Abstract
Purpose
This study aims to (1) describe the activities, function, and health-related quality of life (HRQOL) of a large sample of older adults (age ≥ 65) with cancer, (2) identify the associations with demographics, cancer type, comorbid conditions, and ability to participate in activities and functional status.
Methods
The Health Registry/Cancer Survivorship Cohort is an institutional database designed to aid cancer survivorship research. The registry includes three measures of patient-reported HRQOL: FACT-G and PROMIS® Global measures for physical and mental health. Other measures included in the registry are cancer type, date from diagnosis, number of comorbid conditions and specific conditions and their limitations in daily activity, and self-reported daily activity/function.
Results
Our sample consists of 768 older adults with cancer, mean age 72 years, 60% female, and 90% White. Mean scores for HRQOL: FACT-G (85, range: 25–108), PROMIS-physical (48, range: 16–67) and, PROMIS-mental (51, range: 21–67). In multivariable models, Black race, one or more comorbid conditions, and Gastrointestinal cancer (p < .05), and patient- reported decreased levels of activities/function were all independently associated with poor HRQOL (p < .0001).
Conclusions
Older Black adults with cancer, those that have high comorbidity burden, with gastrointestinal cancers and those that report decreased ability to participate in daily activities/function reported poorer HRQOL. As geriatric oncology moves towards trying to identify who may need supportive services, this study demonstrated that a one question patient-reported level of activities and functional ability were independently associated with physical, mental, and cancer-specific HRQOL.
Keywords: Health-related quality of life, functional status, older adults, supportive services, neoplasm, activities
INTRODUCTION
Cancer is primarily a disease of older adults, with the majority of new cancer diagnoses and cancer deaths occurring in people over the age of 65 years. Given the “aging” of the U.S. population, there is an anticipated 67% increase in cancer incidence among older adults by 2030 [1]. The treatment of older adults with cancer remains complex due to the heterogeneous aging process and associated wide range in treatment tolerability and toxicity [2]. Overall, older adults are at greater risk of treatment-related toxicity [3] and often lack the physiologic reserve necessary to adequately recover from acute toxicities, resulting in prolonged functional deficits and diminished quality of life [4,5].
Health-related quality of life (HRQOL), a multidimensional construct including a person’s subjective assessment of their physical, mental, and social health, [6] has become a frequently-used outcome in clinical cancer studies. However, studies to date in older adults with cancer have a number of limitations including small sample sizes, inclusion of only fitter, younger ‘old’ adults, or measured HRQOL with only one generic (non-cancer specific) questionnaire such as the Short Form-36 [7–11]. Previous work has already shown that 25–30% of older adults report a significant decline in HRQOL within the first year of a cancer diagnosis [12,13]. A deeper understanding of the associations with HRQOL in a large sample of older adults with cancer, including a more diverse mix of cancer types and larger sample sizes, may generate important new insights into associations with poor HRQOL and identify intervention focused factors.
Most older adults value the maintenance of their HRQOL (including cognition and functional ability) higher than their overall survival [14–16]; yet, HRQOL is generally not addressed as part of routine cancer care and referrals to related supportive services in a typical outpatient oncology clinic are underutilized [17,18]. Older adults with cancer report needing and wanting supportive care treatments to help improve their HRQOL, but practical methods to identify patients in need are lacking [19–21].
Cancer-related disability, in contrast to disability related to trauma or neurological conditions such as stroke, is typically a gradual process. This gradual decline can be difficult for providers to detect and is a barrier to patients receiving supportive services during and after cancer treatment [22,23]. Supportive services, such as cancer rehabilitation (occupational and physical therapy), could improve HRQOL and functional status; however, timely identification of patients at risk for poor HRQOL in busy oncology clinics remains problematic [24,22,23]. A deeper understanding of the underlying factors associated with declines in HRQOL, over and above number and type of comorbid conditions, and developing better strategies for identifying patients who may benefit from related supportive interventions quickly is critical to improving the care of older adults with cancer.
This study aims to (1) describe activities, function and HRQOL of a large sample of adults’ age 65 years or older with a cancer diagnosis and (2) identify the associations with demographics, cancer type, comorbid conditions and ability to participate in activities and functional status.
METHODS
Participants
We examined data collected through a large hospital-based observational cohort registry entitled the Health Registry/Cancer Survivorship Cohort (HR/CSC). This registry is an Institutional Review Board-approved (IRB no. 09-0605) database designed to aid cancer survivorship research. The registry includes patient-reported data, biologic specimens, and tumor tissue for adults with cancer. Registry participants are identified and recruited through the North Carolina Cancer Hospital outpatient clinics (2010 – 2014) with the following eligibility criteria: 18 years and older, and English or Spanish language proficiency. Informed consent was obtained from all individual participants included in the study. Our specific sub - study was limited to registry participants’ age 65 years and older and was approved by the Office of Human Research Ethics (IRB no. 13-3791).
Measures and data collection
For the registry, baseline survey interviews were conducted by trained staff using computer-assisted telephone-based interview technology. The baseline survey included 3 standardized measures of HRQOL, demographics, comorbid conditions checklist including questions regarding whether or not the condition limited daily activities, cancer type, date from diagnosis, and self- reported activities/function.
For our study, the following HRQOL measures were included; the Functional Assessment of Cancer Therapy-General (FACT-G), and the National Institutes of Health’s Patient-Reported Outcomes Measurement Information System® (PROMIS®) Global Health short form which includes physical and mental health subscales. The FACT-G consists of 21 questions regarding physical, social/family, emotional, and functional well-being during the past week and was answered on an ordinal response scale between 0 (not at all) to 4 (very much). The total score for the FACT-G ranges from 0 – 108, with higher scores indicating better HRQOL [25]. The average score for adults with cancer 81, adults without cancer is 80 [26]. The minimal clinically importance difference for the FACT-G is 3–7 points [27,28].
The PROMIS Global Health short form version 1 is a 10 - item scale that measures perceptions of global health across domains of physical function, fatigue, pain, emotional distress, and social health [29,30]. The PROMIS Global Health produces 2 different scores. The two larger subdomains (physical health and mental health) consist of eight questions, and have been tested in a large sample of adults living in the US with a standardized mean score of 50 and a standard deviation of 10 [30]. The minimal clinical important difference for PROMIS ranges from 2 to 6 points [31,32].
The HR/CSC also includes one question from the Patient Generated - Subjective Global Assessment (PG- SGA-AF) that asks about activities/function [33]. Specifically, this question asks, “In the past month, how would you generally rate your activity?” Response options range from 0 “normal activity with no limitations” to 4 “pretty much bedridden, rarely out of bed”. For this study, only the activities and function item was used. The full scale (not included here) includes questions regarding nutrition and one question on function. To our knowledge, this is the first time this question has been used alone. This full scale was previously found to be associated with HRQOL in patients undergoing radiation, and it predicted overall survival in patients with advanced cancer [34,35,33].
Statistical Analysis
Descriptive statistics are provided for all study measures. We only used data where we had complete information (768/807 = 95 %). We stratified our sample into two groups to compare and explore potential differences; peri-diagnosis to one year from diagnosis, and patients who were at least one year from diagnosis. Multivariable linear regression modeling was used to evaluate the associations between the outcome variables (HRQOL measures: FACT-G, PROMIS Global physical and mental health) and the following factors available in the dataset: cancer type, date from diagnosis (for this analyses was used as a continuous variable instead of dichotomous), total number of comorbidities (not including primary cancer), type and associated limitations of specific comorbidities chosen for frequency in dataset, and from a consensus of authors of comorbid conditions we hypothesized could potentially decrease HRQOL (diabetes, congestive obstructive pulmonary disease (COPD), arthritis, stroke, depression/anxiety and cardiac conditions) demographics (age, sex, race, living arrangements), and activities/function. We used all variables that were available and no specific model selection was used. All analyses were completed using SAS version 9.3 (Cary, NC).
Results
Descriptive Statistics
There were 768 patients in the registry who were age 65 years or older with cancer. All descriptive statistics for the sample are shown in Table 1. The mean age was 72 years (range: 65 – 93, SD: 6), 60 % (457) were female, a majority of participants lived with at least one other person (64 %), and on average the number of comorbid conditions was 3.2 (range: 0 – 12, SD: 2). The majority were diagnosed with genitourinary cancers (25%), followed by breast and gastrointestinal (24, 22 %), respectfully. Half of the sample reported normal activity, 33% reported “Not my normal self, but able to be up and about with fairly normal activities”, and the remaining 16% reported some level of activities/function limitation. The baseline survey interviews were completed with a median of 76 days from diagnosis. The mean scores for the HRQOL outcome measures are PROMIS Physical 48 (SD: 9.1), PROMIS Mental 51(SD: 8.5), and FACT-G 85(SD: 15.3). The only significant difference between groups of survivors was between cancer types.
Table 1.
Characteristics of the Sample
| Variable | Total (N = 768) | First year* (n = 480) | One-year post (n = 288) | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 311 (40) | 205 (43) | 106 (37) | 0.11 |
| Female | 457 (60) | 275 (57) | 182 (63) | |
| Age (years) | ||||
| 65–70 | 330 (43) | 204 (43) | 126 (44) | 0.12 |
| 70–74 | 201 (26) | 129 (27) | 72 (25) | |
| 75–79 | 139 (18) | 95 (20) | 44 (15) | |
| 80–84 | 63 (8) | 36 (7) | 27 (9) | |
| 85+ | 35 (5) | 16 (3) | 19 (7) | |
| Race | ||||
| White | 689 (90) | 426 (89) | 263 (91) | 0.27 |
| Non-White | 70 (10) | 54 (11) | 25 (9) | |
| Co-morbidities | ||||
| 0 – 1 | 176 (23) | 102 (21) | 74 (26) | 0.10 |
| 2 – 3 | 286 (38) | 194 (40) | 95 (33) | |
| ≥4 | 303 (40) | 184 (39) | 119 (41) | |
| Type of Cancer | ||||
| Genitourinary | 191 (25) | 132 (28) | 59 (20) | <.0001 |
| Breast | 187 (24) | 88 (18) | 99 (34) | |
| Gastrointestinal | 171 (22) | 106 (22) | 65 (23) | |
| Gynecologic | 118 (15) | 98 (20) | 20 (7) | |
| Other | 101 (13) | 56 (12) | 45 (16) | |
| Lived Alone | ||||
| Yes | 186 (24) | 123 (26) | 63 (22) | 0.26 |
| No | 582 (76) | 357 (74) | 225 (78) | |
| Activities/Function | ||||
| Normal | 392 (51) | 230 (48) | 162 (56) | 0.20 |
| Fairly normal | 254 (33) | 165 (34) | 89 (31) | |
| In bed or chair < ½ day | 65 (9) | 46 (10) | 19 (7) | |
| In bed or chair > ½ day | 52 (7) | 35 (7) | 17 (6) | |
| Bedridden | 5 (1) | 4 (1) | 1 (0) | |
| HRQOL^ | ||||
| FACT-G | 85 (15) | 85 (15) | 86 (16) | 0.27 |
| PROMIS- GPH | 48 (9) | 48 (9) | 48 (9) | 0.85 |
| PROMIS- GMH | 51 (8) | 51 (8) | 52 (9) | 0.15 |
Note.
peri-diagnosis to one-year post.
means (standard deviation). FACT-G = Functional Assessment of Cancer Therapy-General, PROMIS Patient-Reported Outcomes Measurement Information System Global Physical Health= GPH, PROMIS Patient-Reported Outcomes Measurement Information System Global Mental Health=PROMIS-GMH.
Multivariable Models of Factors Associated with HRQOL Measures
In the multivariable models (Table 2), alone for each of the three HRQOL measures, the following were significantly associated with worse HRQOL in all measures: Black race (all p < 0.05), and one or more comorbid conditions (all p < .02), For cancer-specific FACT-G, adults with gastrointestinal cancers, activity limiting diabetes and depression/anxiety, and also reported significantly lower HRQOL (p < .05). For PROMIS Global physical health, being male, having genitourinary, gastrointestinal and other cancers, and activity limiting arthritis were separately associated with poor physical HRQOL (p < .001). Lastly, for PROMIS Global mental health, being 85 years or older, having genitourinary, gastrointestinal cancers (compared to breast), reporting depression/anxiety and reporting decreased activities/function were significantly related to poor mental health (p < .05).
Table 2.
Predicting HRQOL in Older Adults with Cancer N = 768
| FACT-G | PROMIS-GPH | PROMIS-GMH | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate | p - value | Estimate | p - value | Estimate | p - value | |
| Sex (ref = Female) | ||||||
| Male | −1.55 | .217 | 1.54 | .031 | 1.17 | .121 |
| Age (ref = 65 – 70 years) | ||||||
| 70 – 74 | 0.26 | .807 | −0.01 | .990 | −0.58 | .375 |
| 75 – 79 | −0.68 | .587 | −0.39 | .585 | −1.13 | .134 |
| 80 – 84 | −0.10 | .951 | −0.70 | .464 | −0.84 | .408 |
| 85 + | −1.41 | .520 | −1.26 | .314 | −2.79 | .034 |
| Race (ref = White) | ||||||
| Non —White | −3.51 | .016 | −1.68 | .047 | −3.64 | <.0001 |
| Living Alone | −1.20 | .250 | 0.09 | .882 | −0.30 | .632 |
| Type of Cancer (ref = Breast) | ||||||
| Genitourinary | −2.47 | .131 | −2.74 | .004 | −2.89 | .004 |
| Gastrointestinal | −2.79 | .059 | −1.85 | .029 | −2.57 | .004 |
| Gynecologic | −0.85 | .561 | −0.68 | .418 | −0.59 | .505 |
| Other | 0.43 | .793 | −2.18 | .019 | −1.41 | .149 |
| Time from diagnosis (years) | 0.09 | .330 | −0.06 | .267 | 0.01 | .916 |
| Number of co-morbid | −1.25 | .001 | −0.66 | .002 | −0.52 | .021 |
| Co-morbidities* | ||||||
| Diabetes | ||||||
| Does not limit activity | 4.64 | .000 | −0.59 | .421 | 0.62 | .420 |
| Limits activity | −4.74 | .046 | −1.44 | .299 | −0.36 | .804 |
| COPD | ||||||
| Does not limit activity | −0.37 | .854 | −0.02 | .986 | −0.69 | .569 |
| Limits activity | 0.16 | .945 | −1.89 | .145 | −1.08 | .432 |
| Arthritis | ||||||
| Does not limit activity | 1.93 | .099 | −0.00 | .997 | −0.21 | .760 |
| Limits activity | 0.75 | .547 | −2.55 | .000 | 0.01 | .989 |
| Stroke | ||||||
| Does not limit activity | 3.11 | .140 | −0.89 | .459 | 0.51 | .691 |
| Limits activity | −3.19 | .367 | −1.31 | .533 | −1.19 | .575 |
| Depression/Anxiety | ||||||
| Does not limit activity | −3.48 | .006 | −0.98 | .173 | −3.76 | <.0001 |
| Limits activity | −16.27 | <.0001 | −2.23 | .089 | −8.54 | <.0001 |
| Cardiac | ||||||
| Does not limit activity | 1.25 | .409 | −0.32 | .712 | −0.63 | .484 |
| Limits activity | 0.20 | .941 | −0.20 | .842 | 0.39 | .810 |
| Activities/Function (ref = none) | ||||||
| Out of bed most of the day | −7.95 | < .0001 | −6.80 | < .0001 | −3.69 | <.0001 |
| In bed, less than half of the day | −15.73 | < .0001 | −10.24 | < .0001 | −5.50 | <.0001 |
| In bed, most of the day | −19.73 | < .0001 | −12.91 | < .0001 | −6.99 | <.0001 |
| Pretty much bedridden | −29.81 | < .0001 | −22.57 | < .0001 | −12.37 | .0002 |
Note. N = 768.
Comparisons are with higher level of function, e.g. no diabetes vs. diabetes with no limitations, diabetes with no limitations vs. diabetes with activity limitation. FACT-G = Functional Assessment of Cancer Therapy-General, PROMIS Patient-Reported Outcomes Measurement Information System Global Physical Health= GPH, PROMIS Patient-Reported Outcomes Measurement Information System Global Mental Health=PROMIS-GMH.
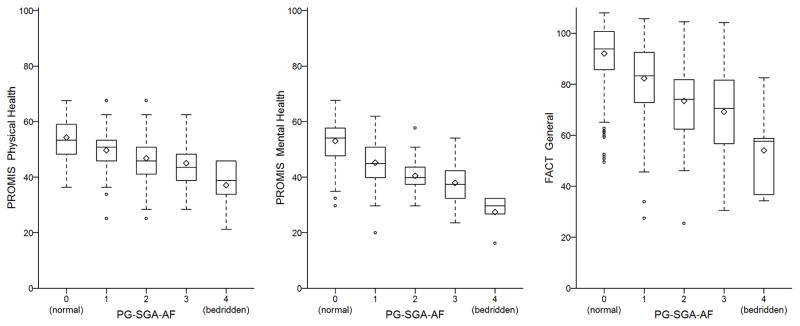
Since the activity/function question was independently associated with all three HRQOL measures even after controlling for the other patient demographic and clinical characteristics (p < .0001), we focused further on this item, see Figure 1. For the PROMIS-Physical, with each decreasing level of activity/function, there was a significant decrease in score compared to the previous level. For instance, those who reported being in the” bed less than half of the day” reported PROMIS physical scores approximately 3.44 points lower than those who reported being “not normal, but out of the bed most of the day”. This similar pattern was seen with both the FACT-G and PROMIS mental.
Figure 1.
DISCUSSION/CONCLUSION
This study describes the activities, function, and the HRQOL of a large sample of older adults (65 years or older) with cancer. On average, our sample of older adults with cancer reported their physical and mental health with PROMIS as similar to the general US population, and better HRQOL with the FACT-G [28]. In our study, Black race, one or more comorbid conditions (p <. 05), and self-reported decreased levels of activities/function were all independently associated with poor HRQOL (p < .001).
The significant association we found between comorbidity burden and lower HRQOL is supported in the literature [8,36,15]. Smith and colleagues found that older adults with cancer were more likely to have at least one comorbid condition compared to same-age counterparts without cancer, and those with two or more comorbidities were more likely to report poor HRQOL. They also found that conditions such as arthritis, lung disease, stroke, and gastrointestinal disease had an even greater role in predicting low HRQOL than cancer. For our sample, older adults with at least one or more condition was associated with a decrease in HRQOL. However, when we added specific conditions that we hypothesized based on the literature to have a potential impact on HRQOL (diabetes, COPD, arthritis, stroke, anxiety/depression, and cardiac conditions) we found that a diagnosis of diabetes, arthritis, and depression/anxiety were significantly associated with HRQOL. We did not find any significant association of HRQOL with COPD, stroke or cardiac conditions.
Interestingly, patients who reported having diabetes with no limitations reported higher physical HRQOL than those without, we suspect that potentially patients with diabetes, with no associated limitations may be more activated within the health care system. This association changed dramatically when patients report that the diabetes limits their activity and it significantly decreased their HRQOL. For adults with arthritis, only their mental health appeared to suffer when arthritis limited their activity level. We did find that having a diagnosis of depression and/or anxiety where it was reported as limiting activity was also significantly associated with poor HRQOL. Adults who reported depression and anxiety reported decreased physical HRQOL on both the PROMIS and the FACT-G, but no decrease in mental health. Those with activity limiting depression and anxiety were significantly 9.4 points lower on the PROMIS scale than those without limitations. This finding, although not surprising, offers a potentially treatable condition which could alleviate decreases in HRQOL for older adults with cancer. Although depression and anxiety can be appropriately managed, psychological treatments are underutilized [37]. Lastly, similar to other studies, higher levels of comorbidity have also been found to be associated with increased number of functional deficits in older adults with cancer [18,8].
In our study, race was significantly associated with HRQOL, even after controlling for other major demographic and clinical variables. This association has been established within the literature, where older Black adults with cancer consistently report lower HRQOL [38]. Recent literature suggests that older Black adults may perceive the diagnosis of cancer to have a greater impact on well-being than White counterparts [39]. In some studies where race was a significant predictor of HRQOL, the limiting factor of certain conditions (i.e. diabetes) was thought to potentially be driving these results or at least interacted with race [40]. In Black Americans, some cancers tend to be diagnosed at a later stage, with increased levels of disability [41]. Multiple levels of systematic and system- level factors not measured in our sample may be contributing to this finding, such as decreased access to care, increased disability burden, or decreased quality of care and cancer care differences [39,42,43]. Future research needs to focus on culturally sensitive interventions aimed at older Black adults with cancer to improve their HRQOL.
We found that asking patients to rate their level of activities and functional ability was significantly associated with HRQOL. Similar studies have found performance status as measured by a clinician to be predictive of survival in some cancer types [44], this study used patient-reported assessments. In studies examining the differences between patient and clinician reported performance status, patient- reported was associated with other factors of disability and concern, such as previous falls [45,2]. Patient-reported activity and functional status is particularly informative as patient-reported outcome measures move from observation to triggering and informing specific types of supportive cancer care services, such as rehabilitation services that directly treat limitations in activities and functional ability. Because our study showed significant and consistent decline in HRQOL related to lower levels of activity and function (see Figure 1), this single-item measure may hold potential as a tool for oncology providers to identify risks for HRQOL concerns in their patients who may require referral for rehabilitation services. Future research is needed to determine the easiest and most effective measure to identify need for cancer rehabilitation services.
Our study has several limitations. At the time of this study we did not have specific cancer and cancer-treatment related variables that may modify our results, such as cancer stage, type and dates of treatment, and length of survival. However, we had a large sample with multiple types of cancers throughout the cancer continuum providing a broad understanding of HRQOL in an older cancer population. We tried to address this by looking at time from diagnosis, assuming patients within the first year of survivorship would be different than those more than a year from diagnosis. We only found differences between cancer types, but when used as a variable within the multivariable model, there was no significant association with HRQOL. Our study was a cross-sectional analysis, so we were not able to examine causational relationships, such as the prediction of HRQOL and longer-term outcomes. We also recognize that our measures of HRQOL have items regarding physical performance which may overlap some with our measure of activities/function. Lastly, this sample was from a single institution, which may not be generalizable to all older adults with cancer, however our institution is similar to other cancer centers, and this study consisted of a wide range of cancer diagnoses and a large diverse sample.
Future research examining how to best identify and treat functional limitations and certain comorbid conditions is necessary to provide the critical supportive services needed to potentially improve HRQOL of older adults with cancer. Clinic friendly, quick, validated screening tools of patient-reported activities of daily living and function within busy oncology clinics could be integrated into the daily oncology provider’s routine. Brief screening tools, such as the question asked in this survey about participating in activities and functional tasks could be useful in identifying patients at risk for poor HRQOL and who could use supportive services including but not limited to occupational and physical therapies, and home health care. In addition, testing of screening tools in a large cancer population will provide data on meaningful cut off values for referral and/or follow-up services. Management of activities of daily living and physical function impact HRQOL and remains an important consideration for the older survivor of cancer.
Acknowledgments
Funding:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA116339 and the University Cancer Research Fund UL1RR025747 Lineberger Comprehensive Cancer Center (LCCC-0916). This work is supported, in part, by the UNC Oncology Clinical Translational Research Training Program NCI 5K12CA120780-07 for Bryant and Williams.
Footnotes
Presented:
Poster presentation at American Society of Clinical Oncology (ASCO) 2015
No disclaimers
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mackenzi Pergolotti, Assistant Professor, Department of Occupational Therapy, College of Health and Human Services, Colorado State University, 800 Oval Drive, Fort Collins, CO 80526 USA.
Allison M. Deal, Biostatistics Core Facility, The University of North Carolina at Chapel Hill, Lineberger Comprehensive Cancer Center, 170 Manning Drive, Chapel Hill, NC 27599 USA.
Grant R. Williams, Division of Hematology/Oncology, School of Medicine, University of Alabama at Birmingham.
Ashley Leak Bryant, School of Nursing, The University of North Carolina at Chapel Hill, Carrington Hall, CB #7460, Chapel Hill, NC 27599-7460.
Jeannette T. Bensen, UNC Lineberger Comprehensive Cancer Center, Campus Box 7295, Chapel Hill 27599 USA.
Hyman B. Muss, Geriatric Oncology Program, The University of North Carolina at Chapel Hill, Lineberger Comprehensive Cancer Center, 170 Manning Drive, Chapel Hill, NC 27599 USA.
Bryce B. Reeve, Department of Health Policy and Management, 1101D McGavran-Greenberg Hall, Campus Box 7411, The University of North Carolina at Chapel Hill, Gillings School of Global Public Health, 135 Dauer Drive, Chapel Hill, NC 27599 USA.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Jolly TA, Deal AM, Nyrop KA, Williams GR, Pergolotti M, Wood WA, Alston SM, Gordon BB, Dixon SA, Moore SG, Taylor WC, Messino M, Muss HB. Geriatric Assessment-Identified Deficits in Older Cancer Patients With Normal Performance Status. Oncologist. 2015 doi: 10.1634/theoncologist.2014-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muss HB, Berry DA, Cirrincione C, Budman DR, Henderson IC, Citron ML, Norton L, Winer EP, Hudis CA. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(24):3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 4.Cohen HJ. Functional assessment and the cancer survivor: something old, something new. J Natl Cancer Inst. 2010;102(19):1450–1451. doi: 10.1093/jnci/djq365. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite D, Satariano WA, Sternfeld B, Hiatt RA, Ganz PA, Kerlikowske K, Moore DH, Slattery ML, Tammemagi M, Castillo A, Melisko M, Esserman L, Weltzien EK, Caan BJ. Long-term prognostic role of functional limitations among women with breast cancer. J Natl Cancer Inst. 2010;102(19):1468–1477. doi: 10.1093/jnci/djq344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cella DF. Measuring quality of life in palliative care. Seminars in oncology. 1995;22(2 Suppl 3):73–81. [PubMed] [Google Scholar]
- 7.Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, Hays RD. Health-related quality of life in older adult survivors of selected cancers: Data from the SEER-MHOS linkage. Cancer. 2015;121(5):758–765. doi: 10.1002/cncr.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith AW, Reeve BB, Bellizzi KM, Harlan LC, Klabunde CN, Amsellem M, Bierman AS, Hays RD. Cancer, Comorbidities, and Health-Related Quality of Life of Older Adults. Health Care Financing Review. 2008;29(4):41–56. [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzsimmons D, Gilbert J, Howse F, Young T, Arrarras J-I, Brédart A, Hawker S, George S, Aapro M, Johnson CD. A systematic review of the use and validation of health-related quality of life instruments in older cancer patients. European Journal of Cancer. 2009;45(1):19–32. doi: 10.1016/j.ejca.2008.07.036. http://dx.doi.org/10.1016/j.ejca.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Reeve BB, Potosky AL, Smith AW, Han PK, Hays RD, Davis WW, Arora NK, Haffer SC, Clauser SB. Impact of cancer on health-related quality of life of older Americans. Journal of the National Cancer Institute. 2009;101(12):860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasetto LM, Falci C, Compostella A, Sinigaglia G, Rossi E, Monfardini S. Quality of life in elderly cancer patients. European Journal of Cancer. 2007;43(10):1508–1513. doi: 10.1016/j.ejca.2006.11.023. http://dx.doi.org/10.1016/j.ejca.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Puts MTE, Monette J, Girre V, Wolfson C, Monette M, Batist G, Bergman H. Quality of life during the course of cancer treatment in older newly diagnosed patients. Results of a prospective pilot study. Annals of Oncology. 2011;22(4):916–923. doi: 10.1093/annonc/mdq446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esbensen BA, Osterlind K, Hallberg IR. Quality of life of elderly persons with cancer: a 6-month follow-up. Scandinavian journal of caring sciences. 2007;21(2):178–190. doi: 10.1111/j.1471-6712.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- 14.Fried TR, Byers AL, Gallo WT, Van Ness PH, Towle VR, O’Leary JR, Dubin JA. Prospective study of health status preferences and changes in preferences over time in older adults. Archives of Internal Medicine. 2006;166(8):890–895. doi: 10.1001/archinte.166.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wedding U, Pientka L, Höffken K. Quality-of-life in elderly patients with cancer: a short review. European Journal of Cancer. 2007;43(15):2203–2210. doi: 10.1016/j.ejca.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Craig BM, Reeve BB, Brown PM, Cella D, Hays RD, Lipscomb J, Simon Pickard A, Revicki DA. US Valuation of Health Outcomes Measured Using the PROMIS-29. Value in Health. 2014;17(8):846–853. doi: 10.1016/j.jval.2014.09.005. http://dx.doi.org/10.1016/j.jval.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine Committee on Psychosocial Services to Cancer Patients/Families in a Community S. The National Academies Collection: Reports funded by National Institutes of Health. In: Adler NE, Page AEK, editors. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. National Academies Press (US) National Academy of Sciences; Washington (DC): 2008. [Google Scholar]
- 18.Pergolotti M, Deal AM, Lavery J, Reeve BB, Muss HB. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. Journal of geriatric oncology. 2015 doi: 10.1016/j.jgo.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montazeri A, Vahdaninia M, Harirchi I, Ebrahimi M, Khaleghi F, Jarvandi S. Quality of life in patients with breast cancer before and after diagnosis: an eighteen months follow-up study. BMC cancer. 2008;8:330. doi: 10.1186/1471-2407-8-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfano CM, Ganz PA, Rowland JH, Hahn EE. Cancer survivorship and cancer rehabilitation: revitalizing the link. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(9):904–906. doi: 10.1200/jco.2011.37.1674. [DOI] [PubMed] [Google Scholar]
- 21.Groessl EJ, Kaplan RM, Rejeski WJ, Katula JA, King AC, Frierson G, Glynn NW, Hsu F-C, Walkup M, Pahor M. Health-Related Quality of Life in Older Adults at Risk for Disability. American Journal of Preventive Medicine. 2007;33(3):214–218. doi: 10.1016/j.amepre.2007.04.031. http://dx.doi.org/10.1016/j.amepre.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheville AL, Beck LA, Petersen TL, Marks RS, Gamble GL. The detection and treatment of cancer-related functional problems in an outpatient setting. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2009;17(1):61–67. doi: 10.1007/s00520-008-0461-x. [DOI] [PubMed] [Google Scholar]
- 23.Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. American Journal of Physical Medicine & Rehabilitation. 2011;90(5):S27–S37. doi: 10.1097/PHM.0b013e31820be3be. [DOI] [PubMed] [Google Scholar]
- 24.Cheville AL. Seminars in oncology. Vol. 2. Elsevier; 2005. Cancer rehabilitation; pp. 219–224. [DOI] [PubMed] [Google Scholar]
- 25.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 26.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General Population and Cancer Patient Norms for the Functional Assessment of Cancer Therapy-General (FACT-G) Evaluation & the Health Professions. 2005;28(2):192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 27.Ringash J, O’Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer. 2007;110(1):196–202. doi: 10.1002/cncr22799. [DOI] [PubMed] [Google Scholar]
- 28.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health and Quality of Life Outcomes. 2003;1:79–79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of clinical epidemiology. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paz SH, Spritzer KL, Morales LS, Hays RD. Age-related Differential Item Functioning for the Patient-Reported Outcomes Information System (PROMIS®) Physical Functioning Items. Primary health care: open access. 2013;3(131):12086. doi: 10.4172/2167-1079.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–516. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition (Burbank, Los Angeles County, Calif) 1996;12(1 Suppl):S15–19. doi: 10.1016/0899-9007(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 34.Isenring E, Bauer J, Capra S. The scored Patient-generated Subjective Global Assessment (PG-SGA) and its association with quality of life in ambulatory patients receiving radiotherapy. Eur J Clin Nutr. 2003;57(2):305–309. doi: 10.1038/sj.ejcn.1601552. [DOI] [PubMed] [Google Scholar]
- 35.Martin L, Watanabe S, Fainsinger R, Lau F, Ghosh S, Quan H, Atkins M, Fassbender K, Downing GM, Baracos V. Prognostic factors in patients with advanced cancer: use of the patient-generated subjective global assessment in survival prediction. Journal of Clinical Oncology. 2010;28(28):4376–4383. doi: 10.1200/JCO.2009.27.1916. [DOI] [PubMed] [Google Scholar]
- 36.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 37.Mehnert A, Koch U. Psychological comorbidity and health-related quality of life and its association with awareness, utilization, and need for psychosocial support in a cancer register-based sample of long-term breast cancer survivors. Journal of Psychosomatic Research. 2008;64(4):383–391. doi: 10.1016/j.jpsychores.2007.12.005. http://dx.doi.org/10.1016/j.jpsychores.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Rao D, Debb S, Blitz D, Choi SW, Cella D. Racial/Ethnic differences in the health-related quality of life of cancer patients. Journal of pain and symptom management. 2008;36(5):488–496. doi: 10.1016/j.jpainsymman.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paskett ED, Alfano CM, Davidson MA, Andersen BL, Naughton MJ, Sherman A, McDonald PG, Hays J. Breast cancer survivors’ health-related quality of life: racial differences and comparisons with noncancer controls. Cancer. 2008;113(11):3222–3230. doi: 10.1002/cncr.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. Jama. 2005;294(14):1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 41.Thorpe RJ, Jr, Wynn AJ, Walker JL, Smolen JR, Cary MP, Szanton SL, Whitfield KE. Relationship Between Chronic Conditions and Disability in African American Men and Women. Journal of the National Medical Association. 2016;108(1):90–98. doi: 10.1016/j.jnma.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolb B, Wallace AM, Hill D, Royce M. Disparities in cancer care among racial and ethnic minorities. Oncology (Williston Park, NY) 2006;20(10):1256–1261. discussion 1261, 1265, 1268–1270. [PubMed] [Google Scholar]
- 43.Skarupski KA, de Leon CF, Bienias JL, Scherr PA, Zack MM, Moriarty DG, Evans DA. Black-white differences in health-related quality of life among older adults. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2007;16(2):287–296. doi: 10.1007/s11136-006-9115-y. [DOI] [PubMed] [Google Scholar]
- 44.Wong E, Chow E, Zhang L, Bedard G, Lam K, Fairchild A, Vassiliou V, Alm El-Din MA, Jesus-Garcia R, Kumar A, Forges F, Tseng LM, Hou MF, Chie WC, Bottomley A. Factors influencing health related quality of life in cancer patients with bone metastases. Journal of palliative medicine. 2013;16(8):915–921. doi: 10.1089/jpm.2012.0623. [DOI] [PubMed] [Google Scholar]
- 45.Williams GR, Deal AM, Nyrop KA, Pergolotti M, Guerard EJ, Jolly TA, Muss HB. Geriatric assessment as an aide to understanding falls in older adults with cancer. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2015;23(8):2273–2280. doi: 10.1007/s00520-014-2598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]