Abstract
Amyotrophic lateral sclerosis (ALS) is the most common adult-onset paralytic disorder. It is characterized by progressive degeneration of the motor neurons controlling voluntary movement. The underlying mechanisms remain elusive, a fact that has precluded development of effective treatments. ALS presents as a sporadic condition 90–95% of the time, i.e. without familial history or obvious genetic mutation. This suggests that ALS has a strong environmental component. Organophosphates (OPs) are prime candidate neurotoxicants in the etiology of ALS, as exposure to OPs was linked to higher ALS incidence among farmers, soccer players, and Gulf War veterans. Also, polymorphisms in paraoxonase 1, an enzyme that detoxifies OPs, may increase individual vulnerability both to OP poisoning and to the risk of developing ALS. Furthermore, exposure to high doses of OPs can give rise to OP-induced delayed neuropathy (OPIDN), a debilitating condition akin to ALS characterized by similar motor impairment and paralysis. The question we pose in this review is: “what can we learn from acute exposure to high doses of neurotoxicants (OPIDN) that could help our understanding of chronic diseases resulting from potentially decades of silent exposure (ALS)?” The resemblances between OPIDN and ALS are striking at the clinical, etiological, neuropathological, cellular, and potentially molecular levels. Here, we critically present available evidence, discuss current limitations, and posit future research. In the search for the environmental origin of ALS, OPIDN offers an exciting trail to follow, which can hopefully lead to the development of novel strategies to prevent and cure these dreadful disorders.
Keywords: organophosphate, delayed neuropathy, neurotoxicity, motor neuron, amyotrophic lateral sclerosis, neurodegeneration
Introduction
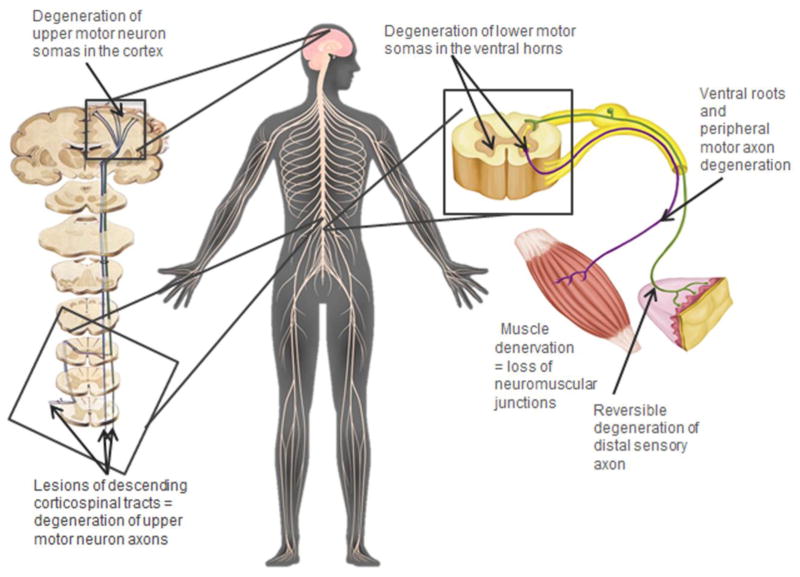
Amyotrophic lateral sclerosis (ALS) is the most common adult-onset paralytic disorder although overall it is relatively rare, with an incidence of 1–2/100,000 persons (Rowland et al. 2010). Clinically, ALS is characterized by spasticity, muscle wasting and weakness, and relentless progression toward complete loss of voluntary movement, culminating in death by respiratory failure within 1–5 years after diagnosis (Rowland et al. 2010). This progressive paralysis is due to the degeneration of the upper (corticospinal) and lower (spinal) motor neurons innervating the skeletal muscles (Fig. 1; Hirano 1996). However, the molecular and cellular mechanisms underlying ALS remain elusive, which has hampered the development of effective treatments to slow or halt the neurodegenerative process.
Figure 1. Anatomy of the voluntary motor system and peripheral sensory system.
Sites of established and potential lesions in ALS and OPIDN.
Fascinatingly, ALS presents as a sporadic condition in 90% of the cases, that is, without any familial history and/or obvious inherited genetic mutation (Andersen and Al-Chalabi 2011). This indicates that ALS likely has a strong environmental component. In support of this hypothesis, many studies have described strikingly high incidences of ALS in small geographic clusters (Plato et al. 2003; Kaji et al. 2012; Oskarsson et al. 2015) or occupational groups in the U.S. and throughout the world (Horner et al. 2003; Chio et al. 2009; Kang et al. 2014). Additionally, several environmental/occupational neurotoxicants have been associated with an increased risk of developing ALS (e.g. heavy metals [lead, manganese, arsenic], organophosphorous [OP] pesticides and the cyanobacterial neurotoxin beta-methylamino alanine [BMAA]) (Oskarsson et al. 2015). Still, none have been clearly demonstrated to cause ALS. In suspected clusters, ALS incidence has been shown to be multiplied by 2–100. However, even at its historically highest reported incidence in the Pacific island of Guam (250/100,000), ALS still only affects a small portion of the population. This indicates that ALS can only be explained by a specific combination of significant neurotoxic exposure and individual susceptibility factor(s), which most probably have a genetic basis. This important aspect of gene-environment interactions in the etiology of ALS has been neglected so far, as 90% of the research is focused on rare hereditary genetic forms. In this new era of precision medicine, the time has come to bolster our effort toward the identification of environmental risk factors for the future development of personalized therapy and prevention, including both environmental/occupational and genetic counseling.
In this review, we hypothesize that insight into environmental exposure leading to ALS could come from the neurological disorder OP-induced delayed neuropathy (OPIDN) which despite its appellation of “neuropathy” is not restricted to peripheral nerves and intriguingly resembles ALS and some other motor neuron diseases at many levels (Table 1). Furthermore, OPs are very unique in the list of neurotoxicants suspected to cause ALS. They have been associated with the disease in the context of both exposure and genetics (specifically, individuals known to be more vulnerable to OPs due to polymorphism-mediated deficient detoxification of OPs). In the following sections, we will detail the scientific premise on which our hypothesis is built, while critically reviewing the strengths and the weaknesses of the literature on OPIDN and OP-associated ALS. We will also emphasize several gaps in knowledge that will need to be addressed by future studies with the hope of elucidating potentially common pathogenic mechanisms between OPIDN and ALS, resulting in a better understanding of the mysterious etiology of the predominant sporadic form of ALS and the development of crucially needed therapies for these two incurable disorders.
Table 1. Systematic comparison between OPIDN and sporadic ALS.
what is established (bold), what is supported by some evidence (underlined) and what is still purely hypothetical or debated (italic). References: ALS (bold), OPIDN (regular).
| Etiology | |||
| •Organophosphate (OP) exposure | -Chronic exposure to low, non-acutely toxic levels of OPs (Gulf War veterans, soccer players, and airline pilots) associated with an increased risk of developing ALS. | -Acute or sub-acute exposure to high levels of OP. | Oskarsson, B. et al., 2015; R.D. Horner et al., 2003; Ronnie D. Horner et al., 2008; Miranda et al., 2008; Chio, A. et al., 1991; Giagheddu, M. et al., 1983; Kalfakis, N. et al., 1991; Kang, H. et al., 2014; Chio, A. et al., 2009; Johnson F.O. & Atchison W. D. 2009; Lacorte E. et al., 2016; de Boer, J. et al., 2015; Nicholas, J.S. et al., 2001; Kaji, R. et al., 2012; Abou-Donia, M.B., 2003. |
| Neuropathology | |||
| •Axonal degeneration |
-Peripheral onset: degeneration of long distal motor axons. -Degeneration of the upper motor neuron axons. -Axonal loss precedes the disappearance of the soma in the spinal cord and cortex. |
-Peripheral onset: degeneration of long distal motor axons and also distal sensory axons (reversible). -Degeneration of the upper motor neuron axons. |
Hirano, A. 1996; Kusaka, H. 1999; Vinsant, S. et al., 2013; el-Fawal, H.A., Jortner, B.S. & Ehrich, M. 1989; Abou-Donia M. B. & Lapadula, D.M. 1990; Jokanović, M. et al., 2011; Lotti, M. & Moretto, A., 2005. |
| •Soma degeneration | -Degeneration of the lower motor neuron and upper motor neuron cell bodies (hallmark). | -Degeneration of the lower motor neuron cell bodies (only “type II”). -Some evidences of lower motor neuron cell body loss with “type I”. -No reported investigation of upper motor neurons cell body loss (only one study with sarin and OPICN). |
Vinsant, S. et al., 2013; Abou-Donia M. B. & Lapadula, D.M. 1990; Abu-Donia, M.B., 1992; Funk, K.A. et al., 1994; Konno, N. et al., 1999; Mou, D. L. et al., 2006; Zou, C. et al., 2013; Beavers, C.T. et al., 2014; Abdel-Rahman, A. et al., 2002; Abu-Donia, M. B., 2003. |
| •Demyelination | -Secondary to axonal degeneration. | -Secondary to axonal degeneration. | Vinsant, S. et al., 2013; M.B. Abou-Donia & Lapadula, 1990. |
| •Gliosis | -Brain and spinal cord. | -Brain and spinal cord. | Vinsant, S. et al., 2013; Nanda, S. & Tapaswi P.K., 1995; Abou-Donia M. B. & Lapadula, D.M. 1990. |
| Clinical phenotype | |||
| •Prognosis | -Fatal within 1–5 years after diagnosis. |
-Non-fatal disorder, except for very severe cases. -Recovery affects mainly sensory nerves, while motor function may be permanently lost. |
Rowland, L.P. et al., 2010; Abu-Donia, M. B., 2003; Beavers, C.T. et al., 2014. |
| •Neuropathological progression of the disease | -Propagates from the periphery to CNS. | -Propagates from the periphery to CNS. | Rowland, L.P. et al., 2010; Nanda, S. & Tapaswi P.K., 1995; Abou-Donia M. B. & Lapadula, D.M. 1990. |
| •Presentation | -Spasticity, muscle wasting and weakness with relentless progression to total paralysis until respiratory failure. |
-Signs within 1–5 weeks after OP exposure. -Sensory symptoms: paresthesia and pain. Rapidly resolved. -Motor signs: cramping in the limbs. Quick progression to muscle weakness and spasticity developing into paralysis of the legs, even quadriplegia (most severe cases). |
Rowland, L.P. et al., 2010; Abou-Donia M. B. & Lapadula, D.M. 1990; Jokanović, M. et al., 2011; Lotti, M. & Moretto, A., 2005; Beavers, C.T. et al., 2014. |
| •Reversibility | -Irreversible. | -The reversibility depends on whether CNS is affected and on the level of exposure and the neurotoxic potency of the OP. | Rowland, L.P. et al., 2010; Hirano, A. 1996; Abou-Donia M. B. & Lapadula, D.M. 1990. |
| Genetic interaction | |||
| •PON1 polymorphisms | - Increase vulnerability to developing ALS. | -Individuals more vulnerable to OPs due to polymorphism-mediated deficient detoxification of OPs. | Ticozzi, N. et al., 2010; Saeed, M. et al., 2006; Slowik, A. et al., 2006; D’Amico, E. et al., 2013; Deakin, S.P. & James, R.W., 2004. |
| Molecular mechanisms proposed | |||
| •Neuropathy target esterase (NTE) | -Not involved in ALS but mutations cause hereditary spastic paraplegia (HSP), disorder affecting preferentially upper motor neurons. | -Molecular target of OP leading to OPIDN. -OPIDN would only be initiated when OP inhibit NTE at least 70%. -Only OPs capable of irreversibly modifying NTE by transfer of R-group adducts induce OPIDN. |
Rainier, S. et al., 2008; Johnson, M.K., Glynn P., 2001; Johnson, M.K. & Lotti, M. 1980; Richardson, R. J. et al., 2013. |
Organophosphates are very hazardous neurotoxicants
OP compounds are a class of widely-used agricultural pesticides that were also developed into warfare nerve agents, and other chemicals with numerous applications in clinical, household and industrial settings (e.g. ophthalmic/antihelmintic agents, solvents, plasticizers, and flame retardants); for review see (Terry 2012). OPs account for one half of the total pesticide usage annually in the USA (Kiely et al. 2004). Accidental and suicidal exposures to high levels of OPs cause ~3 million poisoning cases per year worldwide, ultimately leading to several hundred thousand deaths and debilitated persons, especially in developing countries (Satoh 2006). Victims of OP poisoning can suffer from immediate, and/or more delayed central (CNS = brain and spinal cord) and peripheral nervous system (PNS = peripheral nerves) damages depending on the type of the OP incriminated and the level of intoxication (Fig. 1.; Abou-Donia and Lapadula 1990). For instance, survivors of OP acute neurotoxicity can suffer, successively or independently, from the sudden paralysis and respiratory failure called the intermediate syndrome 24–96 hours later, from the neurodegenerative and paralytic disorder referred to as OPIDN 1–5 weeks later, or from the neuropsychiatric condition termed OP-induced chronic neurotoxicity (OPICN) weeks-years later (Abou-Donia 2003). The acute neurotoxicity of some OPs is well characterized and results from their inhibition of acetylcholine(Ach)esterase(E), the enzyme which is responsible for the breakdown of the neurotransmitter Ach. This leads to massive accumulation of Ach at the cholinergic synapses and dysregulation of their numerous physiological roles (e.g. control of blood pressure, cardiac rhythm, and muscles). Eventually, the cholinergic crisis can culminate in coma and death by respiratory failure. The intermediate syndrome, which can be lethal without respiratory support, is assumed to be due to sustained AchE inhibition (De Bleecker et al. 1994). In contrast, non-cholinergic and more elusive mechanisms contribute to OPIDN and OPICN, as neither is controlled by anticholinergic and/or AchE restoration drugs (Abou-Donia 2003).
OPICN is mainly associated with cognitive symptoms and the main objective of this review is to summarize the evidence, the potential misconceptions, and the gaps in knowledge regarding the neurotoxicity of OPs to the voluntary motor system. As such, we will focus our attention on OPIDN to explore how a better understanding of this disorder may help us to identify the mechanisms by which environmental exposures can lead to ALS. However, it should be briefly mentioned that ALS is purely a motor neuron disease in only half of the patients (Lomen-Hoerth et al. 2003; Murphy et al. 2007). In fact, about 30% of ALS patients will show signs of cognitive impairment while 20% will demonstrate significant behavioral changes and dementia associated with decline in the frontotemporal lobe, which is referred to as the “ALS-frontotemporal dementia (FTD) spectrum”.
OPIDN and neuropathic compounds: an evolving definition and evolving public health concern?
OPIDN is a relatively rare, non-lethal (with the exception of very severe cases (Beavers et al. 2014), but potentially extremely debilitating neurodegenerative disorder observed after repeated or acute intoxication with different types of OPs (Abou-Donia 2003). More than 70,000 cases of OPIDN have been documented over the last 70 years (Johnson and Glynn 2001). However, OPIDN incidence is probably greatly underestimated, as most OP poisonings occur in developing countries yet more than half of OPIDN cases were reported in the U.S. alone. Historically, interest in OPIDN gained momentum in 1930’s, when 50,000 Americans ingested an OP-contaminated alcoholic beverage called “Ginger Jake” and developed OPIDN, nick-named then the “Ginger paralysis” (Morgan 1982). Similar mass incidents were reported with OP-contaminated cooking oil, alcohol, flour, or abortifacients in Netherlands, Yugoslavia, France, Morocco, South Africa, Sri-Lanka, and India (for review see Abou-Donia and Lapadula (1990)). All of these “epidemic” OPIDN cases were associated with the accidental ingestion of a group of OPs classified as triaryl phosphates (e.g. tri-ortho-cresyl phosphate; TOCP; see Table 2 for the list of all the OPs mentioned in this review) which have the particularity to be very weak inhibitors of AchE (Lotti and Moretto 2005). The incapacity of these OPs to trigger the obvious cholinergic toxicity explains why some people had intoxicated themselves for weeks before developing neurotoxic signs, and also accounts for the “epidemic” nature of these poisonings. Based on these observations, “neuropathic” compounds (or compounds “at risk to induce OPIDN”) were originally believed to be restricted to non-cholinergic OPs.
Table 2. Organophosphates and OPIDN.
Summary of characteristics of OPs mentioned in this review: status as AchE and NTE inhibitors and implication in OPIDN and strength of evidence for neurotoxicity to the voluntary motor system. Chlorpyrifos oxon, paraoxon and diazoxon are the toxic metabolites of the parent OPs chlorpyrifos, parathion and diazinon, respectively.
| Organophosphate | AchE inhibition | NTE inhibition/aging | Implicated in OPIDN | Strength of evidence for neurotoxicity to voluntary motor system | References: |
|---|---|---|---|---|---|
| Tri-ortho-cresyl phosphate | Weak inhibitor | Strong inhibitor/Yes | Yes | Strong | Nanda and Tapaswi 1995; Veronesi et al. 1991.; Emerick et al. 2012 |
| Chlorpyrifos/Chlorpyrifos oxon | Strong inhibitor | Strong inhibitor/Yes | Yes | Strong | Capodicasa et al. 1991; Meggs 2003; Thivakaran et al. 2012 |
| Parathion/Paraoxon | Inhibitor | Weak inhibitor/No | No/? | Weak | Emerick et al. 2015; Jokanović et al. 2011; Lotti and Moretto 2005. Yen et at., 2011 |
| Aryl phosphites | Weak inhibitor | Yes/? | Yes | Strong | Abou-Donia and Lapadula 1990; Abou-Donia 1992 |
| Triphenyl Phosphites | Weak inhibitor | Strong inhibitor/Yes | Yes | Strong | Fioroni et al. 1995 |
| Sarin | Strong inhibitor | Yes/? | Yes | Weak | Husain et al, 1993; Abdel-Rahman et al. 2002; |
| Soman | Strong inhibitor | Yes/? | No/? | Weak | Sirin et al. 2012; Crowell et al. 1989 |
| Diazinon/Diazoxon | Inhibitor | No/? | No/? | Weak | Yen et al., 2011 |
| Phenyldipentylphosphinate | Very weak | Yes/No | Only when given after a classic neuropathic OP | Some | Jortner 2002; Read et aI. 2010 |
List of references listed in the table but not cited in the manuscript.
Crowell JA, Parker RM, Bucci TJ, Dacre JC (1989) Neuropathy target esterase in hens after sarin and soman. J Biochem Toxicol 4:15–20.
Fioroni F, Moretto A, Lotti M (1995) Triphenylphosphite neuropathy in hens. Arch Toxicol 69:705–11.
Sirin GS, Zhou Y, Lior-Hoffmann L, et al (2012) Aging Mechanism of Soman Inhibited Acetylcholinesterase. J Phys Chem B 116:12199–12207. doi: 10.1021/jp307790v
Yen J, Donerly S, Linney EA (2011) Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish. Neurotoxicol Teratol 33:735–741. doi: 10.1016/j.ntt.2011.10.004
However, more recently, a growing number of OPIDN cases among survivors of acute poisoning by cholinergic OP pesticides were reported in the literature (Meggs 2003; Thivakaran et al. 2012); for review see (Abou-Donia and Lapadula, 1990; Jokanović et al. 2011; Lotti and Moretto 2005). Particular attention was paid by some authors (Lotti and Moretto 2005) to discern the cases of OPIDN convincingly attributed to this class of OPs (reasonable analytical identification of the chemical, reliable exposure and clinical data) from reports with only partial data. For instance, the capacity of chlorpyrifos to induce OPIDN was clearly established in several cases, whereas the implication of parathion was reported multiple times, but never with compelling evidence (in contrast with “negative evidence”, the “absence of evidence” does not preclude that more OPs will be convincingly associated with OPIDN in the future). This new development had very important implications for the classification of the neurotoxicity and the danger of OPs by regulatory agencies. First, it called into question the definition of the neuropathic compound category, and more importantly, the method that was used for years to predict the neuropathic potential of a compound in the gold standard animal model: the hen (Jamal 1997; Moretto and Lotti 2002). Previously, the neuropathic potential of OPs was tested at levels below the threshold of cholinergic toxicity. Now, in animal models including the hen, cholinergic OPs have been confirmed to induce OPIDN when administrated at doses higher than their LD50 (Abou-Donia and Lapadula 1990; Capodicasa et al. 1991; Lotti and Moretto 2005). In line with this information, a new concern for the field of public health has come to light, regarding recent advances in anticholinergic and AchE reactivation therapy. This therapy allows patients to survive supra-lethal doses of OPs, yet also significantly increases their risk of developing severe and debilitating forms of OPIDN, for which there is currently no treatment (Marrs 1993). Likewise, there is no effective treatment available for ALS, and drug development is hindered by our limited knowledge of its pathogenic mechanisms.
Clinically (for review see Abou-Donia and Lapadula (1990); Lotti and Moretto (2005); Jokanović et al. (2011); Table 1), OPIDN’s first symptoms are cramping, muscle pains in the legs, and distal paresthesia in the limbs. Initial sensory symptoms generally resolve rapidly, though motor signs quickly progress to muscle weakness, first in the lower limbs and then in the hands, causing characteristic foot and wrist drops. Finally, spasticity (upper motor neuron signs) and muscle wasting (lower motor neuron signs) develop, then progress into paralysis of the legs, or even quadriplegia in more severe cases. The motor deficit acquired in OPIDN is rarely reversible, and its extent could depend on the level of exposure and the potency of the OP to cause irreversible CNS lesions. Thus, the clinical picture of OPIDN is intriguingly reminiscent of ALS, explaining why some epidemic cases of OPIDN reported in Italy were qualified as “frozen” ALS (i.e. non-progressing and non-fatal) after 50 years of clinical follow-up (Tosi et al. 1994). The “frozen” metaphor represents a particularly brilliant clinical intuition, as multiple lines of evidence indicate that sporadic ALS and OPIDN may share more than merely a similar clinical presentation.
OPIDN and ALS: a common etiology?
The hypothesis of a common etiology emerged when an association between exposure to OPs and sporadic ALS was proposed following the observation of deployed Gulf War veterans who presented a twofold increase incidence of ALS after receiving prophylactic treatment containing OP-related cholinergic inhibitors intended to guard against nerve gas and insect pests during the war (Horner et al. 2003; Horner et al. 2008; Miranda et al. 2008). Multiple studies around the globe have also linked occupational exposure to OPs with a 2–6 times increased incidence of ALS cases among farmers (Giagheddu et al. 1983; Chió et al. 1991; Kalfakis et al. 1991; Kang et al. 2014) and soccer players (Chio et al. 2009; Johnson and Atchison 2009; Lacorte et al. 2016). Some intriguing yet sparse evidences also suggests the same hypothesis for airline pilots exposed to potentially neurotoxic OPs used as additives in aircraft engine oil (Nicholas et al. 2001; de Boer et al. 2015). However, in contrast with OPIDN, which is caused by acute or sub-acute exposure to significantly high levels of OPs, ALS is believed to be associated with chronic exposure to low, non-acutely toxic levels of OPs.
OPIDN and ALS: neuropathological overlaps
A second similitude between OPIDN and ALS is that they share several key neuropathological features. Notably, they both involve the corticospinal and neuromuscular system comprised of the upper and lower motor neurons (Fig. 1). Observations made in human (autopsied tissues and electrophysiological findings) and in animal models were reviewed in detail for both OPIDN (Abou-Donia and Lapadula 1990; Lotti and Moretto 2005; Jokanović et al. 2011) and ALS (Hirano 1996; Kusaka 1999; Vinsant et al. 2013). Hence, in the next paragraph, only a few specific references will be added to complete the analysis for OPIDN. In summary, for both disorders, the earliest pathological events are detected peripherally and consist of peripheral motor axon degeneration (Vinsant et al. 2013), muscle denervation (El-Fawal et al. 1989), and involve distal motor groups, suggesting a common preferential vulnerability of long axons. A notable difference is that in OPIDN, distal sensory axons are also affected; this explains both the early sensory signs and the other terminology sometimes given to OPIDN, “OP-induced delayed polyneuropathy” (OPIDP). However, peripheral sensory axon degeneration was consistently shown to be transient and reversible. In both disorders, axonal degeneration follows a “dying-back” mechanism with secondary demyelination.
After the peripheral phase, pathology propagates to the CNS and gliosis becomes prominent in the brain (Nanda and Tapaswi 1995) and in the spinal cord (Abou-Donia and Lapadula 1990). Lesions of the descending corticospinal or pyramidal tract (upper motor neuron axons in the spinal cord) are a constant feature in both diseases. Degeneration of lower motor neuron cell bodies in the ventral horn of the spinal cord is a hallmark of ALS (~50% of ventral horn motor neuron loss at end-stage), though in OPIDN, this pathology was reported only when exposure to OPs containing a trivalent phosphorus atom (e.g. aryl phosphites and triphenyl phosphites [TPPi]) have occurred. This is said to be “Type II” OPIDN (Abou-Donia and Lapadula 1990; Abou-Donia 1992). On this basis, two main classifications of OPIDN were proposed: “classical,” or “type I” induced by “type I” OPs with a pentavalent phosphorus atom (i.e. TOCP) and associated mainly with axonal degeneration, and “type II” caused by trivalent phosphines and which also involves neuronal cell body degeneration (Abou-Donia and Lapadula 1990). However, a growing number of studies have reported clear neuronal necrosis, chromatolysis, apoptosis, and cell body loss of ventral horn motor neurons with exposure to type I OPs in animals (Funk et al. 1994; Konno et al. 1999; Mou et al. 2006; Zou et al. 2013) and humans (Beavers et al. 2014). If different classes of OPs cannot account for these data discrepancies in the literature, the dissociation of soma loss from axon degeneration could depend on alternative factors: first, other variables related to the exposure itself (e.g. level, duration, regimen, or route); second, to the age/species/strains/individuals exposed to OPs (Abou-Donia and Lapadula 1990); or third, to the timing of the neuropathological assessment. For instance, in ALS, axonal loss precedes the disappearance of lower motor neuron soma in the spinal cord (Vinsant et al. 2013). Despite the consistently-reported and widely-described degeneration of the corticospinal tract in OPIDN, we could not find any report that effectively investigated the degeneration of the cell bodies of the upper motor neurons (pyramidal neurons) in layers III and V of the motor cortex, whose axons compose the corticospinal tract. In humans it is easily understandable that the generally non-fatal nature of OPIDN (as compared to ALS) limits the timely obtention of autopsied material. However, in animal models of OPIDN, nearly all neuropathological investigations were focused on just the spinal cord. An important implication of this limitation concerns the above-described classification of type I vs. type II OPIDN which is based on the differential vulnerability in the spinal cord of axons (upper motor neurons) and cell bodies (lower motor neurons), which do not belong to the same cells. Nevertheless, OPs may lead to the loss of upper motor neuron cell bodies, knowing that in a study aimed at investigating OPICN, rats exposed to 1 X LD50 sarin were found to exhibit prominent degeneration of upper motor neurons in layers III and V of the motor cortex (Abdel-Rahman et al. 2002; Abou-Donia 2003). Therefore, the dissociation or co-occurrence of motor neuron axon and motor neuron soma degeneration after exposures to different types of OPs must still be formally and kinetically established in future studies. A relevant but simplified and highly controllable system, such as an in vitro model could be a well-suited platform for this type of screening before later confirmation in animal models.
Paraoxonase 1: the Achilles’ heel for developing ALS?
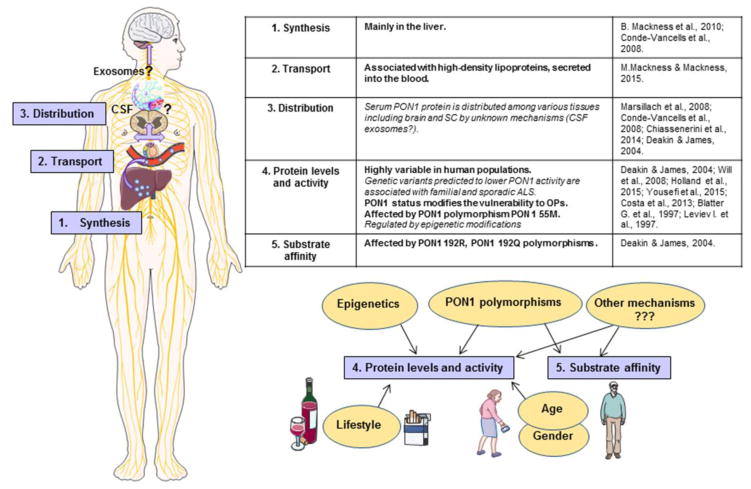
A third convergence for ALS and OPIDN involves several intriguing correlations found in the literature concerning lowered status of an enzyme detoxifying OPs, paraoxonase 1 (PON1), and increased risk to developing ALS. In several studies, gene variants predicted to lower PON1 activity were associated with both familial (Ticozzi et al. 2010) and sporadic ALS (Slowik et al. 2006; Saeed et al. 2006). However, larger-scale meta-analysis did not reach the same conclusion (Wills et al. 2009). This suggests that PON1 polymorphisms may play a role in predisposing only human populations with specific or geographically-limited exposures to ALS (D’Amico et al. 2013). PON1 biology (Fig. 2) and the relationship between PON1 polymorphisms, PON1 protein levels, their stability, activity, and substrate affinity are quite complex and require careful consideration before interpretation of the different data available in the literature. Incidentally, PON1 was at first not appropriately named, as we now know that paraoxon is actually one of its weakest substrates. As demonstrated by the systematic investigation of PON1 mRNA presence in human tissues (Mackness et al. 2010), PON1 is synthesized mainly in the liver before being secreted into the blood where it associates predominantly with high-density lipoproteins (Mackness and Mackness 2015). Serum PON1 protein is then distributed among various tissues including the brain and the spinal cord (Marsillach et al. 2008). The mechanisms allowing PON1 to cross the blood brain barrier (BBB) remain uncertain, yet PON1 was identified within exosomes found in the liver (Conde-Vancells et al. 2008) as well as within cerebrospinal fluid (CSF) (Chiasserini et al. 2014). Exosomes are a type of extracellular vesicles known to very efficiently penetrate into the CNS and so they are considered very promising carriers for brain delivery of therapeutic proteins (Hall et al. 2016). In the context of OP poisoning, ALS, and other diseases associated with PON1 (e.g. arteriosclerosis), past studies have principally focused on serum PON1 protein levels and activity as their highly variability in human populations (up to 40-fold) suggests they could represent a good biomarker for individual risk assessment. Even if the blood constitutes a major early barrier where OPs begin to be detoxified, most OPs are small lipophilic molecules that freely cross membranes and reach the CNS very quickly (minutes to hours). Accordingly, local detoxification of OPs by PON1 in the CNS may also be very important to counteract their neurotoxicity and unraveling the mechanisms controlling PON1 delivery to the CNS may lead to important insights into novel therapeutic strategies and individual vulnerability to OPs.
Figure 2. PON1 Biology.
PON1 synthesis, transport, distribution and mechanisms regulating PON1 protein levels, activity and substrate affinity.
Thus far, it is unknown whether PON1 polymorphisms or other factors can affect PON1 distribution or activity locally in the CNS and contribute to increased vulnerability to OPIDN or ALS. As elegantly reviewed by Deakin and James (2004), variants in the coding, intronic, or promoter regions of PON1 can give rise to increased or decreased serum PON1 levels, associated or not with increased/decreased serum PON1 activity. Yet polymorphisms at the same position (e.g. the common variants 192Q and 192R) can have variable effects on the hydrolysis efficiency of PON1 depending on the substrate. For example, 192R is much more efficient for paraoxon while 192Q is less efficient. 192Q is however much more efficient for sarin, soman, diazinon, and slightly more even for chlorpyrifos. Furthermore, both variants have the same efficiency for detoxifying other substrates such as phenylacetate and dyhydrocoumarin. Without data clearly identifying the OP(s) to which a population has been exposed, it is very difficult to determine the value of negative genetic association studies, or the actual mechanistic relevance of positive association studies. For experiments aiming to compare PON1 activity between two populations (patients vs. controls), the selection of a sound series of substrates is critical for designing a reliable investigation. In the context of ALS, only one study investigated serum PON1 activity in 140 ALS patients versus 153 age/sex/race-matched controls in relation to PON1 polymorphisms. It confirmed the increased frequency of one of the variants (192R) in ALS patients, but could not evidence any reduction in PON1 activity with either paraoxon, diazoxon, or phenylacetate (Wills et al. 2008). These substrates covered the three groups cited above, though the study’s design could have been optimized by taking into consideration exposure data (indirect, if direct is unavailable) and using in the assay the OP(s) to which the target population was most likely exposed. There was also a significant methodological flaw in the way PON1 activity was measured at the time this study was undertaken. A high concentration of NaCl (2mM) was used in the assay buffer because NaCl is known to artificially increase PON1 activity (used in general to help measuring the hydrolysis of weak substrates like paraoxon). However, it is not recommended for measurements at the population level as we now know (Ceron et al. 2014). As an example, NaCl was shown to more greatly stimulate the high-activity allelic form, thereby yielding a higher variability in a healthy population (Furlong et al. 1988). In addition, when used with other substrates such as phenylacetate, NaCl produces a decrease in PON1 activity (Eckerson et al. 1983).
Another level of complexity in PON1 biology is that even within genotypic groups, PON1 serum concentration and activity can vary up to 13-fold, probably due to lifestyle, occupational, and environmental factors (Deakin and James 2004). For instance, smoking decreases PON1 activity (James et al. 2000) and is also one of the strongest risk factors associated with ALS ( >2-fold increase in risk) (Wang et al. 2011). PON1 activity also decreases with age and is lower in males (Costa et al. 2005), which is in direct contrast to the reported incidences of ALS (Rowland et al. 2010). Recently, PON1 status was shown to be regulated by epigenetic modifications, opening a new field for investigation (Huen et al. 2015; Holland et al. 2015). PON1 status clearly modifies the vulnerability of animals to OPs (Costa et al. 2013), but so far the effect of PON1 status on ALS development remains to be experimentally tested.
Neuropathy target esterase (NTE): is NTE for OPIDN what AchE is for the toxidrome?
Fourth and finally, OPIDN’s molecular origin was originally attributed to the inactivation of a serine esterase called neuropathy target esterase (NTE). Mutations in NTE can cause hereditary spastic paraplegia (HSP), a disorder within the same group of motor neuron diseases as ALS, which is characterized by spasticity and upper motor neuron pathology (Rainier et al. 2008). Animal models were developed based on the original observation of OPIDN human epidemics due to non-cholinergic OPs (Morgan 1982; Abou-Donia and Lapadula 1990; Lotti and Moretto 2005) and the suggestion that OPIDN would only be initiated when OPs inhibit NTE by at least 70% (Johnson and Lotti 1980; Johnson and Glynn 2001). On this basis, the neuropathic potential of an OP was defined by its relative capacity to inhibit NTE at doses where AchE inhibition is limited and inferior to its LD50. However, as discussed above, therapeutic improvements in cholinergic crisis management were a game changer, and “non-neuropathic” cholinergic OPs have now been reported to cause OPIDN in humans, as well as in animals when tested above their LD50 (Abou-Donia and Lapadula 1990; Capodicasa et al. 1991; Lotti and Moretto 2005). Furthermore, beyond the cholinergic status of neuropathic OPs, a series of conflicting data in the literature suggest that OPIDN cannot be exclusively explained by NTE inhibition or other NTE-related mechanisms. First, in the hen model of OPIDN, some potent inhibitors of NTE do not cause OPIDN (Abou-Donia and Lapadula 1990; Lotti and Moretto 2005; Richardson et al. 2013). Following this discovery, it was proposed that only those inhibitors capable of irreversibly modifying NTE by transfer of R-group adducts (aging) are capable of inducing OPIDN (Richardson et al. 2013), suggesting that it is not NTE’s loss of function involved in OPIDN, but its gain of some unknown toxic function. However, NTE can be inhibited and aged just as efficiently in animals not demonstrating strong clinical evidence of OPIDN (e.g. rodents) as it is in highly susceptible species such as the hen (Abou-Donia 2003). Intriguingly enough regarding the aging theory, if a non-aging and non-neuropathic NTE inhibitor is given to an animal before a neuropathic compound, it can protect against the development of OPIDN, yet if the same inhibitor is administrated after a neuropathic OP, it instead triggers a worsening of OPIDN, even though NTE inhibition and aging are already maximal (Jortner 2002). In addition, some reports and reviews have described in detail that OPs belonging to the class of phosphines and which do not inhibit NTE can still cause type II OPIDN in diverse animal models (Abou-Donia and Lapadula 1990; Abou-Donia 1992; Abou-Donia 2003). Based on the systematic absence of inhibition of NTE by these neurotoxic phosphines, a third class of OPIDN was proposed and defined as “type III” (Abou-Donia 2005). Other groups who have made the same observation have suggested that despite the extremely similar clinical presentation (ataxia and hind limb paralysis), these forms of OP-induced delayed neurotoxicity should not be referred to as OPIDN, as they are independent from NTE inhibition (Lehning et al. 1996; Davis et al. 1999). As previously mentioned, there exists a series of case reports suggesting that OPs that neither inhibit NTE nor cause OPIDN in the hen can still induce OPIDN in humans, even though analytical demonstration of the OP incriminated was not always confirmed (Abou-Donia and Lapadula 1990; Abou-Donia 2003). In light of this evidence, the use of the hen as a model to predict OP neuropathic potential in humans has been criticized (Moretto and Lotti 2002). Finally, mice engineered to be genetically-deficient for neuronal NTE have shown upper motor neuron/axon lesions, but no peripheral pathology even at a very advanced age, indicating that total inhibition of NTE in neurons (without possible gain of function due to aging) can model at least some aspects of OPIDN (Read et al. 2009; Read et al. 2010). Altogether it appears reasonable that NTE is at least partly involved in the initiation of many types of OPIDN (Lotti and Moretto 2005), yet there is no evidence that NTE inhibition or aging can directly cause degeneration of peripheral axons or motor neuron cell bodies. It can also be inferred that NTE is more involved in pathology of OPIDN in the upper motor neurons than in the lower motor neurons. Mutations in NTE were not evidenced to be causal in ALS and regrettably, there is no epidemiological or clinical data available about the NTE status of patients affected by ALS. In line with the hypothesis we are making for OPIDN, this type of investigation could be particularly relevant to determine whether NTE quantity/activity can help distinguish the forms of ALS that are “upper motor neuron predominant” versus “lower motor neuron predominant”. Regarding the “OPIDN” elicited by non-NTE inhibitors, the fact that authors have referred to it as type II, III, non-classical, or non-OPIDN appears to be a problem of semantics. Whatever the designation, this delayed neurotoxicity is a serious potential hazard derived from OP-exposure and should be investigated as seriously as the originally identified form. In conclusion, if NTE is clearly the key to some familial forms of HSP, there is strong rationale to wonder whether other OP-vulnerable serine esterase(s) or protein(s) could be the key to both OPIDN and ALS. Accumulating evidence has pointed to the existence of molecular entities in the brain other than NTE and AchE with phenyl valerate esterase activity which can be inhibited by OPs (Benabent et al. 2014; Mangas et al. 2016; Estévez et al. 2016). These entities and the consequences of their inhibition by OPs remain to be formally identified, but they could represent relevant candidates to mediate some of the neurotoxic mechanisms pertaining to OPIDN and environment-triggered ALS. Alternatively, cytoskeletal proteins were proposed by Abou-Donia (2003) to be among the neurotoxic targets of OPs. These different attractive hypotheses must be further investigated in future studies.
Cell and animal models to study OPIDN and ALS: what has been done and where should we go next?
Our previous work has pioneered the notion that in familial ALS cases linked to mutations in superoxide dismutase 1 (SOD1), motor neurons degenerate due to the development of a hostile cellular environment. Lower motor neurons produced from mutant SOD1 transgenic mice exhibit some axonal shortening, but can only be killed by soluble factor(s) released by neighboring astrocytes, which undergo a transformation from supporting cells into potent motor neuron killers (Nagai et al. 2007). We recently confirmed that this finding holds true for sporadic ALS and human cells, by showing that astrocytes directly produced from the brain of sporadic ALS patients are killing human embryonic stem cell (ES)-derived motor neurons through the same type of cell death, called necroptosis (or “controlled necrosis”), which we found in our in vitro model of familial ALS (Re et al. 2014). This finding is very encouraging as it indicates that the sporadic and the familial forms of the disease may share common pathogenic mechanisms relevant for therapeutic development. However, it did not help in identifying which environmental and/or occupational neurotoxicant(s) can be responsible for the neurotoxic transformation of astrocytes in sporadic ALS patients, or which risk factor(s) may account for the peculiar vulnerability of some individuals in developing ALS upon a specific exposure. As laid out above, once carefully reviewing the striking resemblance of ALS and OPIDN, we concluded that there is a very strong rationale to investigate exposure to OPs as an environmental cause of sporadic ALS. When reviewing in vitro models of exposure to OPs, we found only 2 reports that investigated OP effects in primary spinal cord cultures. Both were focused on OP acute cholinergic neurotoxicity; the first investigated the short term inhibition of AchE in spinal cells (Goldberg et al. 1980), and the second performed electrophysiological and video recordings of muscle relaxation in co-culture with spinal motor neurons to evaluate the efficacy of AchE reactivation therapy (Drexler et al. 2011). In the context of OPIDN, various efforts were reported as focusing towards the development of in vitro models that could help predict whether a particular OP is capable of causing OPIDN in humans (Emerick et al. 2012; Fernandes et al. 2015; Emerick et al. 2015). Each of these studies used a neuroblastoma clonal cell line (SH-SY5Y) originally derived from a metastatic bone tumor, which exhibits only partial cholinergic features after differentiation. Even after differentiation, these cells do not exhibit neurites comparable to the dendritic tree and the long axon displayed by primary or ES-derived motor neurons. Another concern is that by virtue of their tumor origin and immortalized state, this type of cell line is likely flawed for the study of neurotoxic and neurodegenerative processes. Now, mouse and human ES-derived spinal motor neurons are widely available and possess the same expandability quality before differentiation as immortalized cells, rendering them amenable to high-throughput experiments and material-demanding biochemical or genetic investigations. Both mouse (Wichterle et al. 2002) and human (Boulting et al. 2011) lower ES- motor neurons have been carefully characterized for their morphological, biochemical, and functional resemblance to genuine motor neurons, validating the argument that they represent a much more optimal model for future neurotoxic and neurodegenerative investigations.
Regarding non-cell autonomous mechanisms, previous studies in the context of OPICN have investigated the role of cortical astrocytes in OP neurotoxicity of hippocampal neurons (Pizzurro et al. 2014a; Pizzurro et al. 2014b). They reported that OPs impair the capacity of astrocytes to support neuronal defense against oxidative stress and neurite outgrowth. Therefore, glia-mediated neurotoxicity and/or vulnerabilization of neurons to neurotoxicity are important aspects of ALS and OPICN pathology, respectively, and their roles in OPIDN must be investigated in the future.
We previously discussed that the gold-standard animal model for the study of OPIDN has long been the hen. This was the case until its predictability for human OPIDN was put into question (Abou-Donia and Lapadula 1990; Moretto and Lotti 2002; Abou-Donia 2003). Rodents, particularly mice, are considered poor models of OPIDN because they are highly resistant to the development of ataxia (Abou-Donia and Lapadula 1990; Lotti and Moretto 2005). However, several groups have demonstrated that acute (El-Sebae et al. 1977; Veronesi et al. 1991) or chronic exposure of mice ( Lapadula et al. 1985; Husain et al. 1993) to classic neuropathic OPs (TOCP and MIP), or to OPs less consensually-accepted to be neuropathic (sarin) caused clear degeneration of the lateral and ventral locomotor neurons of the spinal cord including upper motor neuron axons. Peripheral lower motor neuron axon lesions were generally not well demonstrated in mice. Diverse explanations were proposed for this, such as earlier and more active regenerative mechanisms, and differences in the anatomy and/or OP-target enzymes etc. (Veronesi et al. 1991). Yet, the two studies that carried out chronic delivery of sarin (Husain et al. 1993) and TOCP (Lapadula et al. 1985) demonstrated progressive development of muscle weakness, muscle wasting, ataxia, and eventual hind-limb paralysis over the course of exposure, which beyond OPIDN, suggests that exposure to chronic-low doses of OPs could be developed further in order to successfully mimic sporadic ALS in mice. The chronic TOCP study also reported clear degeneration of some peripheral lower motor neuron axons in half of the mice tested, while only vague changes were observed in the other half (Lapadula et al. 1985). Another interesting observation was that three weeks after phenyldipentylphosphinate (PDPP; an OP in the group “non-aging” NTE inhibitors which do not promote OPIDN in the hen except when given after a classic neuropathic OP [Jortner 2002]) was administered to mice, it triggered neurodegenerative lesions located in the trigeminal tract, which contains lower motor neuron axons (Read et al. 2010). Based on this unusual pattern of axonal damages in the mice, the authors suggested that PDPP likely reacts with a serine hydrolase distinct from either NTE or AchE.
Conclusion and perspectives
Mechanistic studies demonstrating the direct implication of OPs as a causative or a risk factor for ALS are still needed. Even so, the hypothesis we presented and discussed in this review is strongly supported by several positive epidemiological and genetic association studies, as well as a series of particularly compelling evidence regarding the overlaps between ALS and OPIDN. Up until now, the majority of research on the pathogenesis of ALS has focused on its rare genetic forms. Naturally, a genetic mutation is more easily tractable and amenable to the generation of animal models than an environmental toxin which needs to be identified from the complex, temporally (and spatially) evolving milieu of life. “Difficult” however is not a synonym of “impossible” and the field of neurotoxicology will have to take on the challenge of investigating the environmental etiology of late adult-onset neurodegenerative diseases by coming up with novel and innovative approaches to address the questions related to human exposure: “What? How much? When? How long?” as well as those related to individual vulnerability: “Why? How?”
Certainly in the case of diseases which remain clinically silent until the fifth to sixth decade of life like ALS, we can wonder why the first symptoms begin to appear so late. What are the mechanisms underlying this late clinical expression? Does the organism reach a threshold of irreparable accumulated damages? Do humans suddenly become vulnerable to an existing neurotoxic insult until recently compensated for by a fitness lost due to aging? Or are clinical signs just the extremely delayed expression of some earlier acute or sub-acute insults programmed to clinically surface one day after similar damage? Until confirmed or disproved, all these hypotheses and variations are valid directions that future research must take.
Intuitively, adult-onset, chronic diseases (even those of relatively short duration such as ALS) are expected to result from chronic exposure to moderate, pathologically silent levels of neurotoxicants. However, there is much more literature available on the consequences of acute massive neurotoxic insults than chronic silent ones. The question we initially posed with this review was: “what can we learn from acute exposure to high doses of neurotoxicants (OPIDN) that may be useful in understanding chronic diseases resulting from silent exposure (ALS)?” The answer we found is: “a lot.” The resemblances between OPIDN and ALS are striking at clinical, etiological, neuropathological, cellular, and potentially molecular levels. During our journey through the OPIDN literature, we found that some of the field was still “frozen” in the original definition of non-cholinergic neuropathic compounds, and regardless of whether semantic or classification changes are needed, scientists need to increase awareness pertaining to the multiplication of OPIDN (or OPIDN-like) cases flourishing in the recent literature of acute poisoning with cholinergic OPs (Meggs 2003; Thivakaran et al. 2012; Beavers et al. 2014). As highlighted by several experts in the field (Abou-Donia and Lapadula 1990; Lotti and Moretto 2005), AchE and NTE cannot account for all the neurotoxic clinical expressions of OP poisoning. Future studies need to unravel whether additional enzyme(s) critical to the function of the voluntary motor system could be selected, targeted, and impaired by OPs. The same enzyme(s) could also become important for the pathogenesis of ALS and its future therapeutic development. The extent of the neurodegenerative insults in OPIDN will also need further investigation and clarification for questions such as: are upper motor neurons soma affected? Or are upper and lower motor neurons more preferentially affected by some OPs than others? Another imperative question for both future prevention and therapy of OPIDN and ALS is how to better understand the biology of PON1 and how its polymorphisms, epigenetic modifications, stability, and transport to the CNS can affect the development of OP-related neurotoxic insults and ALS.
None of these outstanding questions can be soundly addressed until we develop new cell and animal models of OPIDN and ALS. These future models will need to be relevant to human biology and will need to take into consideration all the complex aspects of gene-environment interaction, “real-world” exposure levels, and window(s) of exposure. This is an exciting time for research on environment-linked ALS, as we have a trail to follow for OPs and OPIDN that will surely lead to novel ways for prevention or cures to these dreadful disorders.
Acknowledgments
This work was supported by NIEHS (ES009089), NIGMS (R25GM62454-06) and the Mailman School of Public Health at Columbia University. D.B.R is the recipient of a Career development award and two pilot grants from the NIEHS Center of Northern Manhattan and of the Calderone Prize for Junior Faculty in the Mailman School of Public Health. Y.N. is the recipient of a fellowship from The Initiative for Maximizing Student Development (IMSD) program at Columbia’s Mailman School of Public Health. The figures were created from materials made available by Creative Commons https://creativecommons.org/licenses/by/3.0/# and Mosby’s Medical Dictionary, 8th edition. (2009).
References
- Abdel-Rahman A, Shetty AK, Abou-Donia MB. Acute exposure to sarin increases blood brain barrier permeability and induces neuropathological changes in the rat brain: dose-response relationships. Neuroscience. 2002;113:721–41. doi: 10.1016/s0306-4522(02)00176-8. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB. Organophosphate ester-induced chronic neurotoxicity (OPICN). In: Winder C, editor. Contaminated Air Protection Conference, Proceedings of a Conference, held at Imperial College; London. 20–21 April; Sydney: University of New South Wales; 2005. pp. 59–90. [Google Scholar]
- Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003;58:484–97. doi: 10.3200/AEOH.58.8.484-497. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB. Triphenyl phosphite: A type II organophosphorus compound-induced delayed neurotoxic agent. In: Chamber JELP, editor. Organophosphates Chemistry, Fate, and Effects. Part IV: Toxic Effects-Organismal. Academic Press; San Diego: 1992. pp. 327–51. [Google Scholar]
- Abou-Donia MB, Lapadula DM. Mechanisms of organophosphorus ester-induced delayed neurotoxicity: type I and type II. Annu Rev Pharmacol Toxicol. 1990;30:405–40. doi: 10.1146/annurev.pa.30.040190.002201. [DOI] [PubMed] [Google Scholar]
- Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol. 2011;7:603–615. doi: 10.1038/nrneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- Beavers CT, Parker JJ, Flinchum DA, et al. Pesticide-induced quadriplegia in a 55-year-old woman. Am J Forensic Med Pathol. 2014;35:239–41. doi: 10.1097/PAF.0000000000000108. [DOI] [PubMed] [Google Scholar]
- Benabent M, Vilanova E, Mangas I, et al. Interaction between substrates suggests a relationship between organophosphorus-sensitive phenylvalerate- and acetylcholine-hydrolyzing activities in chicken brain. Toxicol Lett. 2014;230:132–138. doi: 10.1016/j.toxlet.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Boulting GL, Kiskinis E, Croft GF, et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:279–86. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capodicasa E, Scapellato ML, Moretto A, et al. Chlorpyrifos-induced delayed polyneuropathy. Arch Toxicol. 1991;65:150–5. doi: 10.1007/BF02034943. [DOI] [PubMed] [Google Scholar]
- Ceron JJ, Tecles F, Tvarijonaviciute A. Serum paraoxonase 1 (PON1) measurement: an update. BMC Vet Res. 2014;10:74. doi: 10.1186/1746-6148-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasserini D, van Weering JRT, Piersma SR, et al. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics. 2014;106:191–204. doi: 10.1016/j.jprot.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Chio A, Calvo A, Dossena M, et al. ALS in Italian professional soccer players: the risk is still present and could be soccer-specific. Amyotroph Lateral Scler. 2009;10:205–9. doi: 10.1080/17482960902721634. [DOI] [PubMed] [Google Scholar]
- Chió A, Meineri P, Tribolo A, Schiffer D. Risk factors in motor neuron disease: a case-control study. Neuroepidemiology. 1991;10:174–84. doi: 10.1159/000110267. [DOI] [PubMed] [Google Scholar]
- Conde-Vancells J, Rodriguez-Suarez E, Embade N, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–66. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Cole TB, et al. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology. 2013;307:115–22. doi: 10.1016/j.tox.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol. 2005;69:541–550. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- D’Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65:509–27. doi: 10.1016/j.freeradbiomed.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SL, Tanaka D, Aulerich RJ, Bursian SJ. Organophosphorus-induced neurotoxicity in the absence of neuropathy target esterase inhibition: the effects of triphenyl phosphine in the European ferret. Toxicol Sci. 1999;49:78–85. doi: 10.1093/toxsci/49.1.78. [DOI] [PubMed] [Google Scholar]
- De Bleecker J, Lison D, Van Den Abeele K, et al. Acute and subacute organophosphate poisoning in the rat. Neurotoxicology. 1994;15:341–8. [PubMed] [Google Scholar]
- de Boer J, Antelo A, van der Veen I, et al. Tricresyl phosphate and the aerotoxic syndrome of flight crew members--current gaps in knowledge. Chemosphere. 2015;119(Suppl):S58–61. doi: 10.1016/j.chemosphere.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci (Lond) 2004;107:435–47. doi: 10.1042/CS20040187. [DOI] [PubMed] [Google Scholar]
- Drexler B, Seeger T, Grasshoff C, et al. Long-term evaluation of organophosphate toxicity and antidotal therapy in co-cultures of spinal cord and muscle tissue. Toxicol Lett. 2011;206:89–93. doi: 10.1016/j.toxlet.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983;35:1126–38. [PMC free article] [PubMed] [Google Scholar]
- el-Fawal HA, Jortner BS, Ehrich M. Effect of verapamil on organophosphorus-induced delayed neuropathy in hens. Toxicol Appl Pharmacol. 1989;97:500–11. doi: 10.1016/0041-008x(89)90255-x. [DOI] [PubMed] [Google Scholar]
- El-Sebae AH, Soliman SA, Elamayem MA, Ahmed NS. Neurotoxicity of organophosphorus insecticides Leptophos and EPN. J Environ Sci Health B. 1977;12:269–87. doi: 10.1080/03601237709372071. [DOI] [PubMed] [Google Scholar]
- Emerick GL, DeOliveira GH, Oliveira RV, Ehrich M. Comparative in vitro study of the inhibition of human and hen esterases by methamidophos enantiomers. Toxicology. 2012;292:145–50. doi: 10.1016/j.tox.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Emerick GL, Fernandes LS, de Paula ES, et al. In vitro study of the neuropathic potential of the organophosphorus compounds fenamiphos and profenofos: Comparison with mipafox and paraoxon. Toxicol Vitr. 2015;29:1079–1087. doi: 10.1016/j.tiv.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Estévez J, Selva V, Benabent M, et al. Acetylcholine-hydrolyzing activities in soluble brain fraction: Characterization with reversible and irreversible inhibitors. Chem Biol Interact. 2016;259:374–381. doi: 10.1016/j.cbi.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Fernandes LS, Emerick GL, dos Santos NAG, et al. In vitro study of the neuropathic potential of the organophosphorus compounds trichlorfon and acephate. Toxicol Vitr. 2015;29:522–528. doi: 10.1016/j.tiv.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Funk KA, Henderson JD, Liu CH, et al. Neuropathology of organophosphate-induced delayed neuropathy (OPIDN) in young chicks. Arch Toxicol. 1994;68:308–16. doi: 10.1007/s002040050074. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Richter RJ, Seidel SL, Motulsky AG. Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon. Am J Hum Genet. 1988;43:230–8. [PMC free article] [PubMed] [Google Scholar]
- Giagheddu M, Puggioni G, Masala C, et al. Epidemiologic study of amyotrophic lateral sclerosis in Sardinia, Italy. Acta Neurol Scand. 1983;68:394–404. doi: 10.1111/j.1600-0404.1983.tb04849.x. [DOI] [PubMed] [Google Scholar]
- Goldberg AM, Brookes N, Burt DR. The use of spinal cord cell cultures in the study of neurotoxicological agents. Toxicology. 1980;17:233–5. doi: 10.1016/0300-483x(80)90099-2. [DOI] [PubMed] [Google Scholar]
- Hall J, Prabhakar S, Balaj L, et al. Delivery of Therapeutic Proteins via Extracellular Vesicles: Review and Potential Treatments for Parkinson’s Disease, Glioma, and Schwannoma. Cell MolNeurobiol. 2016;36:417–27. doi: 10.1007/s10571-015-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A. Neuropathology of ALS: an overview. Neurology. 1996;47:S63–S66. doi: 10.1212/wnl.47.4_suppl_2.63s. [DOI] [PubMed] [Google Scholar]
- Holland N, Lizarraga D, Huen K. Recent progress in the genetics and epigenetics of paraoxonase: why it is relevant to children’s environmental health. Curr Opin Pediatr. 2015;27:240–7. doi: 10.1097/MOP.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RD, Grambow SC, Coffman CJ, et al. Amyotrophic lateral sclerosis among 1991 Gulf War veterans: evidence for a time-limited outbreak. Neuroepidemiology. 2008;31:28–32. doi: 10.1159/000136648. [DOI] [PubMed] [Google Scholar]
- Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61:742–9. doi: 10.1212/01.wnl.0000069922.32557.ca. [DOI] [PubMed] [Google Scholar]
- Huen K, Yousefi P, Street K, et al. PON1 as a model for integration of genetic, epigenetic, and expression data on candidate susceptibility genes. 2015 doi: 10.1093/eep/dvv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain K, Vijayaraghavan R, Pant SC, et al. Delayed neurotoxic effect of sarin in mice after repeated inhalation exposure. J Appl Toxicol. 1993;13:143–5. doi: 10.1002/jat.2550130212. [DOI] [PubMed] [Google Scholar]
- Jamal GA. Neurological syndromes of organophosphorus compounds. Adverse Drug React Toxicol Rev. 1997;16:133–70. [PubMed] [Google Scholar]
- James RW, Leviev I, Righetti A. Smoking is associated with reduced serum paraoxonase activity and concentration in patients with coronary artery disease. Circulation. 2000;101:2252–7. doi: 10.1161/01.cir.101.19.2252. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Glynn P. Neuropathy target esterase. In: Krieger RI, editor. Handbook of Pesticide Toxicology. Vol. 2. Academic Press; San Diego: 2001. pp. 953–965. [Google Scholar]
- Johnson FO, Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology. 2009;30:761–5. doi: 10.1016/j.neuro.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Lotti M. Delayed neurotoxicity caused by chronic feeding of organophosphates requires a high-point of inhibition of neurotoxic esterase. Toxicol Lett. 1980;5:99–102. doi: 10.1016/0378-4274(80)90155-1. [DOI] [PubMed] [Google Scholar]
- Jokanović M, Kosanović M, Brkić D, Vukomanović P. Organophosphate induced delayed polyneuropathy in man: an overview. Clin Neurol Neurosurg. 2011;113:7–10. doi: 10.1016/j.clineuro.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Jortner ME, BS Organophosphate-Induced Delayed Neuropathy. Handb Neurotoxicology. 2002;1:17–27. [Google Scholar]
- Kaji R, Izumi Y, Adachi Y, Kuzuhara S. ALS-Parkinsonism-Dementia complex of Kii and other related diseases in Japan. Parkinsonism Relat Disord. 2012;18:S190–S191. doi: 10.1016/S1353-8020(11)70059-1. [DOI] [PubMed] [Google Scholar]
- Kalfakis N, Vassilopoulos D, Voumvourakis C, et al. Amyotrophic lateral sclerosis in southern Greece: an epidemiologic study. Neuroepidemiology. 1991;10:170–3. doi: 10.1159/000110266. [DOI] [PubMed] [Google Scholar]
- Kang H, Cha ES, Choi GJ, Lee WJ. Amyotrophic lateral sclerosis and agricultural environments: a systematic review. J Korean Med Sci. 2014;29:1610–7. doi: 10.3346/jkms.2014.29.12.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno N, Horiguchi H, Fukushima M. Delayed neurotoxicity of diisopropylfluorophosphate (DFP): autoradiographic localization of high-affinity [(3)H]DFP binding sites in the chicken spinal cord. Environ Health Prev Med. 1999;4:92–6. doi: 10.1007/BF02932001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka H. Neuropathology of the motor neuron disease--Bunina body. Rinsho Shinkeigaku. 1999;39:65–66. [PubMed] [Google Scholar]
- Lacorte E, Ferrigno L, Leoncini E, et al. Physical activity, and physical activity related to sports, leisure and occupational activity as risk factors for ALS: A systematic review. Neurosci Biobehav Rev. 2016;66:61–79. doi: 10.1016/j.neubiorev.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Lapadula DM, Patton SE, Campbell GA, Abou-Donia MB. Characterization of delayed neurotoxicity in the mouse following chronic oral administration of tri-o-cresyl phosphate. Toxicol Appl Pharmacol. 1985;79:83–90. doi: 10.1016/0041-008x(85)90370-9. [DOI] [PubMed] [Google Scholar]
- Lehning EJ, Tanaka D, Bursian SJ. Triphenyl phosphite and diisopropylphosphorofluoridate produce separate and distinct axonal degeneration patterns in the central nervous system of the rat. Fundam Appl Toxicol. 1996;29:110–8. doi: 10.1006/faat.1996.0012. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Murphy J, Langmore S, et al. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology. 2003;60:1094–7. doi: 10.1212/01.wnl.0000055861.95202.8d. [DOI] [PubMed] [Google Scholar]
- Lotti M, Moretto A. Organophosphate-induced delayed polyneuropathy. Toxicol Rev. 2005;24:37–49. doi: 10.2165/00139709-200524010-00003. [DOI] [PubMed] [Google Scholar]
- Mackness B, Beltran-Debon R, Aragones G, et al. Human tissue distribution of paraoxonases 1 and 2 mRNA. IUBMB Life. 2010;62:n/a–n/a. doi: 10.1002/iub.347. [DOI] [PubMed] [Google Scholar]
- Mackness M, Mackness B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene. 2015;567:12–21. doi: 10.1016/j.gene.2015.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangas I, Estevez J, Vilanova E, França TCC. New insights on molecular interactions of organophosphorus pesticides with esterases. Toxicology. 2016 doi: 10.1016/j.tox.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Marrs TC. Organophosphate poisoning. Pharmacol Ther. 1993;58:51–66. doi: 10.1016/0163-7258(93)90066-m. [DOI] [PubMed] [Google Scholar]
- Marsillach J, Mackness B, Mackness M, et al. Immunohistochemical analysis of paraoxonases-1, 2, and 3 expression in normal mouse tissues. Free Radic Biol Med. 2008;45:146–57. doi: 10.1016/j.freeradbiomed.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Meggs WJ. Permanent paralysis at sites of dermal exposure to chlorpyrifos. J Toxicol Clin Toxicol. 2003;41:883–6. doi: 10.1081/clt-120025357. [DOI] [PubMed] [Google Scholar]
- Miranda ML, Alicia Overstreet Galeano M, Tassone E, et al. Spatial analysis of the etiology of amyotrophic lateral sclerosis among 1991 Gulf War veterans. Neurotoxicology. 2008;29:964–70. doi: 10.1016/j.neuro.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Moretto A, Lotti M. The relationship between isofenphos cholinergic toxicity and the development of polyneuropathy in hens and humans. Arch Toxicol. 2002;76:367–75. doi: 10.1007/s00204-002-0352-8. [DOI] [PubMed] [Google Scholar]
- Morgan JP. The Jamaica ginger paralysis. JAMA. 1982;248:1864–7. [PubMed] [Google Scholar]
- Mou DL, Wang YP, Song JF, et al. Triorthocresyl phosphate-induced neuronal losses in lumbar spinal cord of hens--an immunohistochemistry and ultrastructure study. Int J Neurosci. 2006;116:1303–16. doi: 10.1080/00207450500519655. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Henry RG, Langmore S, et al. Continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Arch Neurol. 2007;64:530–4. doi: 10.1001/archneur.64.4.530. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda S, Tapaswi PK. Biochemical, neuropathological and behavioral studies in hens induced by acute exposure of tri-ortho-cresyl phosphate. Int J Neurosci. 1995;82:243–54. doi: 10.3109/00207459508999804. [DOI] [PubMed] [Google Scholar]
- Nicholas JS, Butler GC, Lackland DT, et al. Health among commercial airline pilots. Aviat Space Environ Med. 2001;72:821–6. [PubMed] [Google Scholar]
- Oskarsson B, Horton DK, Mitsumoto H. Potential Environmental Factors in Amyotrophic Lateral Sclerosis. Neurol Clin. 2015;33:877–88. doi: 10.1016/j.ncl.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzurro DM, Dao K, Costa LG. Diazinon and diazoxon impair the ability of astrocytes to foster neurite outgrowth in primary hippocampal neurons. Toxicol Appl Pharmacol. 2014b;274:372–82. doi: 10.1016/j.taap.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzurro DM, Dao K, Costa LG. Astrocytes protect against diazinon- and diazoxon-induced inhibition of neurite outgrowth by regulating neuronal glutathione. Toxicology. 2014a;318:59–68. doi: 10.1016/j.tox.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plato CC, Garruto RM, Galasko D, et al. Amyotrophic lateral sclerosis and parkinsonism-dementia complex of Guam: changing incidence rates during the past 60 years. Am J Epidemiol. 2003;157:149–57. doi: 10.1093/aje/kwf175. [DOI] [PubMed] [Google Scholar]
- Rainier S, Bui M, Mark E, et al. Neuropathy target esterase gene mutations cause motor neuron disease. Am J Hum Genet. 2008;82:780–5. doi: 10.1016/j.ajhg.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re DB, Le Verche V, Yu C, et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81:1001–8. doi: 10.1016/j.neuron.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read DJ, Li Y, Chao MV, et al. Neuropathy target esterase is required for adult vertebrate axon maintenance. J Neurosci. 2009;29:11594–600. doi: 10.1523/JNEUROSCI.3007-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read DJ, Li Y, Chao MV, et al. Organophosphates induce distal axonal damage, but not brain oedema, by inactivating neuropathy target esterase. Toxicol Appl Pharmacol. 2010;245:108–15. doi: 10.1016/j.taap.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Hein ND, Wijeyesakere SJ, et al. Neuropathy target esterase (NTE): overview and future. Chem Biol Interact. 2013;203:238–44. doi: 10.1016/j.cbi.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Mitsumoto H, Przedborski S. Amyotrophic lateral sclerosis, progressive muscular atrophy, and primary lateral sclerosis. In: Rowland LP, Pedley TA, editors. Merritt’s Neurology. 12. Lippincott, Williams & Wilkins; Philadelphia: 2010. pp. 802–808. [Google Scholar]
- Saeed M, Siddique N, Hung WY, et al. Paraoxonase cluster polymorphisms are associated with sporadic ALS. Neurology. 2006;67:771–6. doi: 10.1212/01.wnl.0000227187.52002.88. [DOI] [PubMed] [Google Scholar]
- Satoh T. Global Epidemiology of Organophosphate and Carbamate Poisosings. Toxicology of Organophosphate and Carbamate Compounds 2006 [Google Scholar]
- Slowik A, Tomik B, Partyka D, et al. Paraoxonase-1 Q192R polymorphism and risk of sporadic amyotrophic lateral sclerosis. Clin Genet. 2006;69:358–9. doi: 10.1111/j.1399-0004.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- Terry AV. Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther. 2012;134:355–65. doi: 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivakaran T, Gamage R, Gunarathne KS, Gooneratne IK. Chlorpyrifos-induced delayed myelopathy and pure motor neuropathy: a case report. Neurologist. 2012;18:226–8. doi: 10.1097/NRL.0b013e318261035b. [DOI] [PubMed] [Google Scholar]
- Ticozzi N, LeClerc AL, Keagle PJ, et al. Paraoxonase gene mutations in amyotrophic lateral sclerosis. Ann Neurol. 2010;68:102–7. doi: 10.1002/ana.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely Timothy, Donaldson D, Arthur Grube P. Pesticides Industry Sales and Usage. US Environ Prot Agency. 2004:1–30. [Google Scholar]
- Tosi L, Righetti C, Adami L, Zanette G. 1942: a strange epidemic paralysis in Saval, Verona, Italy. Revision and diagnosis 50 years later of tri-ortho-cresyl phosphate poisoning. J Neurol Neurosurg Psychiatry. 1994 Oct;57:810–3. doi: 10.1136/jnnp.57.7.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi B, Padilla S, Blackmon K, Pope C. Murine susceptibility to organophosphorus-induced delayed neuropathy (OPIDN) Toxicol Appl Pharmacol. 1991;107:311–24. doi: 10.1016/0041-008x(91)90211-v. [DOI] [PubMed] [Google Scholar]
- Vinsant S, Mansfield C, Jimenez-Moreno R, et al. Characterization of early pathogenesis in the SOD1 G93A mouse model of ALS: part I, background and methods. Brain Behav. 2013;3:335–350. doi: 10.1002/brb3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, O’Reilly ÉJ, Weisskopf MG, et al. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol. 2011;68:207–13. doi: 10.1001/archneurol.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wills A-M, Cronin S, Slowik A, et al. A large-scale international meta-analysis of paraoxonase gene polymorphisms in sporadic ALS. Neurology. 2009;73:16–24. doi: 10.1212/WNL.0b013e3181a18674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills A-M, Landers JE, Zhang H, et al. Paraoxonase 1 (PON1) organophosphate hydrolysis is not reduced in ALS. Neurology. 2008;70:929–34. doi: 10.1212/01.wnl.0000305956.37931.dd. [DOI] [PubMed] [Google Scholar]
- Zou C, Kou R, Gao Y, et al. Activation of mitochondria-mediated apoptotic pathway in tri-ortho-cresyl phosphate-induced delayed neuropathy. Neurochem Int. 2013;62:965–72. doi: 10.1016/j.neuint.2013.03.013. [DOI] [PubMed] [Google Scholar]