Abstract
Objective
Assess the association of initial lactate (L0) with mortality in children with severe sepsis.
Methods
Prospective cohort study of 74 patients <18 years old with severe sepsis admitted to the Pediatric Intensive Care Unit (PICU) of a tertiary, academic children’s hospital with lactate measured within 3 hours of meeting severe sepsis or septic shock. The primary outcome was in-hospital mortality. Secondary outcomes included PICU and hospital length of stay (LOS).
Results
While overall mortality was 10.5% (n=18), patients with L0 measured (n=72) had a higher mortality (16% vs 6%, p=0.03) and higher median PRISM-III risk of mortality scores, (p= 0.02) than those who did not. Median L0 was no different between non-survivors and survivors (3.6 mmol/L [IQR 2.0–9.0] in non-survivors vs. 2.3mmol/L [IQR 1.4–3.5] in survivors, p=0.11). However, L0 was independently associated with PRISM-III score, (coef 1.12, 95% CI 0.4 to 1.8, p = 0.003) with an increase in mean PRISM-III score of 1.12 units for every 1mmol/L increase in L0, with L0 accounting for 12% of the variability in PRISM-III scores between patients. There was no association between L0 and PICU or hospital LOS.
Conclusions
Although our single center study did not demonstrate that an elevated early lactate is associated with mortality in pediatric severe sepsis, L0 did correlate strongly with PRISM-III, the most robust measure of mortality risk in pediatrics. Therefore, early lactate measurement may be important as an early biomarker of disease severity. These data support should be validated in a larger, multicenter, prospective study.
Introduction
Sepsis is a common and often deadly global healthcare problem, and despite advances in vaccination, antibiotics, and intensive care practices, severe sepsis remains one of the leading causes of morbidity and mortality in adult and pediatric patients worldwide. It continues to impose a significant burden on the individual, the family, and the larger society, requiring lengthy and costly hospitalizations and impacting overall quality of life(1–5). While it has been shown that early recognition and intervention reduces the rate of sepsis related mortality (6–9), the initial presentation of sepsis is often subtle, particularly in children. Clinicians and researches alike are, therefore, still searching for a reliable biomarker of early sepsis that can assist in distinguishing those with sepsis from those entering a shock-like state.
In the adult literature, initial lactate is a strong predictor of mortality in septic patients (10–13), trauma patients (14–16) and heterogeneous ICU populations (17,18), and as a result, it has been incorporated into the International Surviving Sepsis Campaign’s care bundles (19). Additionally, New York State law now requires evaluating for lactic acidemia in all septic patients (Sepsis Regulations: Guidance document 405.4 (a) (4)). The association with elevated lactate and mortality in pediatric patients is, however, more controversial, and findings are inconsistent. While some studies have found no correlation between lactate and in-hospital mortality in patients admitted to the pediatric intensive care unit (PICU) (20), others found elevated lactate to be an early predictor of death in children with sepsis and septic shock (21,22) Timing of lactate level studied, however, is variable and often drawn up to 6 to 12 hours after onset of illness or initiation of resuscitation.
The aim of this study is to evaluate the association between early lactate (drawn within 3 hours of severe sepsis/shock onset) and relevant clinical outcomes in pediatric patients with severe sepsis and septic shock using internationally accepted definitions. We hypothesize that higher lactate levels checked within 3 hours of meeting severe sepsis criteria will be associated with higher in-hospital mortality and longer PICU and hospitals stays. Appreciating such an association with a readily available and efficient test will enhance our ability to recognize, treat, and improve outcomes in these critically ill children.
Materials and Methods
Design, Setting and Patients
This was an a priori subgroup analysis of a larger, observational cohort of prospectively identified patients admitted to a large, tertiary care PICU between August 2011 and July 2015. Patients were admitted either from our emergency department or transferred from surrounding community hospitals. We included all patients between the ages of 0–18 years who met criteria for severe sepsis or septic shock and had a lactate drawn within three hours of meeting this criteria. Although our hospital has a protocolized sepsis assessment tool and clinical practice guideline for management of severe sepsis and septic shock, lactate measurement is not required and is measured at the discretion of the clinical team.
We classified severe sepsis and septic shock based on a modified Goldstein criteria (23). Sepsis was defined as the presence of two or more age specific systemic inflammatory response syndrome (SIRS) criteria in the setting of a suspected infection. Severe sepsis was defined as sepsis with either (1) cardiovascular organ dysfunction, (2) Acute Respiratory Distress Syndrome, or (3) dysfunction in two or more organ systems as outlined by Goldstein. Those with cardiovascular dysfunction requiring vasoactive medications were deemed to have septic shock. The modification made to the Goldstein criteria was that definitions could be met regardless of prior fluid resuscitation.
Children transferred from outside hospitals who met severe sepsis criteria before admission to our PICU, and whose first lactate was from the referring hospital, were only included if the normal lactate ranges from that hospital were similar to our laboratory’s normal values.
Measurements and Data Collection
T0 was defined as the earliest time at which severe sepsis or septic shock could be recognized in a patient. L0 was defined as the first lactate drawn within 3 hours of meeting T0. Lactates included were drawn from arterial, venous, or capillary blood samples and measured in mmol/L. Additional information collected included age, gender, prior comorbidity, PRISM-III score (a pediatric risk of mortality score), (24), day 1 PELOD (score of organ dysfunction) (25), need for vasoactive drugs, and need for mechanical ventilation.
Outcomes Measures and Statistical Analysis
Baseline characteristics of the subjects who did and did not have a lactate level within 3 hours of meeting severe sepsis criteria were compared using chi-square comparison for categorical variables and Wilcoxon rank-sum test for non-normally distributed continuous variables. The primary outcome was in-hospital mortality, with secondary outcomes being ICU and hospital lengths of stay (LOS).
In unadjusted analyses, we compared L0 in survivors and non-survivors and evaluated for any correlation between L0 and both ICU and hospital LOS. For in-hospital mortality as the outcome, associations with categorical variables and mortality were analyzed by chi-square test. Non-normally distributed continuous data are reported as median and interquartile range and were compared by Wilcoxon rank-sum test. For correlations between continuous variables and ICU and hospital LOS, the spearman correlation test was utilized. Multivariate logistic regression was performed to evaluate the association between L0 and the odds of death with adjustments made for age, prior comorbidity, PRISM-III score, need for vasoactive medication, and blood source (arterial vs. capillary vs. venous). For multivariate analysis of the secondary outcomes, ICU and hospital LOS, these variables were log transformed. The linear regression analysis was then adjusted for PRISM-III score, need for vasoactive medication, and blood source (arterial vs. capillary vs. venous) to evaluate the association between L0 and lengths of stay. A p-value of < 0.05 was accepted as being statistically significant in all analyses. Analyses were performed using STATA statistical software, version 13.1 (StataCorp, College Station, Texas).
Results
Analyses for Entire Cohort
Over the nearly three year period, 172 patients admitted to the PICU met criteria for severe sepsis or septic shock. The in-hospital mortality of these 172 patients was 11% (n= 18). Seventy-four (43%) of these patients had a lactate measured within 3 hours of meeting severe sepsis criteria (L0), and were therefore included in the study. When comparing to those without an early lactate measurement, those with L0 had a significantly higher median PRISM-III score (9, IQR 3–14 vs. 6, IQR 2–11, p=0.02) and higher mortality (16% vs 6%, p=0.03). There were no differences in age, gender, prior comorbidity, need for vasoactive medication, or mechanical ventilator use between those with and without an early lactate check.
Analyses for those with Early Lactate Measurements
Table 1 displays the baseline characteristics of the 74 patients included in the study. The median L0 was 2.5mmol/L (IQR 1.5–4.4), with the highest L0 being 17.3mmol/L. Nineteen (26%) of the lactate measurements were from an arterial source, 48 (65%) from a venous source, and 6 (8%) from a capillary source. Median lactate measurements were significantly different between the three sources: venous 2.9mmol/L (IQR 1.8–5.2), capillary 2.3mmol/L (IQR 1.5–5.2), and arterial 1.6mmol/L (IQR 1.0–2.7), p = 0.03. There was no association between L0 and age, day 1 PELOD, prior comorbidity, vasoactive use, or mechanical ventilator use. There was, however, a significant, positive correlation between L0 and PRISM-III score (rho 0.33, p<0.01, Figure 1). In unadjusted analysis, higher values of L0 were associated with higher PRISM-III scores, with an increase in mean PRISM-III score of 1.12 units for every 1mmol/L increase in L0 (95% CI 0.4 to 1.8, p = 0.003) with L0 accounting for 12% of the variability in PRISM-III scores between patients.
Table 1.
Baseline characteristics of 74 patients with a lactate drawn within 3 hours of meeting severe sepsis or septic shock criteria
| Characteristic | n = 74 |
|---|---|
| Age, median (IQR), year | 6.1 (1.7–3.9) |
| Male gender, n (%) | 35 (47%) |
| Prior Comorbidity, n (%) | 36 (49%) |
| Oncologic/Immunocompromised, n (%) | 11 (14.9%) |
| Initial lactate, median (IQR), mmol/L | 2.5 (1.5–4.4) |
| PRISM-III a, median (IQR) | 9 (3–14) |
| Day 1 PELOD a, median (IQR) | 11 (10–20) |
| Vasoactive use, n (%) | 38 (51%) |
| Mechanical ventilation, n (%) | 36 (49%) |
| Shock, n (%) | 3 (4%) |
| Mortality, n (%) | 12 (16%) |
| PICU LOS a, median (IQR), days | 4 (2–12) |
| Hospital LOS a, median (IQR), days | 10 (5–22) |
Abbreviations: PRISM-III = pediatric risk of mortality score; PELOD = pediatric logistic organ dysfunction score; LOS = length of stay
Figure 1. Scatter Plot of Lactate levels measured at time of severe sepsis or septic shock diagnosis (L0) and PRISM-III Score.
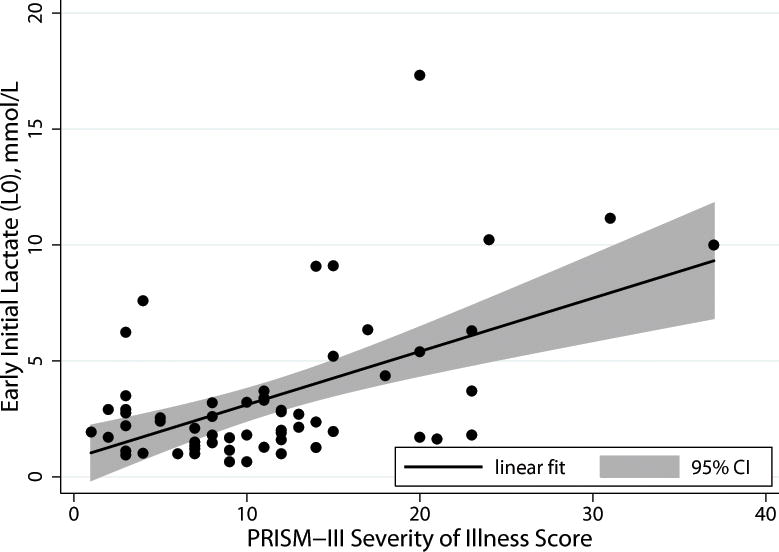
Initial lactate levels correlated with PRISM-III score, a severity of illness score well validated for mortality prediction in pediatric critical illness, rho 0.33, p<0.01
Analyses for Primary Outcome: Mortality
Of the 74 patients with an L0, 12 (16%) died. Table 2 compares the characteristics of the survivors vs. non-survivors. The median L0 in the non-survivors (3.6mmol/L, IQR 2.0–9.0) was not significantly different from that in the survivors (2.3mmol/L, IQR 1.4–3.5), p=0.11. Notably, those who died were younger, had higher median PRISM-III scores, higher median day 1 PELOD scores, and required mechanical ventilation more often. There was no difference in proportion who died based on whether they were transferred from another hospital or admitted directly from our emergency department, nor did mortality differ by whether they received some volume resuscitation and/or antibiotics before or after their first lactate measurement. In multivariate logistic regression assessing for independent association with L0, with adjustment for age, prior comorbidity, PRISM-III, and need for vasoactive medications, only PRISM-III remained statistically significantly associated with in-hospital mortality, with the odds of in-hospital mortality increasing by 12% for every 1 point increase in PRISM-III score (aOR 1.12, 95% CI 1.0–1.26 p=0.05) (Table 3).
Table 2.
Comparison of characteristics between non-survivors and survivors
| Characteristic | Non-Survivors (n=12) |
Survivors (n=62) |
p-value |
|---|---|---|---|
| Age, median (IQR), years | 2.0 (0.9–5.7) | 7.3 (1.9–14.7) | 0.05a |
| Male gender, n (%) | 7 (58%) | 28 (45%) | 0.4b |
| Prior Comorbidity, n (%) | 5 (42%) | 31 (50%) | 0.6b |
| Oncologic/Immunocompromised, n (%) | 1 (8%) | 10 (16%) | 0.49b |
| Initial lactate, median (IQR), mmol/L | 3.6 (2.0–9.0) | 2.3 (1.4–3.5) | 0.11a |
| Vasoactive use, n (%) | 9 (75%) | 29 (47%) | 0.07b |
| Mechanical Ventilation, n (%) | 10 (83%) | 26 (42%) | <0.01b |
| PRISM-III, median (IQR) | 19 (13.5–30.5) | 8 (3–12) | <0.001a |
| Day 1 PELOD, median (IQR) | 21.5 (11–26.5) | 11 (10–13) | <0.001a |
Wilcoxon rank sum,
Chi-square or Fisher exact
Table 3.
Multivariate logistic regression demonstrating the adjusted OR of in-hospital mortality
| Variable | Adjusted OR (95% CI) |
p-value |
|---|---|---|
| Initial lactate, mmol/La | 1.06 (0.84–1.36) | 0.6 |
| PRISM-IIIa | 1.12 (1.00–1.26) | 0.05 |
| Vasoactive use | 1.95 (0.3–13.4) | 0.5 |
| Prior Comorbidity | 0.94 (0.18–4.80) | 0.9 |
| Age, yearsa | 0.87 (0.94–1.03) | 0.1 |
| Lactate source | 0.3 | |
| Arterial | Ref. | |
| Capillary | 1.0 | |
| Venous | 0.4 (0.07–2.3) |
The adjusted OR noted represents the odds of mortality for any 1 unit increase in the variable of interest
Analyses for Secondary Clinical Outcomes
The median PICU LOS was 4 days (IQR 2–12). Neither L0, age, gender, PRISM-III, day 1 PELOD, nor vasoactive use were significantly associated with PICU LOS. The presence of prior comorbidity and the need for invasive mechanical ventilation were the only variables associated with a longer PICU LOS on both univariate and multivariate analyses. The median hospital LOS was 10 days (IQR 5–22). Neither L0, age, gender, vasoactive need, PRISM-III nor day 1 PELOD were significantly associated with hospital LOS. Similar to ICU LOS, children who reported a prior comorbidity or required mechanical ventilation during their illness had a longer hospital LOS.
Discussion
In this cohort of children prospectively diagnosed with severe sepsis or septic shock presenting to the pediatric ICU of a large academic referral hospital we found that an early, initial lactate level was not associated with higher in-hospital mortality, or longer ICU and hospital stays. We did, however, find that early, initial lactate levels significantly correlate with PRISM-III, a well validated severity of illness score collected over the first 24 hours of hospital admission and used for prognostic purposes. In fact an early elevated lactate accounted for 12% of the variability in PRISM-III scores within the cohort, even though it is not a variable used to calculate the score.
Over 75,000 children in the United States develop severe sepsis per year with mortality rates of 10–20% (1–3), with worse outcomes reported in less developed countries. Failure to recognize and treat shock has been identified as a cause of preventable morbidity in children (6,7). Times of transfer from the emergency department to the pediatric ICU or between hospitals are critical hours where invasive monitoring is unavailable and lapses in care may occur, particularly if severity of illness is difficult to assess. Because rapid identification of the children at highest risk is critical to improving care in pediatric sepsis, recent studies have converged to highlight the need for objective measures with which to diagnose pediatric sepsis and monitor progression of disease.
Because preventable morbidity and mortality from sepsis are frequently due to failure to recognize and aggressively treat shock, a physiologic measure that reflects perfusion and metabolic impairments may be valuable for the diagnosis and prognosis of sepsis. Lactate, a by-product of glucose metabolism, is formed by the reduction of pyruvate during periods of anaerobic metabolism (26). In states of decreased oxygen delivery or impaired oxygen utilization, anaerobic metabolism leads to hyperlactatemia, making lactate levels a potentially objective, inexpensive, and minimally invasive measure that clinicians may utilize in real time to diagnose and assess states of shock.
While there is a plethora of adult literature presenting an association between increased early lactate concentration and poor outcome in septic patients (10–13), lactate is not routinely evaluated in pediatric patients. As a result, studies in children with sepsis evaluating the association of elevated lactate levels and poor clinical outcomes are challenging and the scant data available is inconclusive. Duke et al. evaluated the association of serial lactate measurements in 31 pediatric patients admitted to an Australian PICU with the diagnosis of severe sepsis and septic shock (21). They found a positive association between mortality and elevated lactate levels drawn at 12 and 24 hour after PICU admission, but no association with earlier lactate levels, such as those collected upon admission to the PICU. Elevated lactate levels correlated with a greater degree of organ dysfunction and worse survival much sooner than other potential variables, including heart rate, mean arterial pressure, arterial pH and base deficit; yet this association was only noted after 12 hours of ICU admission and not when using lactate levels drawn at sepsis diagnosis. Kim et al. evaluated lactate levels upon admission to the PICU of 65 children with septic shock and found that lactate was significantly higher at PICU admission in the non-survivors compared to the survivors (22). While these studies reveal that elevated lactate may be associated with worse clinical outcomes, delay in measurement of lactate until arrival to the PICU or later allows for confounding by a variety of therapeutic strategies including fluid resuscitation, mechanical ventilation, and vasoactive medication use.
To our knowledge this is the first study to specifically evaluate the association of outcome and early lactate levels drawn in relation to time zero in pediatric patients with severe sepsis and septic shock. Patients were included only if a lactate level was collected within 3 hours of meeting criteria. This three hour time interval was chosen for two reasons; firstly, because it is the time frame recommended in the Surviving Sepsis Campaign (SSC) care bundle (19), and secondly, to minimize the effects of resuscitation and other therapeutic strategies on lactate values. We believe that this early lactate measurement and the resultant reduction of influence by a variety of therapies strengthens our study to appropriately answer the question of association of elevated lactate with in-hospital mortality in pediatric severe sepsis and septic shock. Conducting this study at a large, urban, tertiary, referral hospital provided a very generalizable patient population and further strengthens the quality of this work.
Although our study did not find an association between initial lactate and clinical outcomes, there was a significant association between initial lactate and PRISM-III score. This score is well validated in pediatric critical illness with high PRISM-III scores being significantly correlated with increased mortality risk. Calculating a PRISM-III score, however, is expensive, time consuming, and cannot be calculated until 12 to 24 hours of admission, rendering it ineffective for immediate clinical use and more valuable for research endeavors. Additionally, PRISM-III has not been evaluated for its prognostic ability in disease specific states, such as sepsis or septic shock. A lactate level checked as soon SIRS or sepsis are a concern, in contrast, is rapid, inexpensive, and requires little skill. A statistically significant correlation between lactate levels and these scores indicates the potential for lactate as a prognostic variable measured in the first few hours of critical illness that may enhance efficient and appropriate patient care. Further study of prognostication, prediction and validation by both early and serially elevated lactate, as well as lactate guided clinical management is needed.
Although our study offers new insight into the value of lactate as a potential prognostic marker for pediatric severe sepsis, it does have some limitations. First, this analysis was an a priori identified subanalysis of a larger, prospective pediatric severe sepsis/shock study. As such, the “n” for this analysis was inherently predetermined for the larger study rather than this sub analysis. Nonetheless, our study remains one of the largest studies of lactate in a prospectively identified cohort of pediatric severe sepsis/shock patients to date. Along similar lines, given the relatively low mortality for pediatric sepsis, in general, studies powered to detect differences in mortality require inherently large sample sizes, oft requiring multi-center participation. Indeed, our study supports consideration for other primary outcomes aside from mortality in this population, such as new and progressive multi-organ dysfunction syndrome, long-term morbidity, or impact on quality of life. Additionally, our study only evaluated the association of early elevated lactate and outcomes in patients admitted to the PICU, excluding those admitted to other areas of the hospital. While this focuses the study on patients of greatest concern, further studies are needed before conclusions can be generalized to all patients diagnosed with shock while in the emergency department.
Another limitation was the absence of a current protocolized approach to lactate measurement in our institution. This meant that many patients who either did not have any lactate collected or did not have one collected within 3 hours of meeting severe sepsis criteria were excluded. While the decision to exclude these patients decreased our sample size and may have led to directional bias, it minimized the affect of confounding variables on outcomes and allowed for a more accurate analysis of the association of early elevated lactate on outcomes. Finally, the significant difference between lactates drawn from arterial, venous, and capillary blood sources proved to be another limitation. The decision to include lactates drawn from all vessel sources was made a priori for several reasons. First the blood source may represent a form of selection bias with the sicker patients more likely to have arterial measurements. Secondly, SSC does not specify a particular vessel from which to obtain a lactate in their guidelines, and this reflects current clinical practice. Furthermore, previous research has shown that lactate levels drawn from all three blood sources closely correlate (27,28). Notably, when evaluating those with arterial lactate levels, the correlation between L0 and PRISM-III remains significant (rho 0.7, p<0.01). Further multicenter pediatric sepsis studies should consider simultaneous measurement across at least 2 different sources.
Conclusion
Lactate, while not routinely checked in pediatric patients, may be an early biomarker of disease severity in pediatric severe sepsis. As such, early measurement may provide improved and efficient risk stratification and an opportunity for timelier targeting of specific therapy. Our study did not find any association with early elevated lactate and in-hospital mortality, a finding likely impeded by a small sample size. Taken together, however, these data suggest that early lactate measurement should be included as a biomarker of severe sepsis mortality risk in any forthcoming multi-center, pediatric severe sepsis studies, and evaluated for its prognostic value in these large studies. If included in such biomarker studies, a protocolized approach to measuring lactate levels is warranted.
Acknowledgments
Source of Funding:
National Institutes of Health grants 5T32HD049303-08 (Ward), Charlotte Coleman Frey Fellowship Fund (Ward), Department of Defense TATRC W81XWH (Asselin, Fontana, Heidersbach, Flori). For the remaining authors no funding sources were declared.
Footnotes
Conflicts of Interest:
There are no conflicts of interest to disclose.
References
- 1.Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med. NIH Public Access. 2014 Nov 1;15(9):798–805. doi: 10.1097/PCC.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med. 2014 Nov;15(9):828–38. doi: 10.1097/PCC.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 3.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med. 2013 Sep;14(7):686–93. doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 4.Tiru B, DiNino EK, Orenstein A, et al. The Economic and Humanistic Burden of Severe Sepsis. Pharmacoeconomics. 2015 May 3;33(9):925–37. doi: 10.1007/s40273-015-0282-y. [DOI] [PubMed] [Google Scholar]
- 5.Burchardi H, Schneider H. Economic aspects of severe sepsis: a review of intensive care unit costs, cost of illness and cost effectiveness of therapy. Pharmacoeconomics. 2004 Jan;22(12):793–813. doi: 10.2165/00019053-200422120-00003. [DOI] [PubMed] [Google Scholar]
- 6.Han YY, Carcillo JA, Dragotta MA, et al. Early Reversal of Pediatric-Neonatal Septic Shock by Community Physicians Is Associated With Improved Outcome. Pediatrics. 2003 Oct 1;112(4):793–9. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 7.Inwald DP, Tasker RC, Peters MJ, Nadel S. Emergency management of children with severe sepsis in the United Kingdom: the results of the Paediatric Intensive Care Society sepsis audit. Arch Dis Child. 2009 May 1;94(5):348–53. doi: 10.1136/adc.2008.153064. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Roberts D, Wood KE, Light B, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Haery C, Paladugu B, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006 Jan 15;193(2):251–8. doi: 10.1086/498909. [DOI] [PubMed] [Google Scholar]
- 10.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009 May;37(5):1670–7. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005 May 5;45(5):524–8. doi: 10.1016/j.annemergmed.2004.12.006. Elsevier. [DOI] [PubMed] [Google Scholar]
- 12.Trzeciak S, Dellinger RP, Chansky ME, et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007 Jun;33(6):970–7. doi: 10.1007/s00134-007-0563-9. [DOI] [PubMed] [Google Scholar]
- 13.Howell MD, Donnino M, Clardy P, et al. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007 Jul 6;33(11):1892–9. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 14.Callaway DW, Shapiro NI, Donnino MW, et al. Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. J Trauma. 2009 Apr;66(4):1040–4. doi: 10.1097/TA.0b013e3181895e9e. [DOI] [PubMed] [Google Scholar]
- 15.Vandromme MJ, Griffin RL, Weinberg JA, et al. Lactate is a better predictor than systolic blood pressure for determining blood requirement and mortality: could prehospital measures improve trauma triage? J Am Coll Surg. 2010 May;210(5):861–7. doi: 10.1016/j.jamcollsurg.2010.01.012. 867–9. [DOI] [PubMed] [Google Scholar]
- 16.Guyette F, Suffoletto B, Castillo J-L, et al. Prehospital serum lactate as a predictor of outcomes in trauma patients: a retrospective observational study. J Trauma. 2011 Apr;70(4):782–6. doi: 10.1097/TA.0b013e318210f5c9. [DOI] [PubMed] [Google Scholar]
- 17.Khosravani H, Shahpori R, Stelfox HT, et al. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. 2009 Jan;13(3):R90. doi: 10.1186/cc7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichol AD, Egi M, Pettila V, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. BioMed Central. 2010 Jan;14(1):R25. doi: 10.1186/cc8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013 Feb;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 20.Hatherill M, McIntyre AG, Wattie M, Murdoch IA. Early hyperlactataemia in critically ill children. Intensive Care Med. 2000 Mar;26(3):314–8. doi: 10.1007/s001340051155. [DOI] [PubMed] [Google Scholar]
- 21.Duke TD, Butt W, South M. Predictors of mortality and multiple organ failure in children with sepsis. Intensive Care Med. 1997;23(6):684–92. doi: 10.1007/s001340050394. [DOI] [PubMed] [Google Scholar]
- 22.Kim Ya, Ha EJ, Jhang WK, Park SJ. Early blood lactate area as a prognostic marker in pediatric septic shock. Intensive Care Med. 2013;39(10):1818–23. doi: 10.1007/s00134-013-2959-z. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2005 doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 24.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996 May;24(5):743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Leteurtre S, Martinot A, Duhamel A, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making. Jan;19(4):399–410. doi: 10.1177/0272989X9901900408. [DOI] [PubMed] [Google Scholar]
- 26.Cohen RD, Woods HF. Clinical and Biochemical Aspects of Lactic Acidosis. Blackwell Scientific Publications; 1976. p. 276. [Google Scholar]
- 27.Lavery RF, Livingston DH, Tortella BJ, et al. The utility of venous lactate to triage injured patients in the trauma center. J Am Coll Surg. 2000 Jun;190(6):656–64. doi: 10.1016/s1072-7515(00)00271-4. [DOI] [PubMed] [Google Scholar]
- 28.Pattharanitima P, Tongyoo S, Ratanarat R, et al. Correlation of arterial, central venous and capillary lactate levels in septic shock patients. J Med Assoc Thai. 2011 Feb;94(Suppl 1):S175–80. [PubMed] [Google Scholar]


