Abstract
There is no consensus of treatments for acute acalculous cholecystitis with systemic lupus erythematosus (SLE). The study was aimed to investigate the effect of the corticosteroid for these patients.
A series of patients who were diagnosed as acute acalculous cholecystitis with SLE in the period from January 2012 to December 2016 at our hospital were included. They accepted 2 different conservative treatment strategies initially: the treatment using moxifloxacin (the antibiotic group), and the treatment using corticosteroid combined moxifloxacin (the corticosteroid group). Then clinical manifestations, laboratory features, and outcomes were analyzed.
The study identified 22 women Han Chinese patients with the SLE history of 2.8 ± 1.4 year. There was no significant difference in SLE history, Systemic Lupus Erythematosus Disease Activity Index-2000 (SLEDAI-2000), Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index (SLICC/ACR), hematologic examination results, and corticosteroid dosage between 2 groups. And there was no significant difference in the symptom of acute cholecystitis, duration of the symptoms, white blood level, and the thickness of gallbladder wall between 2 groups either. However, the SLEDAI-2000 of the corticosteroid group was lower than that of the antibiotic group (7.3 ± 1.4 vs 10.7 ± 3.0, P = .03), so was the SLICC/ACR (0.1 ± 0.3 vs 0.3 ± 0.5, P = .01). Moreover, total 11 of 12 patients were successfully treated in the corticosteroid group, only 1 patient got cholecystectomy because no improvement after conservative treatment. While 4 of 10 patients were successfully treated by moxifloxacin alone, 6 patients had to accept cholecystectomy in the antibiotic group. The rate of successful conservative treatment in the corticosteroid group was higher than that of the antibiotic group (P = .02). All patients were followed up at least 6 months, there was no statistical difference in the rate of recurrence of abdominal pain between 2 groups (P = .37).
The corticosteroid plays an important role in the management of the acalculous cholecystitis patient with SLE, and it should be considered as a first line of treatment.
Keywords: acalculous cholecystitis, acute cholecystitis, corticosteroid, systemic lupus erythematosus, treatment
1. Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease with a wide range of clinical and serological manifestations that can affect virtually every organ. Gastrointestinal tracts, kidney, skin, hematologic, and central nervous systems are most common involved, while gallbladder involvement is an uncommon event among SLE patients.[1] Although there are a few cases of acute acalculous cholecystitis (AAC) with SLE reported so far,[2,3] there is no particular consensus of treatment for these patients.[4]
Normally, early cholecystectomy was indicated for all acute cholecystitis in an unselected population, especially the AAC patients. The AAC progresses rapidly with a high incidence of gangrene (>50%) and perforation (>10%).[2] Surgery was considered as an only effective treatment traditionally.[5] However, the corticosteroid treatment has achieved a successful effect in many AAC patients with SLE.[4] Did the corticosteroid play roles in the conservative treatment? Should the corticosteroid be suggested for all AAC patients with SLE? After all there are important roles of the gallbladder in humans and many risks of the cholecystectomy in SLE patients.[6,7] Whereas, no related study was available so far. Considering that these are important questions for physicians and surgeons to make a clinical decision, we observed a series of AAC with SLE patients accepted different treatment strategies to investigate the effect of the corticosteroid, and hoped to find any possible hint for clinical managements and decisions.
2. Subjects and methods
2.1. Patients
A series of AAC patients with SLE in the period from January 2012 to December 2016 at our hospital were included. Our hospital is one of the largest units treating patients with hepatobiliary diseases or autoimmune diseases. The diagnosis of SLE was based on the American College of Rheumatology criteria (ACR criteria)[8] or the 2012 Systemic Lupus International Collaborating Clinics criteria (SLICC 2012).[9] The diagnosis of acute cholecystitis was made by a same hepatobiliary surgeon team according to all eligible medical records, including right upper-quadrant pain, fever, leukocytosis, abnormal liver tests and gallbladder wall thickness on abdominal ultrasonography, CT scan, or MRI.[10,11] The ultimate diagnosis of acute cholecystitis rested on the combination of clinical factors and imaging according to the consensus.[11]
Considering that cholecystectomy could be performed even beyond 3 days[12] and the AAC could be successfully treated using corticosteroid or moxifloxacin alone,[2,3] the AAC patients were conservatively managed firstly. The patients who accepted moxifloxacin[13] alone were referred to the antibiotic group, and the patients who accepted corticosteroid combined moxifloxacin were included into the corticosteroid group. Antibiotics are suggested as supportive care, and the dose of moxifloxacin was according to the consensus,[11] while the corticosteroid (methylprednisolone 0.5–1.5 mg per kilogram of body weight per day) was given by immunological doctors according to the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI-2000) and the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index (SLICC/ACR).[14] There was no steroid-sparing agents (antimalarial drug or immunosuppressant) for these patients during the initial conservative treatment in the study.
Three treatment outcomes of AAC after at least 3-days conservative treatment were defined: (1) successful conservative treatment referred to the complete amelioration of symptom and biochemical results. (2) Disease response was defined as symptomatic or biochemical improvement after the commencement of treatment. The difference from the successful conservative treatment was that the AAC may get worse after initial transient improvement. (3) The surgery (cholecystectomy) was considered,[15] when the patient had no improvement, or suffered persistent fever, vomiting, abdominal pain, tenderness on repeated examinations, and significant leucocytosis after initial conservative treatment. The operation opportunity choice was made by a same hepatobiliary surgeon team. We excluded the probable or possible patients because of their incomplete profiles. Clinical information was collected and analyzed including demographics, clinical presentations, biochemical results, imaging features, and treatment outcomes.
The statistic difference was performed by using Student t test and Chi-square test (SPSS 19, SPSS, Inc, Chicago, IL). Nonparametric quantitative variables were compared using the Mann–Whitney U test. Differences associated with a P value <.05 were considered statistically significant. All procedural protocols were approved by the Ethics Committee of our hospital, and informed consent was signed by each patient.
3. Results
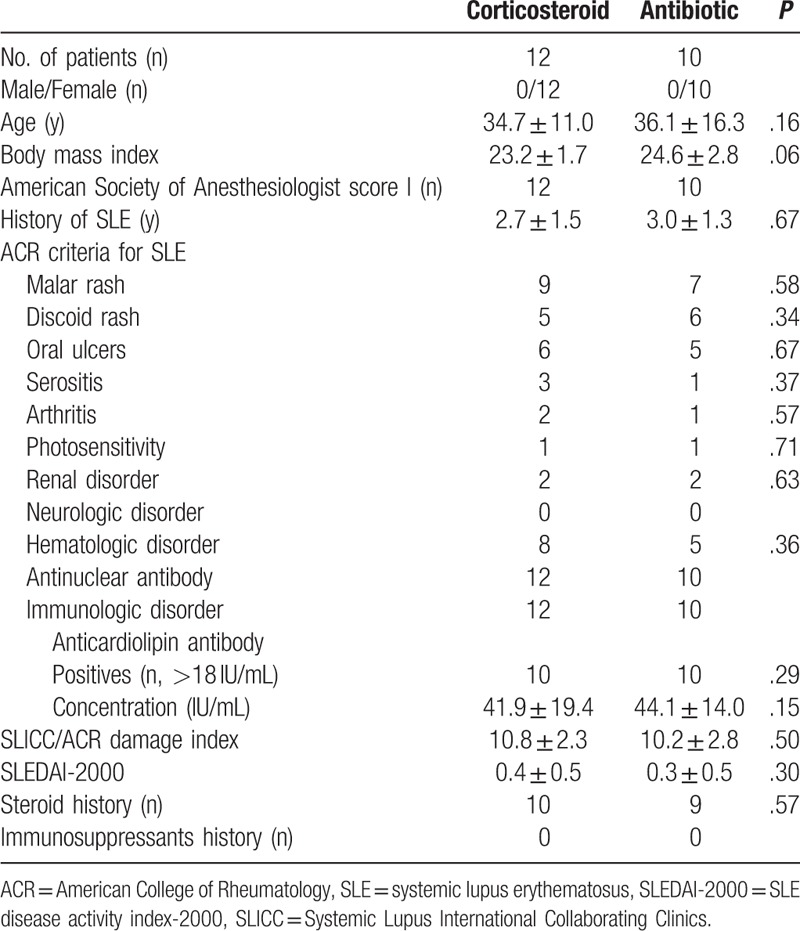
The study identified 22 AAC patients with SLE accepted conservative treatment initially. They were all women Han Chinese (Table 1), and there was no statistical difference in the age and body mass index between 2 groups. There was no patient with pregnancy, diabetes, sickle cell anemia, long-term somatostatin in treatment, and transplant organs. The SLE history of the patients was 2.8 ± 1.4 year. The SLEDAI-2000 and SLICC/ACR damage index were 10.5 ± 2.5 and 0.4 ± 0.5, respectively, when the acute cholecystitis attacked. The concentration of antinuclear antibodies was 2.2 ± 0.7 IU/mL (normal <1.1 IU/mL), the concentration of antidouble stranded DNA was 119.9 ± 18.6 IU/mL (normal <60 IU/mL), and the level of anticardiolipin antibody was 42.9 ± 16.8 IU/mL (normal <18 IU/mL). Most patients were not taking corticosteroid, 2 patients were tapering the corticosteroid dosage, although 19 patients had a history of oral corticosteroid. There was no significant difference in the SLE history, SLEDAI-2000, SLICC/ACR damage index, hematologic examination, and corticosteroid dosage between 2 groups (Table 1).
Table 1.
Characteristics of the systemic lupus erythematosus patients.
The initial major complaints of acute cholecystitis were gastrointestinal discomfort (18/22 cases, Table 2). Twenty patients suffered abdominal pains with positive Murphy sign. Ten patients had fever, but no one suffered any chillness or sweating. We had drawn many blood samples from the patients before and during fever periods and performed bacterial cultures. The results were negative, and no infection was found in any case. There are 11 patients with an increased white blood cell level, and 9 patients showed an increased alanine transaminase or aspartate aminotransferase level. All the patients accept ultrasonography test, 10 of them accept computed tomographic (CT) scan, and no gallbladder stone, microcalculi, or sludge was found. The thickness of gallbladder wall was 5.3 ± 1.2 mm. There was no significant difference in the symptom of acute cholecystitis, duration of the symptoms, white blood level, or thickness of gallbladder wall between 2 groups.
Table 2.
Clinical characteristics and treatment outcomes of the acute acalculous cholecystitis.
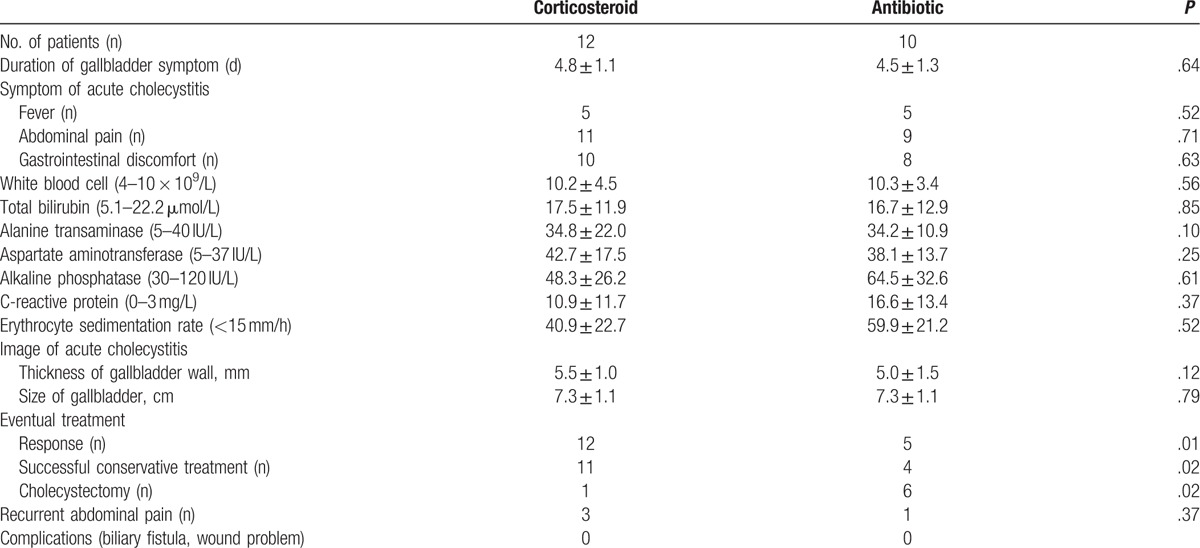
The antibiotic group patients accepted treatment of moxifloxacin alone, and the corticosteroid group patients accepted treatment of corticosteroid combined moxifloxacin. The SLEDAI-2000 of the corticosteroid group was lower than that of the antibiotic group (7.3 ± 1.4 vs 10.7 ± 3.0, P = .03), so was the SLICC/ACR (0.1 ± 0.3 vs 0.3 ± 0.5, P = .01). The concentration of antinuclear antibody in the corticosteroid group was less (1.3 ± 0.4 vs 2.1 ± 0.6 IU/mL, P = .03), so was the concentration of antidouble stranded DNA antibody (40.0 ± 8.3 vs 145.4 ± 25.5 IU/mL, P = .04), and the level of anticardiolipin antibody (17.9 ± 4.5 vs 46.8 ± 13.7 IU/mL, P = .01). All patients in the corticosteroid group exhibited a response as defined, which was more than that in the antibiotic group (5 patients, P = .01). Total 11 of 12 patients had been treated successfully in the corticosteroid group, only 1 patient got cholecystectomy because no improvement after conservative treatment. While 4 of 10 patients were successfully treated by moxifloxacin alone, 6 patients had to accept cholecystectomy in the antibiotic group. The rate of successful conservative treatment in the corticosteroid group was higher than that of the antibiotic group (P = .02). All patients were followed up at least 6 months, there was no statistical difference in the rate of recurrence of abdominal pain between 2 groups (P = .37, Table 2).
4. Discussion
Most acute cholecystitis are due to biliary sludge and stone. Impacted gallstones could cause gallbladder wall trauma, inflammatory mediators are released. Bile stasis and infection further propagate the inflammation, and play important roles in the development of cholecystitis. Although some SLE patients have no existence of gallbladder stones, SLE itself is a chronic inflammatory disease of unknown cause that can affect virtually every organ. Moreover, SLE patients are prone to biliary sludge because of biliary dyskinesia like in other collagen vascular diseases.[3]
The immunological factor might be an etiology of acute cholecystitis. When the acute cholecystitis occurred in our study, the SLEDAI-2000 and the SLICC/ACR damage index were higher, and the level of antinuclear antibody, antidouble stranded DNA antibody, and anticardiolipin antibody were also higher. After the corticosteroid treatment, the symptoms of cholecystitis were mostly remitted, and the indexes of SLE activity were getting down. The concentration of those antibodies in the corticosteroid group decreased sharply compared with the antibiotic group. All of these indicated the causal relationship between the acute cholecystitis and immunological factors from SLE. A variety of vasculitis caused by auto-immunization is a common characteristic of SLE patients.[16] Vessels of all sizes could be involved that lead to a broad clinical spectrum. Vasculitis of the gallbladder might cause the attack of acute cholecystitis. Because the patients after successful conservative treatment did not accepted the surgery, it is difficult to get pathological evidences to support the hypothesis. After all, the gallbladder of the fail conservative treatment might be a little different from that of the successful treatment, and would provide the counter evidence.
Treatment of AAC with SLE has been controversial. Cholecystectomy was considered as a unique treatment because of the high risk of AAC. However, nonoperative treatment has been a first choice in many institutions over the past years. Many case reports in the literature described successful treatments of SLE-induced AAC with high doses of corticosteroid.[17] Shin et al[17] recommended that if the patient's general condition is good and there is no other risk factor for cholecystitis or its serious complications, the high-dose steroid therapy may be considered as a first line of treatment. However, there was no clinical research study to investigate and compare the effectiveness of the corticosteroid treatment due to the limited cases. In our study, 91.7% of the AAC with SLE patients were successfully managed using moxifloxacin and methylprednisolone (0.5–1.5 mg per kilogram of body weight per day) and avoided the cholecystectomy. The rate is much higher than that of the moxifloxacin treatment alone, and also higher than the previous report. In a meta-analysis including 9 randomized controlled trials, a total of 23% had a failure of conservative treatment (without any corticosteroid).[18] The high rate of successful treatment confirmed the effectiveness of the corticosteroid, and the immunological contributing factor of the AAC in the SLE patients. The use of high-dose methylprednisolone plays roles in immune-suppress as well as anti-inflammation for non-infectious etiological factors such as vasculitis or severe inflammation. That might be the reason of the high rate of successful treatment using corticosteroid.
Our study is a single-center and limited sample size study, this is the disadvantage of the study. Although more investigations should be further performed, our study had given a clue for clinical doctors to make clinical decisions. The corticosteroid plays an important role in the management of the acalculous cholecystitis patient with SLE, and it should be considered as a first line of treatment.
Footnotes
Abbreviations: AAC = acute acalculous cholecystitis, SLE = systemic lupus erythematosus, SLEDAI-2000 = Systemic Lupus Erythematosus Disease Activity Index-2000, SLICC/ACR = Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index.
WL and WC have contributed equally to this work.
The authors report no conflicts of interest.
References
- [1].Richer O, Ulinski T, Lemelle I, et al. Abdominal manifestations in childhood-onset systemic lupus erythematosus. Ann Rheum Dis 2007;66:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barie PS, Eachempati SR. Acute acalculous cholecystitis. Gastroenterol Clin North Am 2010;39:343.x–57.x. [DOI] [PubMed] [Google Scholar]
- [3].Basiratnia M, Vasei M, Bahador A, et al. Acute acalculous cholecystitis in a child with systemic lupus erythematosus. Pediatr Nephrol 2006;21:873–6. [DOI] [PubMed] [Google Scholar]
- [4].Xu X, Li B, Zheng C. Hepatobiliary and pancreatic: acute acalculous cholecystitis in systemic lupus erythematosus, successfully treated with corticosteroid. J Gastroenterol Hepatol 2016;31:1673. [DOI] [PubMed] [Google Scholar]
- [5].Yamashita Y, Takada T, Strasberg SM, et al. TG13 surgical management of acute cholecystitis. J Hepatobiliary Pancreat Sci 2013;20:89–96. [DOI] [PubMed] [Google Scholar]
- [6].Turumin JL, Shanturov VA, Turumina HE. The role of the gallbladder in humans. Revista de gastroenterologia de Mexico 2013;78:177–87. [DOI] [PubMed] [Google Scholar]
- [7].Karanikas M, Bozali F, Vamvakerou V, et al. Biliary tract injuries after lap cholecystectomy-types, surgical intervention and timing. Ann Transl Med 2016;4:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- [9].Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huffman JL, Schenker S. Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol 2010;8:15–22. [DOI] [PubMed] [Google Scholar]
- [11].Ansaloni L, Pisano M, Coccolini F, et al. 2016 WSES guidelines on acute calculous cholecystitis. World J Emerg Surg 2016;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roulin D, Saadi A, Di Mare L, et al. Early versus delayed cholecystectomy for acute cholecystitis, are the 72 hours still the rule? A randomized trial. Ann Surg 2016;264:717–22. [DOI] [PubMed] [Google Scholar]
- [13].Schuld J, Glanemann M. Acute cholecystitis. Viszeralmedizin 2015;31:163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tunnicliffe DJ, Singh-Grewal D, Kim S, et al. Diagnosis, monitoring, and treatment of systemic lupus erythematosus: A systematic review of clinical practice guidelines. Arthritis Care Res 2015;67:1440–52. [DOI] [PubMed] [Google Scholar]
- [15].Mendonca JA, Marques-Neto JF, Prando P, et al. Acute acalculous cholecystitis in juvenile systemic lupus erythematosus. Lupus 2009;18:561–3. [DOI] [PubMed] [Google Scholar]
- [16].Barile-Fabris L, Hernandez-Cabrera MF, Barragan-Garfias JA. Vasculitis in systemic lupus erythematosus. Curr Rheumatol Rep 2014;16:440. [DOI] [PubMed] [Google Scholar]
- [17].Shin SJ, Na KS, Jung SS, et al. Acute acalculous cholecystitis associated with systemic lupus erythematosus with Sjogren's syndrome. Korean J Intern Med 2002;17:61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ansaloni L, Pisano M, Coccolini F, et al. Erratum to: 2016 WSES guidelines on acute calculous cholecystitis. World J Emerg Surg 2016;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]


