimate the influence of each individual study on the overall result of the meta-analysis by repeating the random effects model after omitting 1 study at a time. A 2-tailed P value <.05 was considered statistically significant.
3. Results
3.1. Search results and study characteristics
In this meta-analysis, the search strategy generated 462 relevant citations; 85 of these citations were of potential value to be retrieved for detailed evaluation. Because of various reasons, 75 of these 85 articles were excluded from this meta-analysis. Therefore, this process resulted in 10 articles being identified as meeting the rigid inclusion criteria (Fig. 1).
Figure 1.
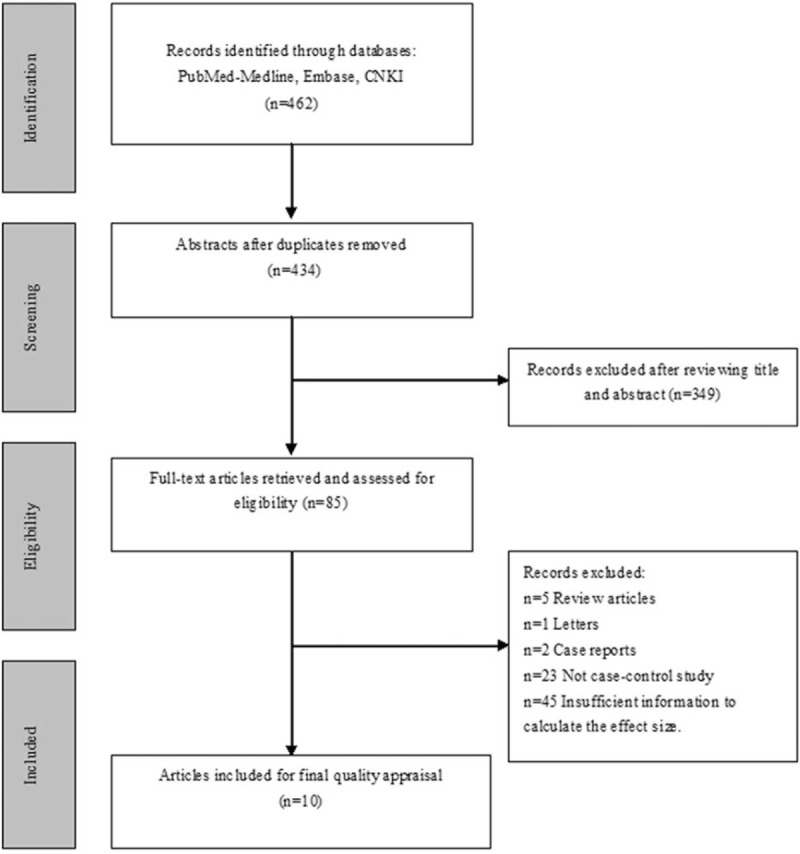
Flow chart of the study selection procedure.
The basic characteristics of selected studies in this meta-analysis are presented in Table 1. One article referred to 2 different control individuals; so, we considered the article as 2 studies in this review. Thus, 11 studies were included in this meta-analysis. All of the studies included 6431 subjects with 520 cases with CRC and 5981 controls.
Table 1.
Characteristics of included studies.
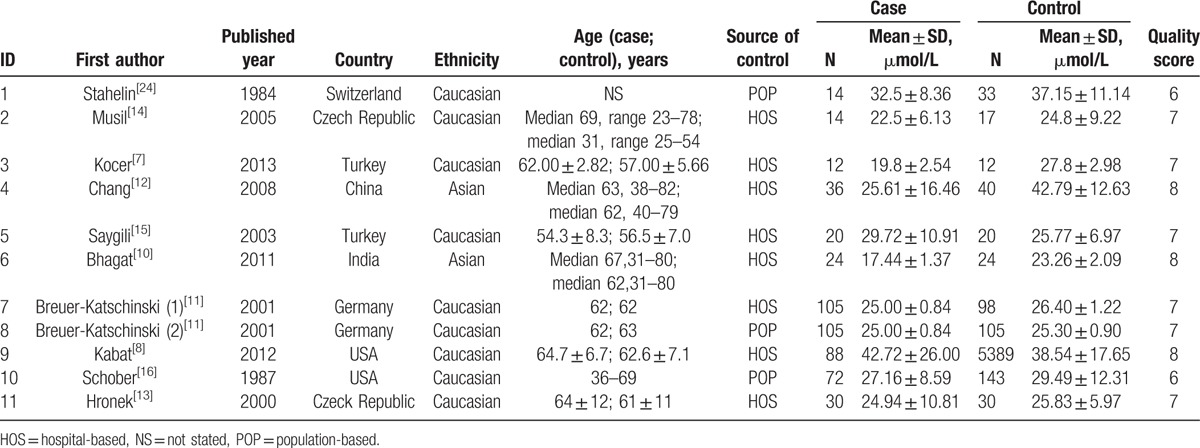
All papers were published in English. The population covered over 7 countries worldwide. The population were of Caucasian (n = 9) and Asian (n = 2) ethnicities. The controls of the 8 studies were hospital-based and those of 3 studies were general population-based; 1 study included female subjects only, 2 studies included male subjects only, and the other studies included both female and male subjects.
3.2. Methodological quality
In this meta-analysis, the NOS indicated that none of the studies obtained 9 stars (the maximum score); 3 studies scored 8 stars, 6 studies scored 7 stars, and 2 studies scored 6 stars (Table 1). Overall, the studies were of moderate to high quality.
3.3. Serum vitamin E concentration in CRC and control subjects
In this comparison, significant heterogeneity was detected (Q = 200.41, P <.001; I2 = 95.0%). Thus, a random effects model was used to pool the effect size (Fig. 2). The mean serum vitamin E concentration of the cases was lower by approximately 2.994 μmol/L (95% CI = −4.395 to −1.593; Z = 4.19, P <.001) than the controls.
Figure 2.
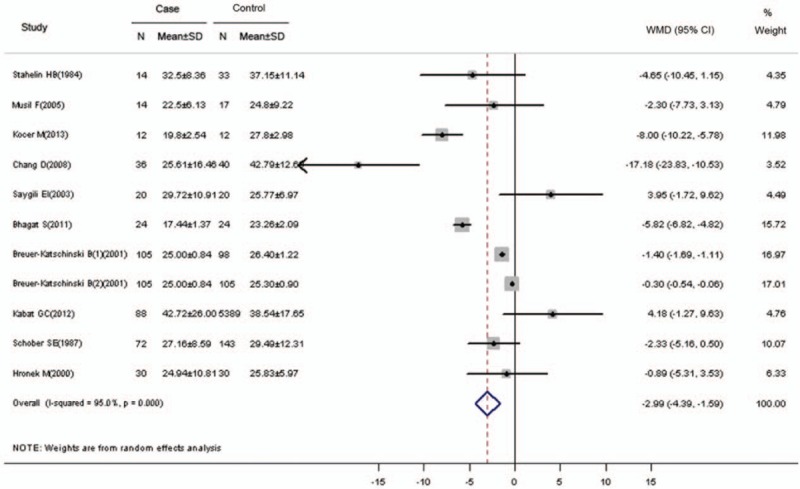
Weighted mean difference and 95% CI of serum vitamin E levels for the risk of colorectal cancer. CI, confidence interval.
3.4. Publication bias
As for publication bias, visual inspection of the Begg funnel plot indicated symmetry (Fig. 3). In addition, Begg test revealed no risk of publication bias (continuity-corrected Z = 0.16, P = .876, >.05).
Figure 3.
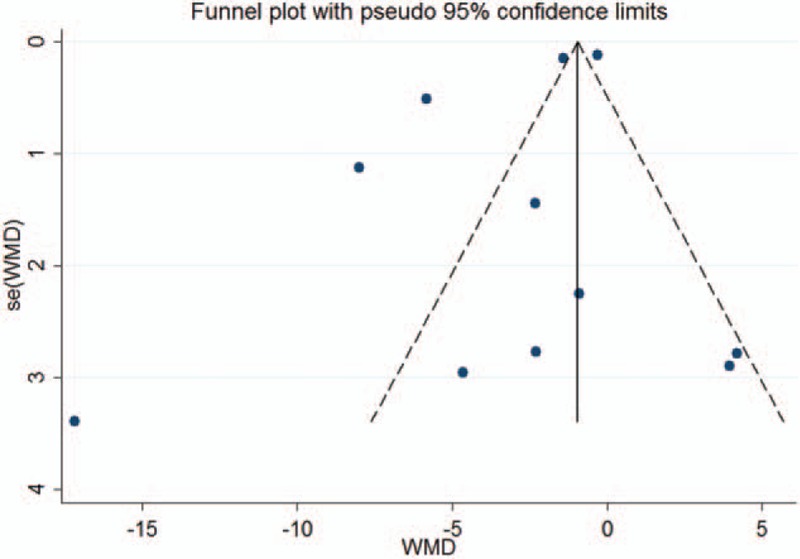
Funnel plot of 11 case–control studies in this meta-analysis.
3.5. Subgroup and sensitivity analysis
Significant heterogeneity in our dataset was explored as fully as possible by subgroup analysis and sensitivity analysis.
Ethnicity subgroup analysis (see Fig. 4) indicated that in the Caucasian subgroup with 9 studies, serum vitamin E concentration was lower in patients with CRC than in the controls (WMD = −1.82 μmol/L, 95% CI = −3.00 to −0.65, P = .002, <.01). However, the pooled effect size showed no significant difference in the Asian subgroup with 2 studies (WMD = 11.01 μmol/L, 95% CI = −22.09 to 0.09, P = .052, >.05). In this meta-analysis, significant association was observed in subgroup with hospital-based controls (WMD = −3.43 μmol/L, 95% CI = −6.27 to −0.59, P = .018, <.05). However, no association was found in the subgroup with population-based controls. With regard to the influence of gender on CRC, we analyzed the relationship between different genders and CRC. In this meta-analysis, 8 studies were conducted among males and females, and significant difference in pooled effect size was indicated (WMD = −3.66 μmol/L, 95% CI = −5.16 to −2.16, P <.001), but no significant difference in the pooled effect size was indicated in the male-only or female-only subgroups (WMD = −0.33 μmol/L, 95% CI = −8.76 to 8.10, P = .939, >.05; WMD = 4.18 μmol/L, 95% CI = −1.27 to 9.63, P = .133, >.05).
Figure 4.
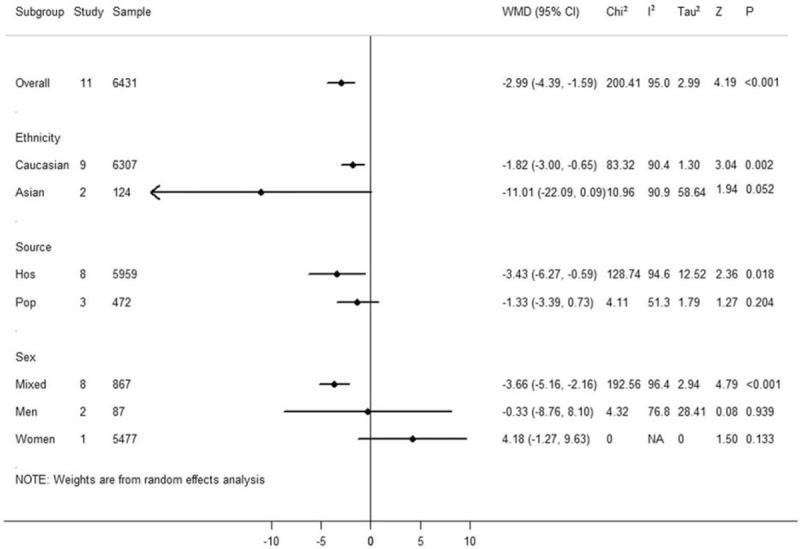
Subgroup meta-analysis of 11 case–control studies in this study.
In this meta-analysis, we performed sensitivity analysis to evaluate the reliability and stability by omitting each study and recalculating the pooled WMD for the remaining studies. Figure 5 shows that no significant difference in the pooled effect size was found when any 1 study was omitted. This result suggests that inter-study heterogeneity was not reduced significantly when a certain study was excluded.
Figure 5.
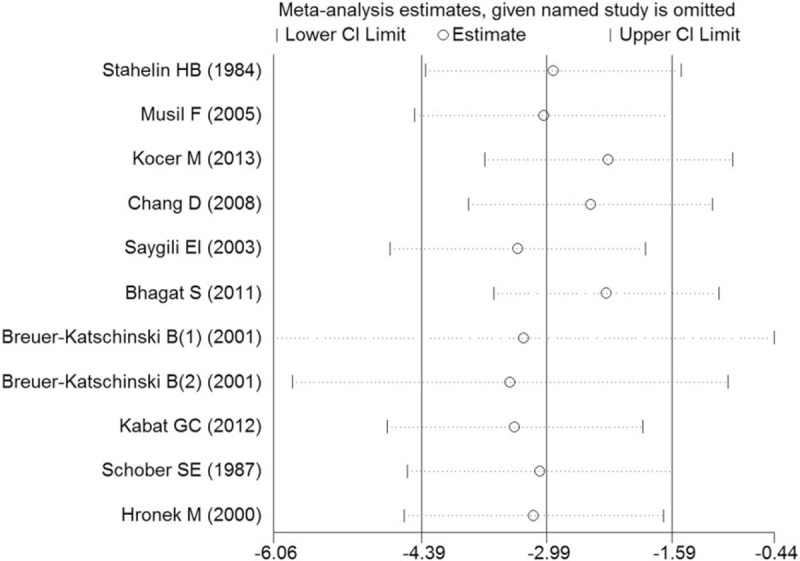
Sensitivity analysis of 11 case–control studies in this meta-analysis.
4. Discussion
To the best of our knowledge, this meta-analysis is the first to investigate serum vitamin E levels in patients with CRC. In this meta-analysis, 11 studies were included, which contained 6431 subjects with 520 cases and 5981 controls. The present meta-analysis suggests that patients with CRC show a lower serum level of vitamin E, compared with healthy controls, and the pooled effect size (WMD) for CRC cases versus controls was −2.994 μmol/L (95% CI = −4.395 to −1.593, P <.001). Considering the large sample size of this review and the better quality of the included studies, we believe that the results we obtained are highly accurate.
The major function of vitamin E in our body is to act as an antioxidant.[24,25] Several previous studies have been conducted on the variation of incidence rates of CRC in different ethnicities.[3,26,27] Regardless, no evidence-based systematic review has been reported regarding serum vitamin E level and the risk of CRC based on different ethnicities. Ingles et al[28] evaluated the relationship between plasma vitamin E concentration and colorectal adenomas in a multiethnic population. They found a significant difference in plasma vitamin E concentration in patients with CRC among 4 ethnic groups: White Americans, African–American, Hispanic, and Asian. In this meta-analysis, we found that serum vitamin E concentration was significantly lower in patients with CRC than in healthy controls among Caucasians (WMD = −1.82 μmol/L, 95% CI = −3.00 to −0.65, P <.01). Notably, only 2 studies reported on the relationship between serum vitamin E levels and the risk of CRC among Asians in this review. However, no significant difference was found between patients with CRC and healthy controls among Asians. The reasons may be attributed to a small number of subjects which resulted in a wide range of 95% CI for WMD. What's more, though the 2 studies conducted Asian ethnicity, some fine differences between Asian[12] and India[10] people were not found. Even the different matching factors might influence the accuracy of our results. For example, Bhagat et al[10] had matched the characters of age and sex between the cases and controls, however, Chang et al[12] did not match any factors in his study. Thus, some studies among Asians should be conducted to verify the current results in the future.
According to the United States National Cancer Institute and Centers for Disease Control and Prevention, the incidence rate of CRC was higher among males than females; the age-adjusted incidence rate of new cases was 47.1 per 100,000 persons in males and 36.0 per 100,000 persons in females.[4,29] Some studies in other countries have found similar results. Considering the difference in the incidence of CRC between the 2 genders, we ask whether a relation existed between serum vitamin E levels and the risk of CRC in different gender persons. In Japan, a case–control study was performed by Jiang et al,[19] which found no difference in serum α-tocopherol concentration in males or in females. However, in this meta-analysis, only 2 studies reported the relationship between serum vitamin E concentration and the risk of CRC among males, and only 1 study reported on the association of serum vitamin E concentration with the risk of CRC among females. Given the smaller number of included studies in the subgroup, the pooled effect size (WMD) had wide 95% CI. Thus, the results of the subgroup meta-analysis for males or females were not accurate. In the future studies should explore the relationship between serum vitamin E level and the risk of CRC among different gender individuals.
Breuer-Katschinski et al[11] simultaneously conducted a population-based case–control study and a hospital-based case–control study with the same patients to evaluate the relationship between serum vitamin E concentration and the risk of colorectal adenoma. The main advantage of the said study was that 2 control groups and 1 case group were selected at the same time. However, no significant association between serum vitamin E concentration and risk of colorectal adenoma was determined. In this meta-analysis, we pooled the effect size of the 8 studies with hospital-based controls and a significantly lower serum vitamin E concentration in patients with CRC than in the healthy controls. Considering the prominent advantage of the population-based case–control study that could better react with the characteristics of the source population, we also performed a subgroup meta-analysis based on the general population-based studies. No relationship between serum vitamin E concentration and the risk of CRC was indicated.
However, this meta-analysis included several limitations that should not be ignored. Firstly, this review only searched published studies, thus ignoring studies that have not been published. Secondly, this review only included papers published in English and Chinese, which might have generated results that are biased toward English-speaking countries. Thirdly, the studies included in this meta-analysis mainly focused on case–control design, and few were based on the cohort studies about Vitamin E and CRC, causality as implied in this review could not be firmly established. Thus, in the future, prospective cohort studies with larger samples are needed to determine the association between serum vitamin E levels and the risk of CRC.
In conclusion, this meta-analysis suggests that serum vitamin E concentration was lower in patients with CRC than in healthy controls. Reduced serum vitamin E levels may be a risk factor for CRC. However, prospective cohort studies are still needed to assess the risk of serum vitamin E on CRC in the future.
Acknowledgment
The authors thank Ph.D Z Zhang of School of Public Health, Anhui Medical University for his statistical contributions in this manuscript.
Footnotes
Abbreviations: CI = confidence interval, CNKI = China National Knowledge Infrastructure, CRC = colorectal cancer, NOS = Newcastle–Ottawa Scale, WMD = wighted mean difference.
YD, YL, YS, XC, and JH contributed equally to this manuscript.
Funding: This study was supported by Science and Technology Planning Project of Health and Family Planning Commission of Jiangxi Province (No. 20162001).
The authors have no conflicts of interest to disclose.
References
- [1].Pinol-Felis C, Fernandez-Marcelo T, Vinas-Salas J, et al. Telomeres and telomerase in the clinical management of colorectal cancer. Clin Transl Oncol 2016;19:10–07. [DOI] [PubMed] [Google Scholar]
- [2].Mousavinezhad M, Majdzadeh R, Akbari SA, et al. The effectiveness of FOBT vs. FIT: A meta-analysis on colorectal cancer screening test. Med J Islam Repub Iran 2016;30:366. [PMC free article] [PubMed] [Google Scholar]
- [3].Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet 2016;7:105–14. [PMC free article] [PubMed] [Google Scholar]
- [4].National Cancer Institute. Suirveillance, Epidemiology, and End Results Program. Available at: http://seer.cancer.gov/statfacts/html/colorect.html. Accessed January 5, 2017. [Google Scholar]
- [5].Heine-Broring RC, Winkels RM, Renkema JM, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J Cancer 2015;136:2388–401. [DOI] [PubMed] [Google Scholar]
- [6].Xu X, Yu E, Liu L, et al. Dietary intake of vitamins A, C, and E and the risk of colorectal adenoma: a meta-analysis of observational studies. Eur J Cancer Prev 2013;22:529–39. [DOI] [PubMed] [Google Scholar]
- [7].Kocer M, Naziroglu M. Effects of 5-fluorouracil on oxidative stress and calcium levels in the blood of patients with newly diagnosed colorectal cancer. Biol Trace Elem Res 2013;155:327–32. [DOI] [PubMed] [Google Scholar]
- [8].Kabat GC, Kim MY, Sarto GE, et al. Repeated measurements of serum carotenoid, retinol and tocopherol levels in relation to colorectal cancer risk in the Women's Health Initiative. Eur J Clin Nutr 2012;66:549–54. [DOI] [PubMed] [Google Scholar]
- [9].Longnecker MP, Martin-Moreno JM, Knekt P, et al. Serum alpha-tocopherol concentration in relation to subsequent colorectal cancer: pooled data from five cohorts. J Natl Cancer Inst 1992;84:430–5. [DOI] [PubMed] [Google Scholar]
- [10].Bhagat SS, Ghone RA, Suryakar AN, et al. Lipid peroxidation and antioxidant vitamin status in colorectal cancer patients. Indian J Physiol Pharmacol 2011;55:72–6. [PubMed] [Google Scholar]
- [11].Breuer-Katschinski B, Nemes K, Marr A, et al. Relation of serum antioxidant vitamins to the risk of colorectal adenoma. Digestion 2001;63:43–8. [DOI] [PubMed] [Google Scholar]
- [12].Chang D, Wang F, Zhao YS, et al. Evaluation of oxidative stress in colorectal cancer patients. Biomed Environ Sci 2008;21:286–9. [DOI] [PubMed] [Google Scholar]
- [13].Hronek M, Zadak Z, Solichova D, et al. The association between specific nutritional antioxidants and manifestation of colorectal cancer. Nutrition 2000;16:189–91. [DOI] [PubMed] [Google Scholar]
- [14].Musil F, Zadak Z, Solichova D, et al. Dynamics of antioxidants in patients with acute pancreatitis and in patients operated for colorectal cancer: a clinical study. Nutrition 2005;21:118–24. [DOI] [PubMed] [Google Scholar]
- [15].Saygili EI, Konukoglu D, Papila C, et al. Levels of plasma vitamin E, vitamin C, TBARS, and cholesterol in male patients with colorectal tumors. Biochemistry (Mosc) 2003;68:325–8. [DOI] [PubMed] [Google Scholar]
- [16].Schober SE, Comstock GW, Helsing KJ, et al. Serologic precursors of cancer. I. Prediagnostic serum nutrients and colon cancer risk. Am J Epidemiol 1987;126:1033–41. [DOI] [PubMed] [Google Scholar]
- [17].Stahelin HB, Rosel F, Buess E, et al. Cancer, vitamins, and plasma lipids: prospective Basel study. J Natl Cancer Inst 1984;73:1463–8. [PubMed] [Google Scholar]
- [18].Nair S, Norkus EP, Hertan H, et al. Serum and colon mucosa micronutrient antioxidants: differences between adenomatous polyp patients and controls. Am J Gastroenterol 2001;96:3400–5. [DOI] [PubMed] [Google Scholar]
- [19].Jiang J, Suzuki S, Xiang J, et al. Plasma carotenoid, alpha-tocopherol and retinol concentrations and risk of colorectal adenomas: a case-control study in Japan. Cancer Lett 2005;226:133–41. [DOI] [PubMed] [Google Scholar]
- [20].Szwed R, Grzebieniak Z, Saleh Y, et al. Cysteine peptidase and its inhibitor activity levels and vitamin E concentration in normal human serum and colorectal carcinomas. World J Gastroenterol 2005;11:850–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [22].Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 2012;31:3805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: an insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer 2008;123:739–52. [DOI] [PubMed] [Google Scholar]
- [25].Peh HY, Tan WS, Liao W, et al. Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther 2016;162:152–69. [DOI] [PubMed] [Google Scholar]
- [26].Qurieshi MA, Khan SM, Masoodi MA, et al. Epidemiology of cancers in Kashmir, India: an analysis of hospital data. Adv Prev Med 2016;2016:1896761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhu J, Tan Z, Hollis-Hansen K, et al. Epidemiological Ttends in colorectal cancer in China: an ecological study. Dig Dis Sci 2016;62:235–43. [DOI] [PubMed] [Google Scholar]
- [28].Ingles SA, Bird CL, Shikany JM, et al. Plasma tocopherol and prevalence of colorectal adenomas in a multiethnic population. Cancer Res 1998;58:661–6. [PubMed] [Google Scholar]
- [29].Centers for Disease Control and Prevention. Colorectal (Colon) Cancer. Available at: http://www.cdc.gov/cancer/colorectal/index.htm. Accessed January 10, 2017. [Google Scholar]


