Abstract
Introduction:
Early diagnosis of acute kidney injury (AKI) remains a challenge. Recently, [TIMP-2]·[IGFBP7], which is a combination of urine tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor (IGF) binding protein 7 (IGFBP7), has been identified as a potential biomarker of AKI. We performed this meta-analysis to assess the diagnostic accuracy of urinary [TIMP-2]·[IGFBP7] for AKI in adult patients.
Methods:
We searched the PubMed, Embase, and Cochrane Library databases from database inception to March 2017. Two authors independently screened articles based on inclusion and exclusion criteria and assessed the methodological quality of each included study using the Quality Assessment of Diagnostic Accuracy Studies 2 criteria. Review Manager and STATA were used for all statistical analyses.
Results:
Nine studies (n = 1886) satisfied the inclusion criteria. Pooled analyses demonstrated that urinary [TIMP-2]·[IGFBP7] exhibited fair diagnostic accuracy for AKI (sensitivity [SEN] 0.83 [95% CI 0.75–0.89], specificity [SPE] 0.72 [95% CI 0.56–0.84], and area under the summary receiver operating characteristic [SROC] curve 0.86 [95% CI 0.82–0.88]) and AKI stage ≥ 2 (according to the 2012 Kidney Disease: Improving Global Outcomes [KDIGO] 2012 classification system; SEN 0.92 [95% CI 0.81–0.96], SPE 0.63 [95% CI 0.49–0.74], and area under the SROC curve 0.88 [95% CI 0.85–0.91]) in adult patients.
Conclusion:
Our findings indicate that urinary [TIMP-2]·[IGFBP7] may be a reliable biomarker for the early detection of AKI. However, given the significant heterogeneity among the included studies, clinicians should be aware of the utility and limitations of this biomarker in clinical practice. Additional high-quality studies examining a larger sample of patients are required.
Keywords: [TIMP-2]·[IGFBP7], acute kidney injury, biomarker, diagnosis, insulin-like growth factor binding protein 7, meta-analysis, tissue inhibitor of metalloproteinase 2
1. Introduction
Acute kidney injury (AKI) is a common but complex clinical syndrome that often occurs in critically ill or postoperative patients, is difficult to predict, and is inevitably associated with adverse clinical outcomes. In addition, AKI significantly increases hospital costs and the incidences of dialysis and chronic kidney disease (CKD).[1–5] As understanding of the etiology and pathology of AKI has advanced, various biomarkers have been evaluated for the early and preclinical detection of AKI in different patients. These biomarkers include plasma and urine neutrophil gelatinase-associated lipocalin,[6] urine interleukin 18,[7] urine liver-type fatty acid-binding protein,[8] and urine kidney injury molecule 1.[9] However, none of these potential markers has been widely used in clinical practice because they do not exhibit acceptable accuracy for the early diagnosis of kidney injury and the early identification of at-risk patients.[10]
Fortunately, the novel AKI-related biomarker [TIMP-2]·[IGFBP7], which is a combination of urine tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor (IGF) binding protein 7 (IGFBP7), was approved by the US Food and Drug Administration (FDA) for AKI-related marketing.[11,12] TIMP-2 and IGFBP7 are biomarkers of G1 cell cycle arrest, and the levels of these proteins increase during the early period after renal tubular cell injury.[13,14] TIMP-2 is an important component in the pathophysiology of ischemia–reperfusion injury,[15] and IGFBP7 is a secreted protein that regulates the bioavailability of IGFs through direct low-affinity binding.[16] Therefore, [TIMP-2]·[IGFBP7] has potential value for the prediction of early AKI.
Recently, an increasing number of studies have evaluated the value of urinary [TIMP-2]·[IGFBP7] in the diagnosis of AKI. To fully understand the diagnostic accuracy of urinary [TIMP-2]·[IGFBP7] for AKI, we conducted this meta-analysis to assist physicians in making clinical decisions.
2. Methods
2.1. Search strategy
We searched the PubMed, Embase, and Cochrane Library databases from database inception to March 2017. The search terms were as follows: (“TIMP-2” or “tissue inhibitor metalloproteinase-2” or “IGFBP7” or “IGF-binding protein 7” or “insulin-like growth factor binding protein 7” or “cycle arrest biomarkers”) and (“AKI” or “acute kidney injury”). The search was limited to human studies with no language restrictions. The reference lists of selected studies were searched by hand to identify potentially relevant citations. Ethical approval was not required because the meta-analysis was based on published articles.
2.2. Study selection
Two investigators (CL and ZM) independently conducted the study selection. Any disagreement was resolved by consultation with a third party (FZ). The inclusion criteria were as follows: a diagnostic value of urinary [TIMP-2]·[IGFBP7] for AKI morbidity in adult patients (≥18 years old) was reported; a 2 × 2 contingency table could be extracted; AKI was adjudicated using the RIFLE (risk, injury, failure, loss, and end-stage renal disease), Acute Kidney Injury Network (AKIN) or Kidney Disease: Improving Global Outcomes (KDIGO) consensus criteria (based on the RIFLE/AKIN definitions for AKI)[17]; and a prospective controlled design was used. The exclusion criteria were as follows: a review, letter, commentary, correspondence, case report, conference abstract, expert opinion, editorial, or animal experiment; a duplicated study; insufficient information to calculate accurate estimates; the involvement of pediatric patients; and the inclusion of patients with pre-existing chronic renal failure.
2.3. Data extraction and quality assessment
One investigator extracted details regarding the first author, year of publication, study design, inclusion criteria, definition of AKI, definition of a positive test result, number of patients, average age, time of marker detection, cut-off points, true positives, false positives (FPs), false negatives, true negatives, sensitivity (SEN), and specificity (SPE) from the included studies.
Two investigators (CL and ZM) independently assessed the methodological quality of each included study using the QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies 2) criteria and evaluated each of these studies in 4 domains: patient selection; index test; reference standard; and patient flow and test timing.[18] Any disagreements in the quality assessment were resolved by discussion and consensus.
2.4. Statistical analysis
All statistical analyses were conducted using Review Manager, version 5.1.2 (RevMan; The Cochrane Collaboration, Oxford, UK) and STATA, version 12.0 (Stata Corporation, College Station, TX). A bivariate random-effects regression model was used to calculate the pooled SEN, SPE, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with 95% confidence intervals (CIs). We also constructed a summary receiver operating characteristic (SROC) curve by plotting individual and summary points for SEN and SPE to assess the overall diagnostic accuracy.[19,20] Between-study heterogeneity was assessed using the I2 index, with an I2 ≥ 50% regarded as indicative of substantial heterogeneity among studies. P values < .05 were considered significant. In addition, sensitivity and subgroup analyses were conducted to investigate potential sources of between-study heterogeneity. Fagan nomogram was used to calculate the post-test probability (PTP), and Deek funnel plot was employed to detect publication bias.
3. Results
3.1. Search results and study characteristics
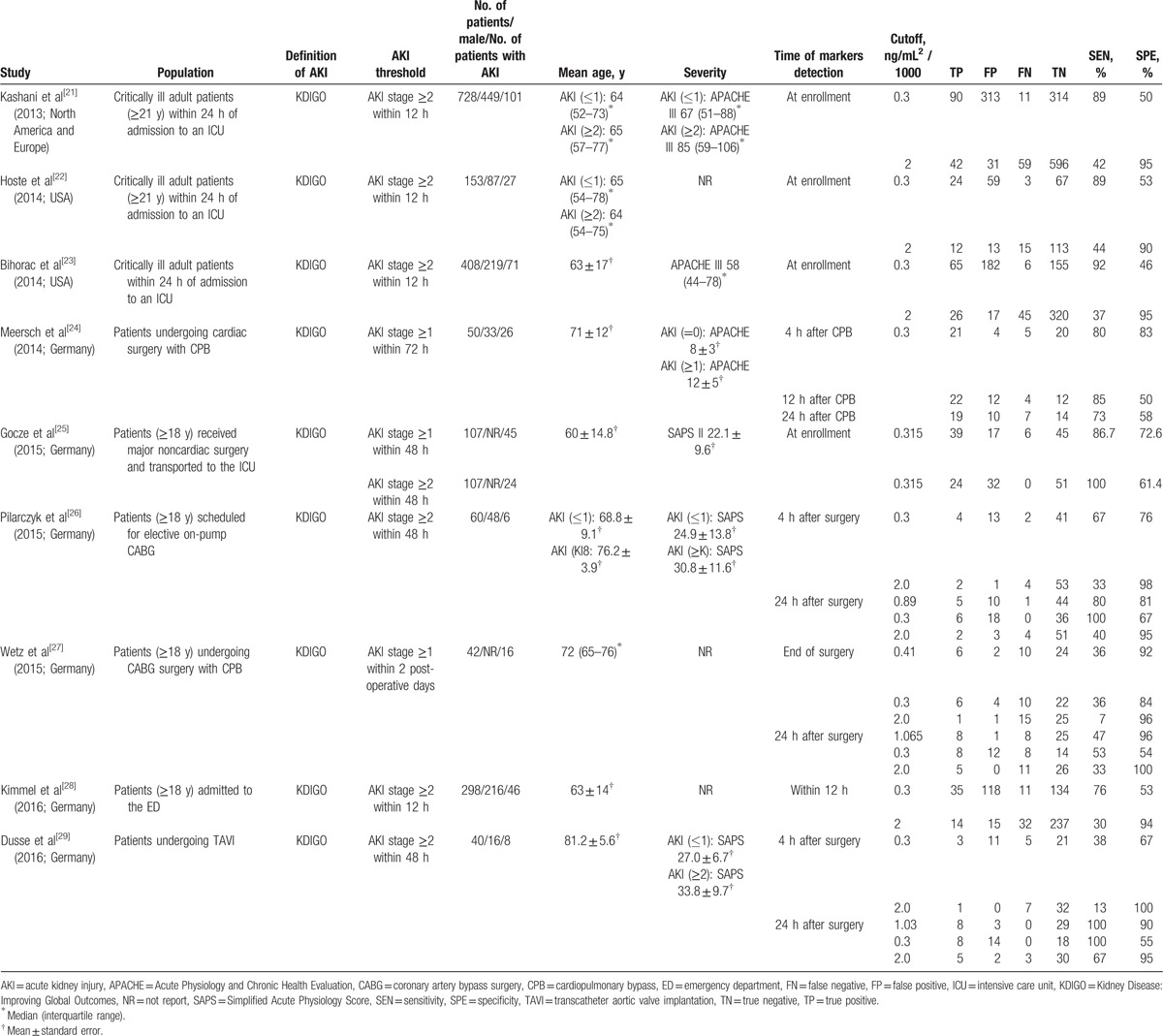
The literature flow diagram (Fig. 1) summarizes the search for and selection of studies. In total, 9 studies satisfied the inclusion criteria.[21–29] One valuable study was excluded from this meta-analysis due to an inability to extract a 2 × 2 contingency table from the available data,[30] and 3 other studies were excluded due to the inclusion of infants[31] and pediatric patients.[32,33] Details regarding all 9 studies are presented in Table 1. All these studies were published between 2013 and 2016, and a total of 1886 patients were included in this meta-analysis. Six of the included studies were conducted in Germany,[24–29] 2 were conducted in the United States,[22,23] and the remaining study was conducted in North America and Europe.[21] Four studies focused on patients who had undergone cardiac surgery,[24,26,27,29] 1 study included noncardiac surgery patients,[25] 4 studies included critically ill patients,[21–23,25] and 1 study included emergency department patients.[28] All studies defined AKI based on the KDIGO criteria,[17] and urinary [TIMP-2]·[IGFBP7] was measured using the commercially available and FDA-approved NephroCheck Test.
Figure 1.
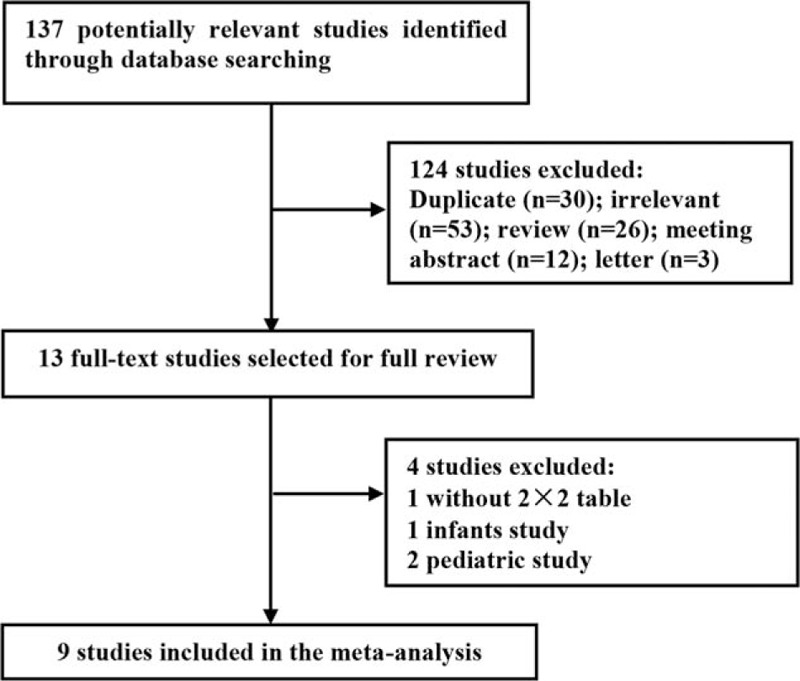
Flow chart depicting the study selection procedure.
Table 1.
Characteristics of the included studies.
3.2. Study quality and publication bias
The QUADAS-2 tool was used to assess the risk of bias in the 9 included studies (Fig. 2). The results revealed that 1 study[24] had a high risk in patient selection, and 2 studies[26,27] had a high risk in flow and timing. Deek funnel plot is shown in Fig. 3. Significant publication bias was observed (P = .04).
Figure 2.
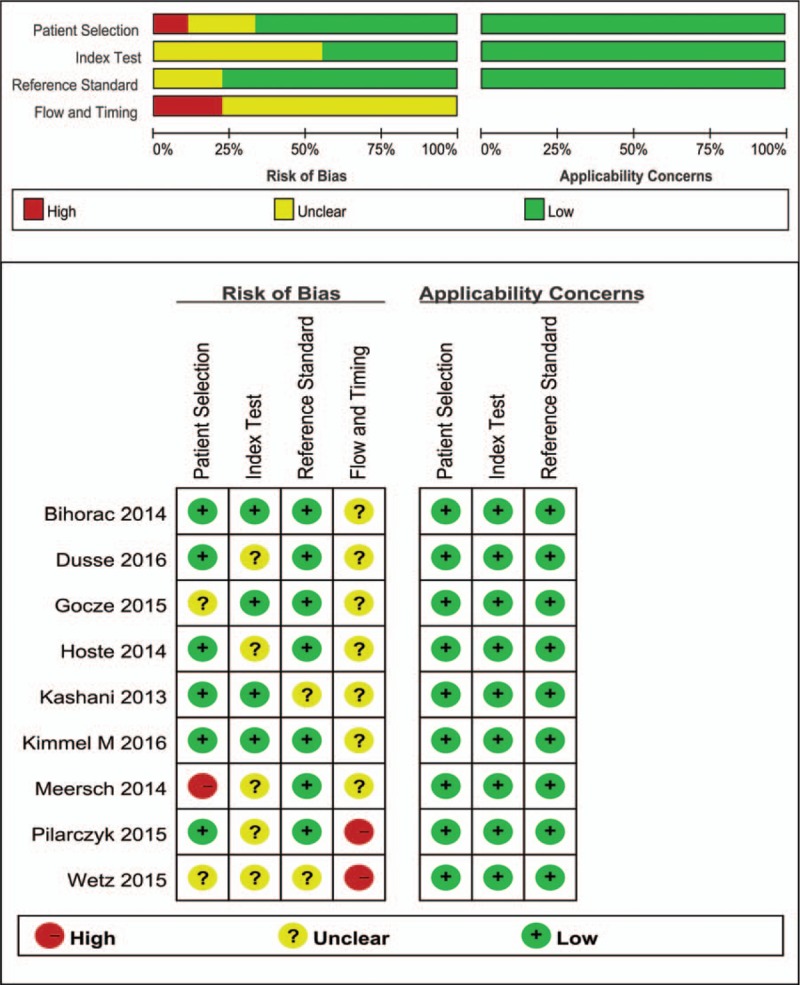
Summary of the methodological quality of the studies according to the QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies-2) criteria.
Figure 3.
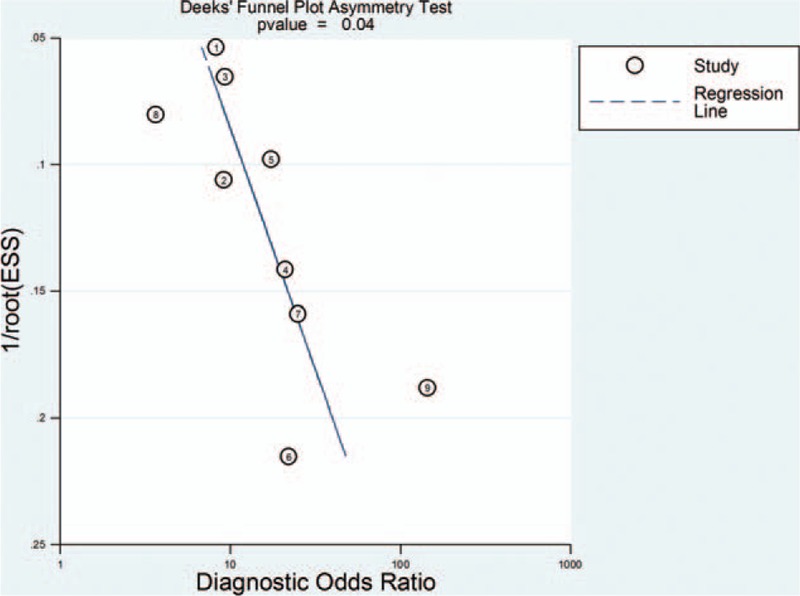
Deek funnel plot asymmetry test for publication bias.
3.3. Diagnostic value of urinary [TIMP-2]·[IGFBP7] for AKI prediction
The pooled SEN and SPE values were 0.83 (95% CI 0.75–0.89) and 0.72 (95% CI 0.56–0.84), respectively (Fig. 4). The PLR and NLR were 3.0 (95% CI 1.9–4.7) and 0.24 (95% CI 0.17–0.33), respectively (Fig. 5). The DOR was 12 (95% CI 7, 22). The area under the SROC curve for urinary [TIMP-2]·[IGFBP7] was 0.86 (95% CI 0.82, 0.88; Fig. 6). Fagan nomogram was applied to estimate the diagnostic value of urinary [TIMP-2]·[IGFBP7] for AKI (Fig. 7). When 50% was selected as the pretest probability of AKI, the results indicated that the use of [TIMP-2]·[IGFBP7] for the detection of AKI increased the post-test probability to 75% when the [TIMP-2]·[IGFBP7] results were positive; the observed PLR of 3 indicated that a person with AKI was 3 times more likely to have a positive diagnosis than a healthy individual. By contrast, when the [TIMP-2]·[IGFBP7] results were negative, the post-test probability decreased to 19%; the NLR was 0.24, suggesting that the combination of TIMP-2 and IGFBP7 was a useful biomarker for the diagnosis of AKI.
Figure 4.
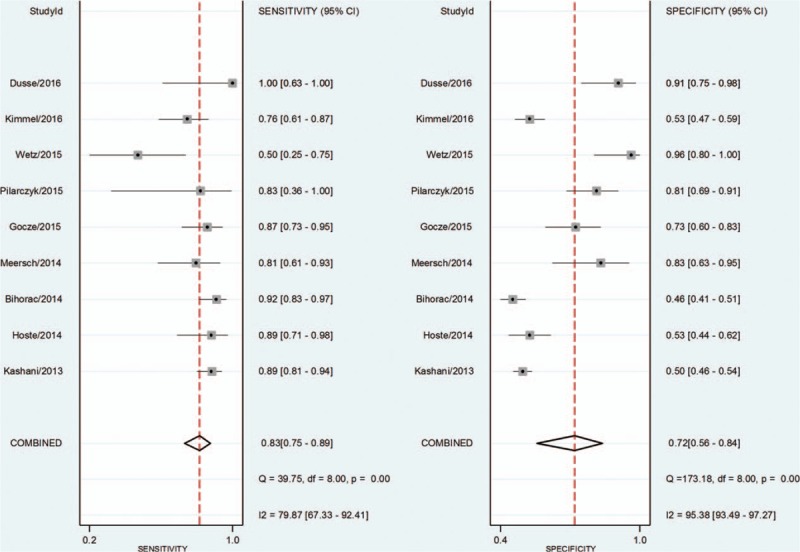
Forest plot of the sensitivity and specificity of urinary [TIMP-2]·[IGFBP7] for the diagnosis of acute kidney injury.
Figure 5.
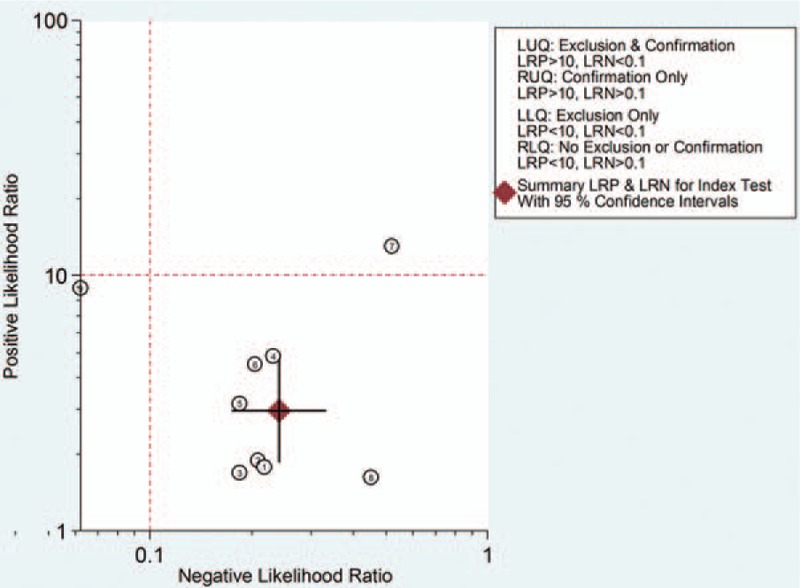
Likelihood ratio scattergram of urinary [TIMP-2]·[IGFBP7] for the diagnosis of acute kidney injury. The positive likelihood ratio and negative likelihood ratio were 3.0 (95% CI 1.9–4.7) and 0.24 (95% CI 0.17–0.33), respectively. CI = confidence interval, LLQ = left lower quadrant, LRN = likelihood ratio negative, LRP = likelihood ratio positive, LUQ = left upper quadrant, RLQ = right lower quadrant, RUQ = right upper quadrant.
Figure 6.
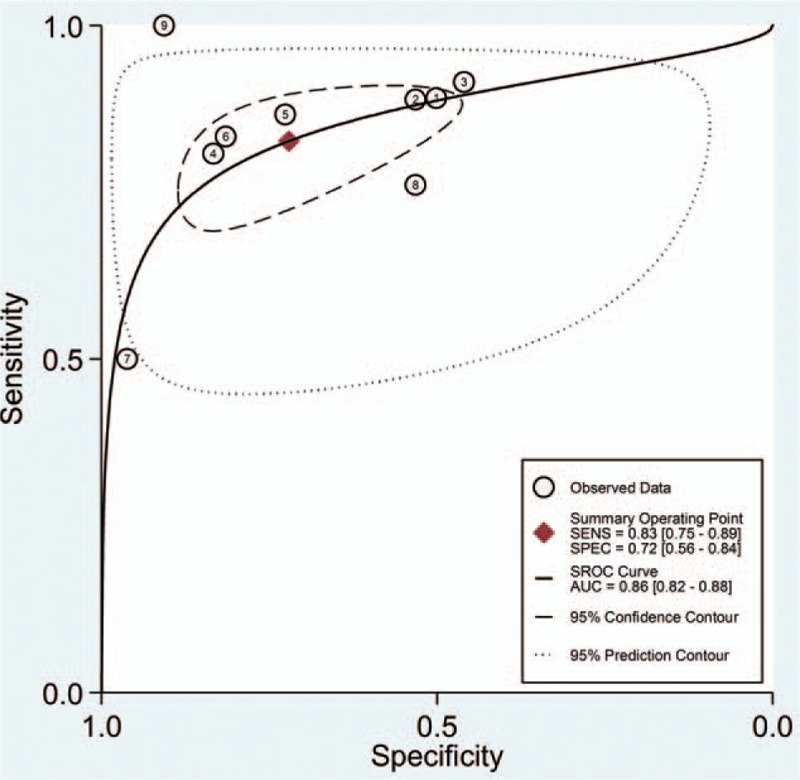
Summary receiver operating characteristic graph for the included studies. AUC = area under curve, SEN = sensitivity, SPE = specificity.
Figure 7.
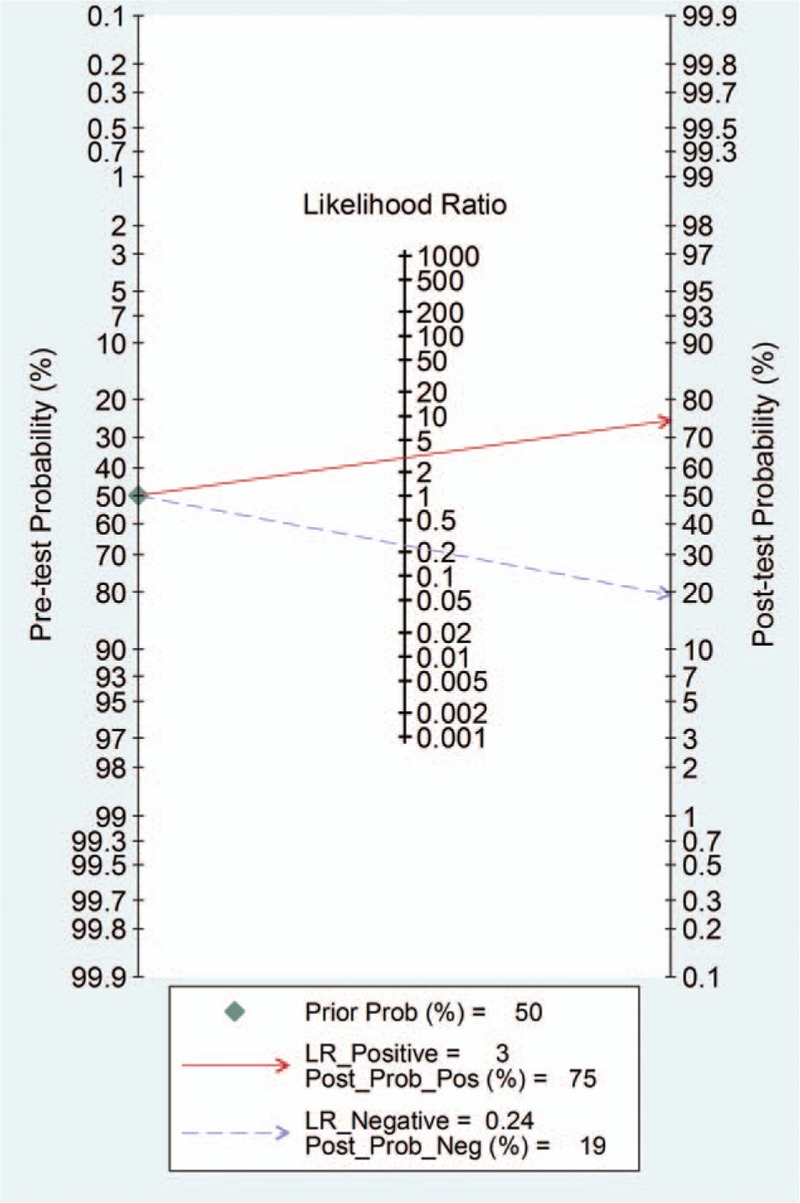
Fagan nomogram of urinary [TIMP-2]·[IGFBP7] for the diagnosis of acute kidney injury.
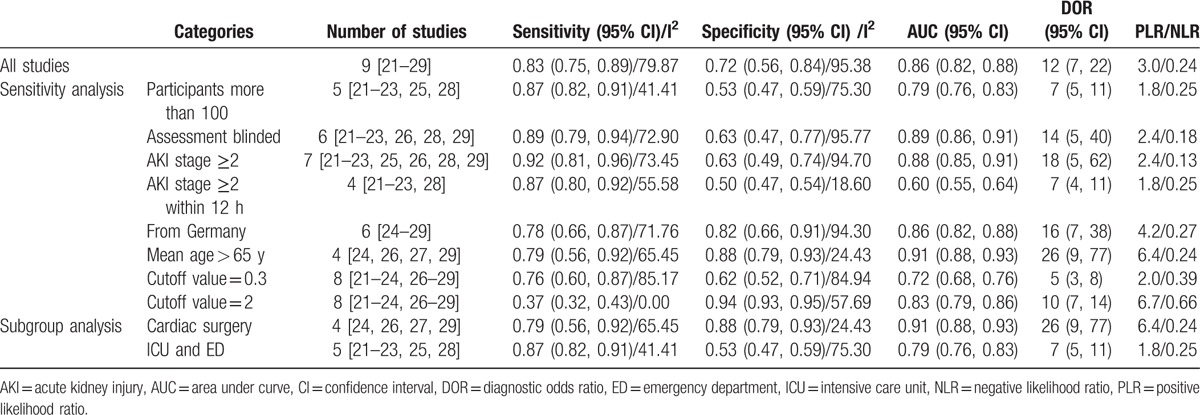
The I2 values for the pooled SEN and SPE were 79.87% (95% CI 67.33–92.41; P < .01, Fig. 4) and 95.38% (95% CI 93.49–97.27; P < .01; Fig. 4), respectively. The overall I2 result for the bivariate model was 94% (95% CI 89–99). The proportion of heterogeneity likely caused by the threshold effect was small (P = .86). Some of the observed heterogeneity was likely caused by population differences and the use of different definitions of positive test results, different cut-off values, or different assessment times. Therefore, we performed a sensitivity analysis and a subgroup analysis to explore the sources of potential heterogeneity in the SEN and SPE (Table 2). However, due to the limited number of included studies, significant heterogeneity was observed among those groups. The results suggested that the cardiac surgery group and the elderly group (mean age > 65 years) had a higher area under curve (AUC 0.91), although these 2 groups included the same studies. Patients who will develop moderate and severe AKI (stage 2 and 3 AKI, respectively, according to the 2012 KDIGO classification)[17] also have a higher AUC (SEN 0.92 [95% CI 0.81–0.96], SPE 0.63 [95% CI 0.49–0.74], and area under the SROC curve 0.88 [95% CI 0.85–0.91]).
Table 2.
Results of sensitivity analysis and subgroup analysis.
4. Discussion
This meta-analysis evaluated the diagnostic accuracy of urinary [TIMP-2]·[IGFBP7] for AKI in adult patients. Overall, [TIMP-2]·[IGFBP7] exhibited fair diagnostic accuracy for AKI (AUC = 0.86, SEN = 0.83, and SPE = 0.72) and AKI ≥ stage 2 (according to the 2012 KDIGO classification[17]; AUC = 0.88, SEN = 0.92, and SPE = 0.63), suggesting that [TIMP-2]·[IGFBP7] is a valuable biomarker for the early detection of AKI. However, current evidence indicates that early recognition cannot prevent the progression of AKI or reduce AKI-associated costs; moreover, FPs may increase unnecessary expenditures.[34]
A previous meta-analysis[35] focused on this topic included 10 full-text prospective studies showing that the estimated sensitivity of urine [TIMP-2]·[IGFBP7] for the early diagnosis of AKI was 0.84 (95% CI 0.80–0.88) and the SPE was 0.57 (95% CI 0.55–0.60). The SROC analysis showed an AUC of 0.88.[35] The results from our meta-analysis were similar, but this meta-analysis included a subgroup analysis[36] of the enrolled studies,[21,23] which might influence the accuracy of the analysis results. We also performed more sensitivity analyses to explore the sources of heterogeneity and collected more data in Table 1 to enable the readers to acquire more valuable information. Furthermore, we used the STATA software to draw Fagan nomogram (Fig. 7) to analyze the index tests and draw a likelihood ratio scattergram (Fig. 5) to evaluate the clinical utility.
AKI is a common complication among hospitalized patients and is associated with significant morbidity and mortality.[37] Early identification of AKI can provide better opportunities for preventive interventions.[38] TIMP-2 and IGFBP7 are novel urinary G1 cell cycle biomarkers released by cellular stress during the early phase of tubular cell injury[39] and have potential value for the early recognition of AKI.[39] However, the product of [TIMP-2]·[IGFBP7] showed a small reverse correlation with age,[40] and diabetes was independently associated with higher [TIMP-2]·[IGFBP7] levels.[30] Therefore, clinicians should be aware of both the utility and limitations of this biomarker in clinical practice.
In this meta-analysis, significant between-study heterogeneity was observed. Although we performed sensitivity and subgroup analyses to explore the sources of potential heterogeneity, the between-study heterogeneity was not significantly decreased. Additional high-quality studies examining a larger sample of patients are required. In addition to its predictive value for AKI, urinary [TIMP-2]·[IGFBP7] has potential for the prediction of the use of renal replacement therapy (RRT) in high-risk patients. One study[25] included in this meta-analysis revealed that the AUC for the use of RRT was 0.83.
Our meta-analysis excluded patients with pre-existing chronic renal failure. However, 1 study compared the reference intervals (inner 95%) for [TIMP-2]·[IGFBP7] in apparently healthy subjects with those for chronic comorbid subjects without AKI (including patients with stable CKD) and found no significant difference (P = .42).[40] Therefore, an analysis of the urinary [TIMP-2]·[IGFBP7] results for CKD patients would be interesting.
Several limitations of this meta-analysis should be considered. First, only 9 studies with marked between-study heterogeneity were included in this meta-analysis; additional subgroup analyses could not be performed to reduce and interpret the heterogeneity. This issue will limit the widespread clinical use of urinary [TIMP-2]·[IGFBP7]. Second, differences in sample collection times may have affected the detection results and led to bias in the findings of this analysis. Third, publication bias was produced because several valuable studies were excluded from this meta-analysis due to an inability to extract a 2 × 2 contingency table from the available data. Fourth, most of the patients were associated with 2 authors (J.A. Kellum and A. Bihorac), most of whom were from German studies; this issue could also have influenced the publication bias. Further research addressing the diagnostic accuracy of the examined biomarker in patients of other ethnicities and regions may be required.
5. Conclusions
Despite the aforementioned limitations, the results of this meta-analysis indicated that urinary [TIMP-2]·[IGFBP7] may be a reliable biomarker for the early detection of AKI. However, given the significant heterogeneity among the included studies, clinicians should be aware of the utility and limitations of this biomarker in clinical practice. Additional high-quality studies examining a larger sample of patients are required.
Footnotes
Abbreviations: AKI = acute kidney injury, AUC = area under curve, CKD = chronic kidney disease, DOR = diagnostic odds ratio, FDA = Food and Drug Administration, FP = false positive, IGFBP7 = insulin-like growth factor binding protein 7, NLR = negative likelihood ratio, PLR = positive likelihood ratio, QUADAS-2 = Quality Assessment of Diagnostic Accuracy Studies 2, RRT = renal replacement therapy, SEN = sensitivity, SPE = specificity, SROC = summary receiver operating characteristic, TIMP-2 = tissue inhibitor of metalloproteinase 2.
The datasets analyzed during the present study are available in the PubMed, Embase, and Cochrane Library databases.
CL and XL contributed equally to this work.
CL and XL contributed equally to this work. CL participated in the design, selected trials, extracted data, performed the statistical analyses, and drafted the manuscript. XL participated in the design, selected trials, performed the statistical analyses, and drafted the manuscript. ZM helped draft the manuscript and assessed the risk of bias of the trials. HK helped draft the manuscript and assisted with interpretation of the data. HL contributed to data collection. LP participated in the analysis and interpretation of data. LW helped draft the manuscript and assessed the risk of bias of the trials. FZ collected the data, performed the statistical analyses, and supervised the study. All authors read and approved the final manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998;104:343–8. [DOI] [PubMed] [Google Scholar]
- [2].Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg 2009;249:851–8. [DOI] [PubMed] [Google Scholar]
- [3].Chawla LS, Amdur RL, Shaw AD, et al. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol 2014;9:448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 2011;79:1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- [6].Zhang A, Cai Y, Wang PF, et al. Diagnosis and prognosis of neutrophil gelatinase-associated lipocalin for acute kidney injury with sepsis: a systematic review and meta-analysis. Crit Care 2016;20:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin X, Yuan J, Zhao Y, et al. Urine interleukin-18 in prediction of acute kidney injury: a systemic review and meta-analysis. J Nephrol 2015;28:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Susantitaphong P, Siribamrungwong M, Doi K, et al. Performance of urinary liver-type fatty acid-binding protein in acute kidney injury: a meta-analysis. Am J Kidney Dis 2013;61:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shao X, Tian L, Xu W, et al. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS ONE 2014;9:e84131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ronco C. Cell cycle arrest biomarkers: new weapons for a new battle. Blood Purif 2014;38:I–II. [DOI] [PubMed] [Google Scholar]
- [11].U.S. Food and Drug Administration. FDA allows marketing of the first test to assess risk of developing acute kidney injury [Press release]; 2014. Available from: http://www.fda.gov/NewsEvents/Newsroom/Press Announcements/ucm412910.htm. Accessed October 12, 2014. [Google Scholar]
- [12].Vijayan A, Faubel S, Askenazi DJ, et al. Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis 2016;68:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int 2009;76:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang QH, Liu DW, Long Y, et al. Acute renal failure during sepsis: potential role of cell cycle regulation. J Infect 2009;58:459–64. [DOI] [PubMed] [Google Scholar]
- [15].Wang Z, Famulski K, Lee J, et al. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int 2014;85:82–93. [DOI] [PubMed] [Google Scholar]
- [16].Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 1999;20:761–87. [DOI] [PubMed] [Google Scholar]
- [17].KDIGO Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–38. [Google Scholar]
- [18].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [19].Arends LR, Hamza TH, van Houwelingen JC, et al. Bivariate random effects meta-analysis of ROC curves. Med Decis Mak 2008;28:621–38. [DOI] [PubMed] [Google Scholar]
- [20].Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982–90. [DOI] [PubMed] [Google Scholar]
- [21].Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hoste EA, McCullough PA, Kashani K, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant 2014;29:2054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014;189:932–9. [DOI] [PubMed] [Google Scholar]
- [24].Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS ONE 2014;9:e93460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gocze I, Koch M, Renner P, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS ONE 2015;10:e0120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, et al. Urinary [TIMP-2]∗[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care 2015;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wetz AJ, Richardt EM, Wand S, et al. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care 2015;19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kimmel M, Shi J, Latus J, et al. Association of renal stress/damage and filtration biomarkers with subsequent AKI during hospitalization among patients presenting to the emergency department. Clin J Am Soc Nephrol 2016;11:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dusse F, Edayadiyil-Dudásova M, Thielmann M, et al. Early prediction of acute kidney injury after transapical and transaortic aortic valve implantation with urinary G1 cell cycle arrest biomarkers. BMC Anesthesiol 2016;16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bell M, Larsson A, Venge P, et al. Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Markers 2015;2015:158658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gist KM, Goldstein SL, Wrona J, et al. Kinetics of the cell cycle arrest biomarkers (TIMP-2∗IGFBP-7) for prediction of acute kidney injury in infants after cardiac surgery. Pediatr Nephrol 2017;DOI: 10.1007/s00467-017-3655-y. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [32].Meersch M, Schmidt C, Van Aken H, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS ONE 2014;9:e110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Westhoff JH, Tönshoff B, Waldherr S, et al. Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) • insulin-like growth factor-binding protein 7 (IGFBP7) predicts adverse outcome in pediatric acute kidney injury. PLoS ONE 2015;10:e0143628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lameire N, Vanmassenhove J, Van Biesen W, et al. The cell cycle biomarkers: promising research, but do not oversell them. Clin Kidney J 2016;9:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Su Y, Gong Z, Wu Y, et al. Diagnostic value of urine tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for acute kidney injury: a meta-analysis. PLoS ONE 2017;12:e0170214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Honore PM, Nguyen HB, Gong M, et al. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med 2016;44:1851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu KD, Vijayan A, Rosner MH, et al. Clinical adjudication in acute kidney injury studies: findings from the pivotal TIMP-2∗IGFBP7 biomarker study. Nephrol Dial Transplant 2016;31:1641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med 2017;DOI: 10.1515/cclm-2016-0973. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [39].Ortega-Loubon C, Fernández-Molina M, Carrascal-Hinojal Y, et al. Cardiac surgery-associated acute kidney injury. Ann Card Anaesth 2016;19:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chindarkar NS, Chawla LS, Straseski JA, et al. Reference intervals of urinary acute kidney injury (AKI) markers [IGFBP7]·[TIMP2] in apparently healthy subjects and chronic comorbid subjects without AKI. Clin Chim Acta 2016;452:32–7. [DOI] [PubMed] [Google Scholar]


