Abstract
Voxel-based morphometry (VBM) studies of Parkinson’s disease (PD), have yielded mixed results, possibly due to several studies not accounting for common nuisance variables (age, sex, and total intracranial volume [TICV]). TICV is particularly important because there is evidence for larger TICV in PD. We explored the influence of these covariates on VBM by 1) comparing PD patients and controls before adding covariates, after adding age and sex, and after adding age, sex and TICV, and 2) by comparing controls split into large and small TICV before and after controlling for TICV, with age and sex accounted for in both analyses. Experiment 1 consisted of 40 PD participants and 40 controls. Experiment 2 consisted of 88 controls median split by TICV. All participants completed an MRI on a 3T scanner. TICV was calculated as gray+white+CSF from Freesurfer. VBM was performed on T1 images using an optimized VBM protocol. Volume differences were assessed using a voxel-wise GLM analysis. Clusters were considered significant at >10 voxels and p<.05 corrected for familywise error. Before controlling for covariates, PD showed reduced GM in temporal, occipital, and cerebellar regions. Controlling for age and sex did not affect the pattern of significance. Controlling for TICV reduced the size of the significant region although it still contained portions of bilateral temporal lobes, occipital lobes and cerebellum. The large TICV group showed reduced volume in temporal, parietal, and cerebellar areas. None of these differences survived controlling for TICV. This demonstrates that TICV influences VBM results independently from other factors. Controlling for TICV in VBM studies is recommended.
Introduction
Structural MRI is a powerful tool for assessing brain volume differences, particularly in the context of neurodegenerative disease such as Parkinson’s disease (PD). It offers insight into neuroanatomical differences in PD and informs how different presentations of PD (for example, PD with and without dementia) may differ in terms of whole brain or regional volumes. There are several methods for determining structural or volumetric brain differences. One popular method is voxel-based morphomety (VBM), commonly used because it does not rely on pre-determined brain structures.
VBM has been employed in several studies of PD with mixed results ranging from widespread volumetric declines in PD to no differences between individuals with PD and demographically matched peers (Table 1). Several researchers have reported regional volumetric decreases in temporal, parietal, and occipital areas in idiopathic PD relative to peers. In a comparison of patients with PD with short disease duration (mean disease duration=3.1 years) and low disease severity (mean Hoehn & Yahr =1.8) and control individuals, Lee et al (2013) found smaller volumes in PD participants in the frontal lobes and parieto-occipital regions. Xia et al. (2013) also compared non-demented early PD patients to controls and reported decreased volume in the bilateral temporal lobes, bilateral occipital lobes, bilateral parietal lobes, bilateral amygdalae, right uncus, and right posterior lobe of the cerebellum. However, the authors noted poorer general cognition in the PD group in their sample, which may indicate brain differences independent of PD. Beyer et al. (2007) examined PD patients with dementia (PDD) and PD patients without dementia (PDND) and reported PDND patients had reduced gray matter volume compared to controls in the left frontal and bilateral temporal lobes after controlling for age, gender, and duration of the disease. Summerfield et al. (2013) also investigated PDD and PDND patients with similar disease duration and symptom severity to Beyer et al’s participants and found reduced gray matter volume in the right hippocampus, left anterior cingulate, and left superior temporal gyrus in PDND compared to controls.
Table 1.
Previous studies investigating brain differences in idiopathic PD using VBM. Studies were considered as accounting for TICV if TICV or total brain volume was used as a covariate or if regional brain volume was divided by TICV or total brain volume.
| Paper | N | Disease Duration (Yrs) |
H&Y | UPDRS III | Covariates | Regions of Significance | Significance Threshold |
|---|---|---|---|---|---|---|---|
| Burton et al 2004 | 31 | 3.6; 2.9 | 25.8; 11.1 | TICV | None | p<.001 uncorrected | |
| Beyer et al 2007 | 20 | 12.0; 6.3 | 2.4; 0.6 | NA | Age, Sex | right superior temporal gyrus; None after familywise correction |
FWE p<.05 based on # of voxels in ROI |
| Ramirez-Ruiz et al 2007 | 20 | 10.6; 4.3 | 2.5; .7 | 24.5; 14 | TICV, Cognition, Symptom Severity |
right frontal gyrus, left superior temporal gyrus |
p<.001; only p<.05 FWE corrected reported |
| Dalaker et al 2010 | 10 | 2.7; .5-7 | NA | 19.2; 11.4 | Age, Sex, TICV, Symptom Severity |
None | p<.05; FDR corrected |
| Jubault et al 2009 | 23 | 6.33; 3.93 | NA | 29.07; 8.97 | Age, Sex, Grey Matter Volume |
None | p<.05; FDR corrected |
| Martin et al 2009 | 26 | 3.0; 1.7 | 1.5; .5 | 15.8; 6.9 | Age, Sex, TICV | None | p<.05; FDR corrected; one-tailed; k>30 |
| Kostic et al 2010 | 24 | 5 (1-19 range) | 2 (1-3 range) | 19 (10-35 range) | Age, Sex, TICV | bilateral frontal and parietal lobes | p<.05 uncorrected; k>20 |
| Nishio et al 2010 | 27 | 3.7; 2.9 | 2.5; .5 | 18.3; 8.0 | Age, Sex, TICV | medial frontal, perisylvian, posterior lateral temporal, medial occipital |
p<.001; 100 voxels |
| Meppelink et al 2011 | 11 | 7.9; 2.4 | NA | NA | Grey Matter Volume | left occipital, left parietal, left temporal, left frontal orbicularis, angular gyrus |
p<.001; considered significant if survived familywise correction |
| Agosta et al 2013 | 13 | 10.0; 7.1 | 2.4; 0.7 | 28.3; 11.4 | TICV | None | p<.05; FWE corrected |
| Lee et al 2013 | 40 | 3.1; 3.2 | 1.8; 0.7 | 17.7; 10.7 | None | frontal and parieto-occipital regions | p<.05 FWE corrected |
| Lin et al 2013 | 10 | 2.85; 2.47 | 2.20; .26 | 22.5; 8.29 | None | lentiform nucleus, insula, middle frontal gyrus, cerebellum |
p<.05 FWE corrected, k<=30 |
| Summerfield et al 2013 | 13 | 10.61; 7.41 | 2.73; 0.72 | 24.5; 12.04 | None | right hippocampus, left anterior cingulate gyrus, left superior temporal gyrus |
p<.001 uncorrected; k>10 voxels |
| Xia et al 2013 | 32 | 1-11 yrs | 1.98; 0.81 | NA | None | bilateral temporal lobe, bilateral occipital lobe, bilateral parietal lobe, bilateral frontal lobe, bilateral insular lobe, bilateral parahippocampal gyrus, bilateral amygdale, right uncus, cerebellum |
p<.005 uncorrected; k>200 voxels |
| Planetta et al 2015 | 14 | 5.9; 5.5 | NA | 29.6; 5.3 | Gray Matter Volume | None | p<.005 uncorrected; k=1611 |
These findings suggest gray matter reductions occur in early PD with relatively mild symptoms. However, other investigators report little to no volumetric change in idiopathic PD. Burton et al. (2004) compared individuals with PDND, PDD, Alzheimer’s disease, and dementia with Lewy bodies to controls. They reported PD patients showed reduced volumes only in the right frontal lobe. Dalaker et al. (2010) investigated PD patients with mild cognitive impairment, comparing brain volume as well as assessing the association between brain volume and cognitive ability in memory, visuospatial, and attentional/executive tasks. They reported no volumetric differences between non-demented PD patients and controls. Agosta et al. (2013) compared mild, moderate, and severe PD patients categorized by Hoehn & Yahr stage to control individuals; they found no volumetric differences between mild and moderate PD patients and controls, despite these groups having similar disease severity and duration to the previously mentioned studies. Planetta et al. (2015) investigated functional and volumetric differences between PD patients, individuals with multiple system atrophy and controls and found no volumetric differences between PD patients and controls.
A close review of these contrasting findings reveals that these differences may have arisen from a lack of consistency in the methods of comparison for the different studies. Investigators have demonstrated that VBM results can be highly influenced by several factors, including type of statistical correction used, smoothing kernel size, and software version (Henley et al, 2010; Shen et al, 2012). Additionally, Barnes et al. (2010) found that results from VBM analyses are significantly influenced by gender, age, and head size. A lack of a significant difference in these covariates between groups is frequently assumed to imply that these factors will not influence results, but Henley et al. (2010) demonstrate this is a faulty assumption.
Whether or not covariates are accounted for is relevant for Parkinson’s disease research; of the studies cited above, those that controlled for the three covariates suggested by Henley et al 2010 (age, sex, and head size) showed little to no difference between groups (Burton et al. 2004; Agosta et al. 2013; Planetta et al. 2015), whereas those that do not control for these variables show widespread differences (Summerfield et al. 2005; Lin et al. 2013; Lee et al. 2013). This pattern of findings suggests that differences in reported patterns of gray matter atrophy in non-demented PD may be largely attributable to variability in age, sex, and head size.
The most consistent method of controlling for head size in these studies is total intracranial volume (TICV), defined as the volume within the cranium including gray matter, white matter, meninges, and cerebrospinal fluid. Controlling for TICV is a common practice in studies comparing whole brain regions as it reduces inter-individual variations in brain volume due to head size difference (Whitwell et al., 2001). In VBM analyses, controlling for TICV is widespread, but not universal.
Controlling for TICV may be particularly important in studies of PD. Several studies (Krabbe et al. 2005; Gallagher et al. 2013; Tanner et al. 2015), have reported larger TICV in PD patients compared to controls. Furthermore, several genetic studies have suggested that genetic variation at 17q21 is associated with infant head circumference (Taal et al. 2013), intracranial volume (Early Growth Genetics Consortium, 2012), and PD (Simon-Sanchez et al. 2009).
The present study aims to assess the issue of covariates in VBM analyses through two separate comparisons. First, VBM will assess voxelwise differences in brain volume between a sample of PD patients and matched controls before and after controlling for age, sex, and TICV. We hypothesize this analysis will show diffuse volumetric differences between non-demented PD and non-PD peers before controlling for covariates, but these differences will not survive controlling for covariates. Second, we will examine whether this pattern persists in a population composed purely of controls, median-split by TICV. We hypothesize this analysis will produce results similar to those comparing PD patients and controls before controlling for age, sex, and TICV.
Methods
Participants
This retrospective analysis utilizes a federally funded dataset for investigating neuroanatomical and cognitive profiles in idiopathic PD relative to non-PD matched peers. The study was IRB approved for human participant investigation, required consent, and followed the protocol of the Declaration of Helsinki. Recruitment for individuals with PD involved a combination of: 1) brochure mailings to individuals identified through a research database within the UF Center for Neurorestoration and Movement Disorders (UF CNMDC), 2) UF CNMDC direct neurology referrals, 3) advertisement at different PD support symposiums. Control participants were recruited through community fliers, free community memory screenings, and mail-outs to targeted individuals in local counties who met demographic inclusion criteria. All individuals were screened via telephone or in person and completed baseline cognitive testing to ensure they met cognitive screening criteria.
All participants were required to be right-handed, speak fluent English, and show no signs of dementia (Telephone Screening for Cognitive Status [TICS] >34; Dementia Rating Scale-Revised [DRS-R] score in the average range [age and education scale score>8]; Mini Mental State Exam [MMSE] >27 [Folstein et al. 1975]). Individuals with PD were diagnosed by a movement disorder fellowship trained neurologist, met criteria outlined by the UK Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria (Hughes, Ben-Shlomo et al. 1992) and had a Hoehn and Yahr (1967) scale ranging from 1-3. Medical exclusions included cancer (other than non-melanoma skin cancer) requiring treatment in past 5 years, serious infectious diseases (e.g., self-reported HIV), myocardial infarction or cerebrovascular accident in the last six months, congestive heart failure, chronic hepatitis, history of organ transplantation, seizure disorders and head trauma resulting in intensive care, and any other medical condition likely to limit lifespan. Additional exclusion criteria included existence of a deep brain stimulator, secondary or atypical Parkinsonism as a result of 1) history of major stroke(s) associated with cognitive sequelae, 2) exposure to toxins or neuroleptics, 3) history of encephalitis, 4) neurological signs of upper motor neuron disease, cerebellar involvement, supranuclear palsy, or significant orthostatic hypertension, signs of a dementia as indicated by the neurological/ neuropsychological assessment (DSM-IV criteria and DRS corrected scale score <8), major psychiatric disorder as assessed by the psychiatric and neurological team with the Structured Clinical Interview for DSM-IV, and a history of Major Depressive Disorder. We did not exclude patients reporting mild depression or anxiety because many PD patients report such symptoms. Other exclusions include less than five years of normal education, inability to read or write, self-reported hearing difficulty that interferes with standardized test administration, claustrophobia, non-medical bodily metal, and a pace-maker device.
MRI Metrics
Neuroimaging data were prospectively acquired with a Siemens 3T Verio scanner using an 8-channel head coil. For gray and white matter analyses we acquired 2 T1-weighted scans (176 contiguous sagittal slices, 1mm3 voxels, 256×256 matrix, TR/TE= 2500/3.77ms, 7/8 Partial Fourier, acquisition time 9:22). The first of the two T1 scans was analyzed for this analysis. For skull segmentation, T2-weighted images were acquired (176 contiguous sagittal slices, 1mm3 voxels, 256×256 matrix, TR/ TE= 3200/ 409ms, GRAPPA, acquisition time 4:43). Images were visually examined for excessive motion, and images showing more than a moderate degree of motion were excluded from the analyses.
For all participants in the first analysis (PD vs control), TICV was measured by exporting the inner surface of the skull from FSL version 4.1 Brain Extraction Tool (BET; Smith 2002). These initial intracranial masks were then manually edited by expert raters to fill the enclosure within the inner surface of the skull. The inferior portion of the mask terminated on a line between the bottom of the occipital bone and the clivus. Reliability was high (intra-rater and inter-rater reliability DSC > 0.99). The final variable of interest was TICV in mm3. For participants added for the large vs small TICV analysis, TICV was measured using an automated method from Freesurfer (Fischl et al 2002), which combines all voxels labeled as white matter, gray matter, and CSF to create a volume that represents TICV.
A VBM analysis was performed on T1 structural images to investigate voxel-wise grey matter changes between PD patients and control participants. Structural data was analyzed with FSL-VBM (Douaud et al. 2007), an optimized VBM protocol (Good et al. 2001) carried out with FSL tools (Smith et al. 2004). First, structural images were brain-extracted and grey matter-segmented before being registered to the MNI 152 standard space using non-linear registration (Andersson et al. 2007). The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific grey matter template. Second, all native grey matter images were non-linearly registered to this study-specific template and "modulated" to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation. The modulated grey matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm.
Statistical Analysis Approaches
Statistical calculations were performed using a commercially available software package (SPSS, version 22; SPSS, Inc). Variables were confirmed for normality and statistical assumptions prior to all analyses. Parametric analyses were used to complete demographic analyses. Due to violations of normality, Mann-Whitney U tests were used to compare UPDRS scores. Effect sizes are based on Cohen’s guidelines (small: r= .01-.23; medium: r= .24-.36; large: r=.37 or larger).
For VBM analyses, a voxel-wise general linear model (GLM) analysis was applied on the smoothed images using permutation-based nonparametric testing with multiple comparison correction across space. A cluster was considered significant if it contained at least 10 consecutive voxels with p<.05, corrected for familywise error. This small cluster size was selected in order to detect any reasonable clusters of significant volume differences.
Results
Of 186 people phone screened, 43 individuals with PD and 41 non-PD peers met criteria. Four enrolled participants could not complete MRI (i.e., claustrophobia, metal artifact). Our final sample consisted of 40 PD patients and 40 controls. For the large vs small TICV analysis, additional controls were added from a separate, federally funded study with identical exclusion criteria to the initial study for a total of 88 participants.
Participant Demographic Characteristics
Details of participant characteristics for the PD vs controls comparison are found in Table 2. PD and controls were statistically similar in demographics, comorbidity, premorbid intellect, and general cognition estimates. All participants were independent in instrumental activities of daily living (i.e., telephone, financial management), and all but one PD individual independently managed their medications. In the PD group, the sample was largely unilateral tremor dominant (70% Hoehn & Yahr stage≤ 1.5).
Table 2.
Parkinson’s disease (PD) and non-PD “control” peers demographic, motor, and general cognition characteristics
| Measure | PD (n=40) | Non-PD Peers (n=40) | t | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 67.80 ± 5.44, 60/79 | 68.18 ± 4.64, 62/79 | −0.33 | 0.74 |
| Education | 16.28 ± 3.03, 10/20 | 16.75 ± 2.35, 12/20 | −0.78 | 0.44 |
| Sex (M:F) | 32:8 | 33:7 | 0.08 | 0.78 |
| Charlson Comorbidity Index |
0.30 ±0 .72, 0/4 | 0.28 ± .61, 0/2 | 0.12 | 0.91 |
| UPDRS-III | 17.58 ± 10.74, 3/46 | 2.75 ± 3.36*; 0/15 | 83.50 | <0.001 |
| H&Y | 1.64±.76, 1/3 | -- | -- | -- |
| Disease Duration (yrs) | 7.50 ± 5.15, 0/26 | -- | -- | -- |
| < 10 years duration | 33 of 40; 83% | -- | -- | -- |
| TICV | 1745 ± 170 cc | 1644 ± 125 cc | 3.024 | 0.003 |
Charlson Comorbidity Index is a measure of comorbidities and their severities; UPDRS Total = United Parkinson’s Disease Rating Scale Total score; H&Y=Hoehn and Yahr stage; cc=Cubic Centimeters
Details for participant characteristics for the large vs small TICV comparison are found in Table 3. Large and small TICV participants were not statistically different in age, education, and general cognition as measured with the Montral Cognitive Assessment (MOCA) (Nasreddine et al 2005). There were significantly more males in the large TICV group than small TICV group (p<.001).
Table 3.
Large TICV and small TICV demographic and general cognition characteristic
| Measure | Large TICV (n=44) | Small TICV (n=44) | t | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 69.59 ± 5.47 | 68.68 ± 5.65 | −0.767 | 0.445 |
| Education | 16.91 ± 1.97 | 15.95 ± 2.89 | −1.808 | 0.074 |
| Sex (M:F) | 35:9 | 18: 26 | 3.984 | <0.001 |
| MoCA | 26.40 ± 2.02 | 25.89 ± 2.23 | −1.113 | 0.269 |
| TICV | 1708 ± 90 cc | 1477 ± 76 cc | 12.919 | <0.001 |
MoCA=Montreal Cognitive Assessment; cc=Cubic Centimeters
PD vs. Controls with No Covariates
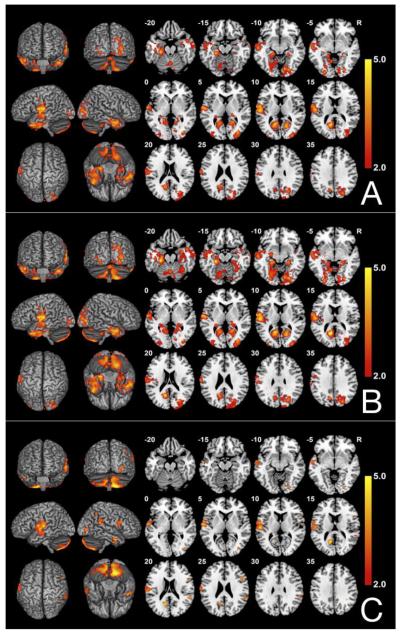
The results of the between-group VBM analysis comparing PD participants and controls before adding covariates are shown in Figure 1A. Compared to controls, PD participants had reduced GM volumes in a large cluster of voxels with peaks in the left and right temporal poles (BA 40 and 20/21, respectively), inferior temporal gyrus (BA 20), left parahippocampus (BA 30), left and right middle occipital lobe (BA 18), right superior occipital lobe (BA 19), bilateral calcarine (BA 17), and bilateral cerebellum (Table 4). These analyses were re-run without two individuals with longer years of disease duration as well as a control individual with higher rate of depression. The findings remained unchanged.
Fig. 1.
FSL-VBM results comparing 40 individuals with PD to 40 matched healthy controls (A) Before adding covariates; (B) Controlling for age and sex; (C) Controlling for age, sex, and TICV. Contrast T map shows significant clusters (P<.05, FWE corrected) where reduced GM densities are located in individuals with PD. Neurological convention (L is L).
Table 4.
Local peaks of significant clusters (FWE-corrected P-value<.05) showing reduced gray matter in 40 individuals with PD compared to 40 matched healthy controls
| Cluster | Voxels | Brain Region | Side | BA | T value | MNI (mm) |
||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| 1 | 17571 | Calcarine Sulcus | L | 17 | 4.69 | −14 | −60 | 14 |
| Superior Temporal Lobe/Pars Opercularis |
L | 44, 48 | 4.52 | −56 | −18 | 10 | ||
| Inferior Temporal Gyrus | L | 20 | 4.02 | −42 | 8 | −36 | ||
| Inferior Temporal Gyrus | R | 20 | 3.49 | 52 | 8 | −42 | ||
| Middle Temporal Gyrus | L | 21 | 3.79 | −60 | −16 | −10 | ||
| Inferior Temporal Gyrus | R | 20 | 4.53 | 50 | 2 | −30 | ||
| Retrosplenial Area | L | 30 | 4.38 | −26 | −30 | −18 | ||
| Cerebellum | L | - | 3.54 | −16 | −66 | −60 | ||
| Cerebellum | R | - | 3.79 | 18 | −58 | −56 | ||
| Precuneus | L | 7 | 3.48 | −12 | −68 | 36 | ||
| Secondary Visual Cortex | R | 18 | 3.24 | 20 | −74 | 22 | ||
| Postcentral | L | 43 | 3.30 | −66 | −14 | 24 | ||
| Fusiform Gyrus | L | 37 | 2.62 | −22 | −44 | −10 | ||
| Fusiform | R | 18 | 4.12 | 26 | −82 | −6 | ||
| Superior Occipital Lobe | R | 19 | 3.63 | 24 | −76 | 34 | ||
| Middle Occipital | R | 18 | 3.62 | 32 | −90 | 8 | ||
| Lingual Gyrus | R | 18 | 3.92 | 14 | −78 | −10 | ||
| 2 | 507 | Calcarine Sulcus | R | 17 | 4.13 | 18 | −66 | 8 |
| Retrosplenial Area | R | 30 | 3.07 | 24 | −34 | −12 | ||
Age and Sex Corrected PD vs. Controls
Figure 1B. After correction for age and sex, the results were largely unchanged with one large significant peak encompassing parts of bilateral calcarine sulcui (BA 17), left superior temporal gyrus (BA 22/48), bilateral middle temporal gyrus (BA 22/48/22/38), bilateral inferior temporal gyrus (BA 20/21), left superior occipital lobe (BA 19), left parahippocampus (BA 30), left retrosubicular area (BA 48), bilateral cerebellum, bilateral fusiform gyrus (BA 30 and 18), left hippocampus (BA 20), right lingual gyrus (BA 18), and left precuneus (BA7) (Table 5).
Table 5.
Local peaks of significant clusters (FWE-corrected P-value<.05) showing reduced gray matter in 40 individuals with PD compared to 40 matched healthy controls after correcting for age and sex. BA=Brodmann area.
| Cluster | Voxels | Brian Region | Side | BA | T Value | MNI (mm) |
||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| 1 | 27460 | Primary Visual Cortex | L | 17 | 5.45 | −12 | −60 | 16 |
| Primary Visual Cortex | R | 17 | 4.19 | 16 | −64 | 8 | ||
| Superior Temporal Gyrus | L | 22/48 | 4.34 | −62 | −8 | 4 | ||
| Superior Temporal Gyrus | L | 22/38 | 4.45 | −46 | 16 | −36 | ||
| Middle Temporal Gyrus | R | 20/21 | 4.8 | 50 | 2 | −30 | ||
| Inferior Temporal Gyrus | L | 20 | 4.25 | −42 | 10 | −36 | ||
| Inferior Temporal Gyrus | R | 20/21 | 4.21 | 58 | −6 | −38 | ||
| Superior Occipital Lobe | R | 19 | 3.86 | 28 | −76 | 20 | ||
| Middle Occipital Lobe | R | 19 | 4.52 | 32 | −78 | 2 | ||
| Parahippocampus | L | 30 | 5.27 | −26 | −28 | −18 | ||
| Retrosubicular Area | L | 48 | 4.83 | −58 | −4 | 12 | ||
| Cerebellum | L | 4.55 | −24 | −28 | −30 | |||
| Cerebellum | R | 4.47 | 16 | −58 | −56 | |||
| Fusiform | L | 30 | 4.94 | −25 | −32 | −16 | ||
| Fusiform | R | 18 | 4.36 | 26 | −82 | −6 | ||
| Hippocampus | L | 20 | 4.19 | −36 | −10 | −18 | ||
| Lingual Gyrus | R | 18 | 3.91 | 14 | −78 | −10 | ||
| Precuneus | L | 7 | 3.85 | −12 | −68 | 36 | ||
Age, Sex and TICV Corrected PD vs. Controls
The results comparing the same sample after controlling for age, sex and TICV are shown in Figure 1C. Individuals with PD showed smaller volumes than controls in the cerebellum bilaterally, in a cluster consisting of several regions of left temporal lobe, a cluster in the left calcarine sulcus (BA 17), a cluster in the right superior temporal gyrus (BA 42) and right supramarginal gyrus (BA 48), a cluster in the right middle occipital lobe (BA 19), fusiform and lingual gyrus (BA 18), and small clusters in the right inferior frontal gyrus (BA 48), right middle temporal gyrus (BA 20), right angular gyrus (BA 40), and right inferior temporal lobe (BA 20) (Table 6).
Table 6.
Local peaks of significant clusters (FWE-corrected P-value<.05) showing reduced gray matter in 40 individuals with PD compared to 40 controls after correcting for age, sex and TICV. BA=Broadmann area.
| Cluster | Voxels | Brain Region | Side | BA | T Value | MNI (mm) |
||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| 1 | 3984 | Cerebellum | R | 5.2 | 16 | −60 | −56 | |
| Cerebellum | L | 2.99 | −48 | −64 | −50 | |||
| 2 | 1125 | Superior Temporal Gyrus | L | 48 | 4.6 | −56 | −18 | 10 |
| Retrosubicular Area | L | 48 | 3.84 | −54 | −16 | 18 | ||
| Retrosubicular Area | L | 48 | 3.76 | −62 | −20 | 20 | ||
| Middle Temporal Gyrus | L | 21 | 3.47 | −66 | −6 | −10 | ||
| Superior Temporal Gyrus | L | 48 | 2.85 | −48 | 0 | 0 | ||
| 3 | 213 | Calcarine Sulcus | L | 17 | 4.83 | −12 | −60 | 18 |
| 4 | 148 | Superior Temporal Gyrus | R | 42 | 4.03 | 54 | −42 | 20 |
| Retrisubicular Area | R | 48 | 3.28 | 58 | −38 | 28 | ||
| 5 | 130 | Superior Occipital Lobe | R | 19 | 4.07 | 34 | −78 | 2 |
| Fusiform Gyrus | R | 18 | 3.93 | 26 | −82 | −8 | ||
| Lingual Gyrus | R | 18 | 3.27 | 14 | −78 | −10 | ||
| 6 | 100 | Retrosubicular Area | R | 48 | 3.76 | 50 | 16 | 22 |
| 7 | 80 | Inferior Temporal Gyrus | R | 20 | 4.32 | 50 | 2 | −30 |
| 8 | 25 | Angular Gyrus | R | 40 | 3.67 | 44 | −52 | 48 |
| 9 | 12 | Inferior Temporal Gyrus | R | 20 | 4.1 | 58 | −6 | −38 |
Large TICV vs Small TICV Controls with No Covariates
The results of the between-group VBM analysis comparing controls with large TICV and controls of small TICV are shown in Supplementary Figure 1. Compared to small TICV, large TICV showed reduced GM volumes in several clusters, the largest of which included bilateral parahippocampus (BA 30), right hippocampus (BA 20/37), right superior temporal lobe (BA 22), bilateral thalamus, and bilateral cerebellum. Other clusters included areas of the left superior temporal lobe (BA 22), left inferior parietal lobe (BA 40), left angular gyrus (BA 39), right postcentral gyrus (BA 43), bilateral insula (BA 48), and left cingulate cortex (Supplementary Table 1).
Age, Sex, and TICV Corrected Large TICV vs Small TICV Controls
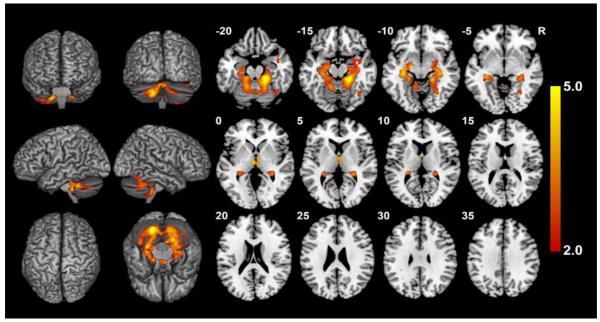
The results of the same analysis after controlling for age and sex are shown in Figure 2. Compared to small TICV, the large TICV group showed differences in a large cluster consisting of regions of bilateral cerebellum, bilateral hippocampus (BA 20), left parahippocampus (BA 30), and right fusiform (BA 37) as well as a small cluster in bilateral thalamus (Table 7). No volumetric differences survived after controlling for TICV.
Fig. 2.
FSL-VBM results of 88 control participants split between those with large and small TICV before controlling for TICV, controlling for age and gender. Contrast T map shows significant clusters (p<.05, FWE corrected) where large TICV participants showed smaller volumes than small TICV. No region remained significant following correction for TICV. Neurological convention (L is L).
Table 7.
Local peaks of significant clusters (FWE-corrected P-value<.05) showing reduced gray matter in 44 large TICV controls compared to 44 small TICV controls before correcting for TICV and after controlling for age and sex. No areas survived TICV correction. BA=Broadmann area.
| Cluster | Voxels | Brain Region | Side | BA | T Value | MNI (mm) |
||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| 1 | 8458 | Cerebellum | R | 5.7 | 20 | −36 | −18 | |
| Cerebellum | L | 5.45 | −18 | −70 | −38 | |||
| Hippocampus | L | 20 | 4.75 | −32 | −24 | −8 | ||
| Hippocampus | R | 20/37 | 3.92 | 26 | −38 | 6 | ||
| Parahippocampus | L | 30 | 3.87 | −26 | −30 | −14 | ||
| Fusiform | R | 37 | 3.73 | 30 | −62 | −8 | ||
| 2 | 168 | Thalamus | L | 4.67 | −4 | −14 | 0 | |
| Thalamus | R | 4.14 | 4 | −16 | 0 | |||
Post-Hoc Analysis on Lobe Volume and TICV associations
In order to determine if TICV had differential relationships with different brain regions, we conducted a post-hoc analysis examining correlations between the volumes of the four major lobes (frontal, parietal, temporal and occipital) and the cerebellum relative to TICV within the control participant sample from aim 2. These five regions were chosen because the frontal and parietal lobes showed little to no differences in our analysis while the occipital lobes showed moderate differences, and the temporal lobes and cerebellum showed widespread differences before accounting for TICV. Table 8. Two-tailed Pearson Product Moment Correlation analyses showed a significant association between TICV and bilateral frontal lobe volume (r(88) = 0.744, p<0.001), TICV and bilateral parietal lobe volume (r(88) = 0.722, p<0.001), TICV and bilateral temporal lobe volume (r(88) = 0.539, p<0.001), TICV and occipital lobe volume (r(88) = 0.645), and TICV and cerebellum volume (r(88) = 0.585, p<0.001). A Fisher Z showed that the temporal lobe correlation was significantly weaker than the frontal lobe correlation (p = 0.019) and significantly weaker than the parietal lobe correlation (p = 0.042). The cerebellum was not significantly weaker than the frontal or parietal correlations but showed a trend towards being significantly weaker than the frontal lobe correlation (p=0.056). Within group correlations are shown in Table 8. TICV relationships with frontal and parietal volume were significantly higher in the large TICV group (r(88) = 0.664 and r(88) = 0.675, respectively) than in the small TICV group (r(88) =0.324 and r(88) = 0.321, respectively). The correlation between TICV and temporal lobe, occipital lobe, and cerebellar volume w similar between the large and small TICV groups (r(88) = 0.397 and r(88) = 0.394 for temporal lobe, respectively; r(88) = 0.336 and r(88) = 0.404 for the occipital lobe and cerebellum, respectively; r(88) = 0.441 and r(88) = .383, respectively).
Table 8.
Pearson Product Moment correlations between the four major lobes (frontal, parietal, temporal and occipital) and cerebellum and TICV for the total control sample (N=88) and the small and large groups (N=44 for both groups).
| Lobe | Total (N=88) | Large TICV (n=4440) | Small TICV (n=4440) |
|---|---|---|---|
| Frontal Lobes | 0.744** | 0.664** | 0.324* |
| Parietal Lobes | 0.722** | 0.675** | 0.321* |
| Temporal Lobes | 0.539** | 0.397* | 0.394* |
| Occipital Lobes | 0.645** | 0.441** | 0.383* |
| Cerebellum | 0.336* | 0.336* | 0.404* |
= p<.05;
= p<.00
Discussion
We conducted two separate investigations that examined the influence of correction for several common covariates on volumetric group-based comparisons. Our first experiment showed that without correction for the covariates, particularly before correcting for TICV, individuals with PD showed numerous volumetric differences relative to non-PD peers. These differences or “atrophy” were within the temporal lobe, occipital lobe, hippocampus, and cerebellum. Our second experiment showed that when controls with large head size were compared to those with small head size, similar areas of morphometric “atrophy” were identified in the larger head size group. For both experiments, the areas of “atrophy” diminished slightly after controlling for age and sex, and greatly diminished or were eliminated when we corrected for TICV. All three of these covariates altered the final results with the most influential variable involving TICV.
This result invites the question: why did TICV correction alter these findings to such a high degree? Additionally, why did the “atrophy” uncorrected for TICV for both experiments occur in the temporal lobe and ventral regions (e.g., the hippocampus and cerebellum)? A retrospective correlation analysis between lobe volume and TICV within the control groups provides some insight. In individuals with larger heads, TICV highly associates with frontal and parietal lobe volumes. It was less strongly associated with the temporal lobe and cerebellum, with the TICV-temporal association significantly lower than that of the frontal/parietal-TICV associations. Importantly, these associations were not observed in individuals with smaller heads; in these participants, the correlations are small to moderate in all four regions we measured. This difference in proportionality by region has been previously noted in Barnes et al. (2010). We postulate that the larger TICV causes each voxel to represent a smaller area when it has been converted into template space, resulting in lower gray matter density values per voxel. However, because a larger brain volume appears to be largely driven by the frontal and parietal lobes, these smaller values over-correct in areas that do not correlate as strongly with the larger head size. The resulting images thus represent smaller volumes than are truly present.
The issue of differential use of TICV in VBM studies is important in research generally, but may be particularly pertinent to investigations of PD. Several studies have reported individuals with PD have significantly larger TICVs than controls (Krabbe et al., 2005; Gallager et al., 2013), including our own sample (Schwab et al., 2015). There is also genetic evidence indicating PD may be linked to larger head size. In a genome-wide association study, Ikram et al. (2012) found common variants of genes located at 17q21 were associated with total intracranial volume. Some research links genes near this site to PD (Simon-Sanchez et al., 2009). Another recent genome-wide association study directly correlated TICV and risk for PD (Adams et al, 2016). This potential difference in TICV between PD patients and their peers is not widely recognized in VBM studies of PD. Indeed, it is difficult to even quantify the issue, as many studies do not report TICV in their results; of the fifteen studies listed in Table 1, only one (Martin et al., 2009) reported TICV.
As shown in previous VBM analyses cited in this study, failure to correct for nuisance variables in neurodegenerative samples runs the risk of creating false positives. Investigators who did not control for TICV reported numerous areas of decreased volumes in PD relative to non-PD groups. For example, Summerfield et al. (2013) found decreased volumes in the right hippocampus, left anterior cingulate gyrus and left superior temporal gyrus in idiopathic non-dementia PD relative to non-PD peers. These differences are surprising given that these are non-demented PD. Although the idiopathic non-demented PD participants in this study were more advanced in their disease pathology than many of the other studies cited in this paper (mean disease duration of 10.61 years, mean Hoehn and Yahr stage of 2.73, and mean UPDRS of 25.50), it is unlikely that volume would decrease in the frontal lobe at this stage in the disease given what is understood about the progression of PD pathology (Braak et al 2005). Xia et al (2014) reported differences in many areas of the brain including bilateral temporal lobe, bilateral occipital lobe, bilateral parietal lobe, bilateral frontal lobe, bilateral insular lobe, bilateral parahippocampal gyrus, bilateral amygdale, right uncus, and cerebellum. However, it is important to note that the PD participants in this study had poorer scores than controls on the MMSE and MoCA, indicating some level of global cognitive dysfunction. In addition, a very liberal significance threshold (p<0.005) was used in their analysis. Studies that control for TICV, age and sex reported fewer volumetric differences between groups.
This is not to assume that there are no structural differences in PD. Indeed, we identified differences in several regions of temporal lobes as well as areas of the occipital lobes, parietal lobes, and cerebellum in our idiopathic PD sample relative to matched peers even after correcting for nuisance variables. The volumetric differences observed in the left temporal lobe fit well with other recent neuroimaging investigations reporting left temporal abnormalities in PD (Tanner et al., 2015; Goldman et al., 2012) and some reports of disease progression to the inferior and lateral temporal cortices (Braak et al 2005; Jellinger et al 1991). The region of smaller volume in cerebellar areas has been noted in other VBM studies in PD (Lin et al 2013; Xia et al 2013). Parietal and occipital lobes have also been implicated in several studies (Kostic et al 2010, Nishio et al 2010, Meppelink et al 2011, Lee et al 2013, Xia et al 2013). However, it is important to note that the majority of these areas of atrophy are fairly small (k<200 voxels) and have shrunk dramatically when compared to the non-corrected comparison of PD and non-PD peers. Thus, controlling for TICV decreased the pattern of “atrophy” in our findings from widespread to more restricted in areas that have been previously reported in VBM studies of PD.
Although our decreased temporal lobe volume finding fits with the existing PD literature, side of symptom onset may have been a contributor. Parkinson’s disease motor symptoms typically begin on one side of the body and then generalize over the course of the disease (Hoehn & Yahr, 1967). Our study sample was composed of 25 (62%) right-side onset participants, meaning that their pathology might be more severe in the left side of their brain. Future studies with larger sample sizes should attempt to separate left and right side onset in order to assess volumetric differences between these groups, or attempt to balance right and left onset participants in their analysis.
Although this study has strengths, including a large sample size and a thorough investigation of several covariates, it is not without its weaknesses. We used FSL to analyze data, while the majority of the studies cited used SPM. Given that even software version can affect the results of VBM analyses (Barnes et al. 2010), it is likely that different software packages would alter results, and different software packages may also show differential influence of the covariates used. The study was also limited by the use of only a single smoothing kernel size (3mm). This is slightly problematic as smoothing kernel size and statistical threshold can influence VBM results (Shen & Sterr, 2012). However, the smoothing size used in this analysis was found to be most accurate by Shen and Sterr (2012). In addition, the choice of a small cluster threshold, while providing a very thorough result displaying many significant clusters, may have been small enough to display coincidental clusters of significant voxels. In light of these limitations, future studies should focus on comparing multiple software packages and comparing results using different smoothing kernel sizes.
Accounting for differences in TICV is far from ubiquitous in VBM research, but the present study demonstrates that differences in TICV can significantly alter results and interpretation. This is particularly concerning when authors then make inferences regarding disease profiles. Consistent with our other publications (e.g., Schwab et al., 2014) studying morphological-brain association patterns, as well as studies showing the influence of several variables on VBM outcomes, we argue that clinical researchers in particular need to be educated on neuroimaging pitfalls that alter analyses and interpretation. At minimum, researchers utilizing VBM are encouraged to consider controlling for TICV, age, and gender, even if no significant difference in these variables exists between groups being studied. In light of findings from other studies (Shen & Sterr 2012; Barnes et al 2010; Henley et al 2010), researchers should carefully consider statistical threshold and smoothing kernel size when conducting VBM studies. Finally, researchers conducting MRI research in PD are strongly encouraged to report TICV metrics for publication consumer review due to evidence showing significantly larger TICV in individuals with PD.
Supplementary Material
Supplementary Table 1: Local peaks of significant clusters (corrected P-value<.05) showing reduced gray matter in 44 large TICV controls compared to 44 small TICV controls before correcting for TICV. No areas survived TICV correction. BA-Broadmann area.
Supplementary Fig. 1 FSL-VBM results of 88 control participants split between those with large and small TICV before controlling covariates. Contrast T map shows significant clusters (p<.05, FWE corrected) where large TICV participants showed smaller volumes than small TICV. Neurological convention (L is L).
Footnotes
Disclosures: Samuel Crowley, Haiqing Huang, Jared Tanner, Qing Zho, Nadine Schwab, Loren Hizel, Daniel Ramon, Babette Brumback, Mingzhou Ding and Catherine Price declare that they have no conflict of interest.
Consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
References
- Adams HH, Hibar DP, Chouraki V, Stein JL, Nyquist PA, Rentería ME, Beecham AH. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nature Neuroscience. 2016 doi: 10.1038/nn.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Canu E, Stojković T, Pievani M, Tomić A, Sarro L, Filippi M. The topography of brain damage at different stages of Parkinson's disease. Human brain mapping. 2013;34(11):2798–2807. doi: 10.1002/hbm.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford. 2007;2 [Google Scholar]
- Barber M, Stott DJ. Validity of the Telephone Interview for Cognitive Status (TICS) in post- stroke subjects. International journal of geriatric psychiatry. 2004;19(1):75–79. doi: 10.1002/gps.1041. [DOI] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, Fox NC. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53(4):1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell and tissue research. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(3):254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127(4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Cook SE, Marsiske M, McCoy KJ. The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. Journal of geriatric psychiatry and neurology. 2009;22(2):103–109. doi: 10.1177/0891988708328214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalaker TO, Zivadinov R, Larsen JP, Beyer MK, Cox JL, Alves G, Aarsland D. Gray matter correlations of cognition in incident Parkinson's disease. Movement Disorders. 2010;25(5):629–633. doi: 10.1002/mds.22867. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. The anatomical basis of conduction aphasia. Brain. 1980;103(2):337–350. doi: 10.1093/brain/103.2.337. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130(9):2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Early Growth Genetics (EGG) Consortium Common variants at 6q22 and 17q21 are associated with intracranial volume. Nature genetics. 2012;44(5):539–544. doi: 10.1038/ng.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallagher C, Bell B, Bendlin B, Palotti M, Okonkwo O, Sodhi A, Harding S. White matter microstructural integrity and executive function in Parkinson's disease. Journal of the International Neuropsychological Society. 2013;19(03):349–354. doi: 10.1017/S1355617712001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JG, Stebbins GT, Bernard B, Stoub TR, Goetz CG, deToledo- Morrell L. Entorhinal cortex atrophy differentiates Parkinson's disease patients with and without dementia. Movement Disorders. 2012;27(6):727–734. doi: 10.1002/mds.24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. http://doi.org/10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Henley SMD, Ridgway GR, Scahill RI, Klöppel S, Tabrizi SJ, Fox NC, EHDN Imaging Working Group Pitfalls in the use of voxel-based morphometry as a biomarker: examples from huntington disease. American Journal of Neuroradiology. 2010;31(4):711–719. doi: 10.3174/ajnr.A1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1998;50(2):318–318. doi: 10.1212/wnl.50.2.318. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease A clinicopathologic study. Neurology. 1992;42(6):1142–1142. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology of Parkinson’s disease. Molecular and Chemical Neuropathology. 1991;14(3):153–197. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- Jubault T, Brambati SM, Degroot C, Kullmann B, Strafella AP, Lafontaine AL, Monchi O. Regional brain stem atrophy in idiopathic Parkinson's disease detected by anatomical MRI. PloS one. 2009;4(12):e8247. doi: 10.1371/journal.pone.0008247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostić VS, Agosta F, Petrović I, Galantucci S, Špica V, Ječmenica-Lukic M, Filippi M. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology. 2010;75(10):857–863. doi: 10.1212/WNL.0b013e3181f11c1d. [DOI] [PubMed] [Google Scholar]
- Krabbe K, Karlsborg M, Hansen A, Werdelin L, Mehlsen J, Larsson HB, Paulson OB. Increased intracranial volume in Parkinson's disease. Journal of the neurological sciences. 2005;239(1):45–52. doi: 10.1016/j.jns.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Lee EY, Sen S, Eslinger PJ, Wagner D, Shaffer ML, Kong L, Huang X. Early cortical gray matter loss and cognitive correlates in non-demented Parkinson's patients. Parkinsonism & related disorders. 2013;19(12):1088–1093. doi: 10.1016/j.parkreldis.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Chen CM, Lu MK, Tsai CH, Chiou JC, Liao JR, Duann JR. VBM Reveals Brain Volume Differences between Parkinson's Disease and Essential Tremor Patients. Front Hum Neurosci. 2013;7:247. doi: 10.3389/fnhum.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Wieler M, Gee M, Camicioli R. Temporal lobe changes in early, untreated Parkinson's disease. Movement Disorders. 2009;24(13):1949–1954. doi: 10.1002/mds.22680. [DOI] [PubMed] [Google Scholar]
- Meppelink AM, de Jong BM, Teune LK, van Laar T. Regional cortical grey matter loss in Parkinson's disease without dementia is independent from visual hallucinations. Movement Disorders. 2011;26(1):142–147. doi: 10.1002/mds.23375. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Hirayama K, Takeda A, Hosokai Y, Ishioka T, Suzuki K, Mori E. Corticolimbic gray matter loss in Parkinson’s disease without dementia. European journal of neurology. 2010;17(8):1090–1097. doi: 10.1111/j.1468-1331.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- Planetta PJ, Kurani AS, Shukla P, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Distinct functional and macrostructural brain changes in Parkinson’s disease and multiple system atrophy. Human Brain Mapping. 2015;36(3):1165–1179. doi: 10.1002/hbm.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Ruiz B, Martí MJ, Tolosa E, Gimenez M, Bargallo N, Valldeoriola F, Junque C. Cerebral atrophy in Parkinson's disease patients with visual hallucinations. European journal of neurology. 2007;14(7):750–756. doi: 10.1111/j.1468-1331.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- Schwab NA, Tanner JJ, Nguyen PT, Schmalfuss IM, Bowers D, Okun M, Price CC. Proof of principle: Transformation approach alters caudate nucleus volume and structure-function associations. Brain imaging and behavior. 2015;9(4):744–753. doi: 10.1007/s11682-014-9332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Krüger R. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nature genetics. 2009;41(12):1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Niazy RK. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Shen S, Sterr A. Is DARTEL-based voxel-based morphometry affected by width of smoothing kernel and group size? A study using simulated atrophy. Journal of Magnetic Resonance Imaging. 2013;37(6):1468–1475. doi: 10.1002/jmri.23927. [DOI] [PubMed] [Google Scholar]
- Summerfield C. Structural Brain Changes in Parkinson Disease With Dementia. Arch Neurol. 2005;62 doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- Taal HR, St Pourcain B, Thiering E, Das S, Mook-Kanamori DO, Warrington NM, Geller F. Common variants at 12q15 and 12q24 are associated with infant head circumference. Nature genetics. 2012;44(5):532–538. doi: 10.1038/ng.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JJ, Mareci TH, Okun MS, Bowers D, Libon DJ, Price CC. Temporal Lobe and Frontal-Subcortical Dissociations in Non-Demented Parkinson’s Disease with Verbal Memory Impairment. PloS one. 2015;10(7):e0133792. doi: 10.1371/journal.pone.0133792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreyt N, Nys GM, Santens P, Vingerhoets G. Cognitive differences between patients with left-sided and right-sided Parkinson’s disease. A review. Neuropsychology review. 2011;21(4):405–424. doi: 10.1007/s11065-011-9182-x. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. American Journal of Neuroradiology. 2001;22(8):1483–1489. [PMC free article] [PubMed] [Google Scholar]
- Xia J, Wang J, Tian W, Ding H, Wei Q, Huang H, Tang L. Magnetic resonance morphometry of the loss of gray matter volume in Parkinson ’ s disease patients. Neural Regeneration Research. 2013;8(27):2557–2565. doi: 10.3969/j.issn.1673-5374.2013.27.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Local peaks of significant clusters (corrected P-value<.05) showing reduced gray matter in 44 large TICV controls compared to 44 small TICV controls before correcting for TICV. No areas survived TICV correction. BA-Broadmann area.
Supplementary Fig. 1 FSL-VBM results of 88 control participants split between those with large and small TICV before controlling covariates. Contrast T map shows significant clusters (p<.05, FWE corrected) where large TICV participants showed smaller volumes than small TICV. Neurological convention (L is L).