Abstract
All immune cells depend on specific and efficient metabolic pathways to mount an appropriate response. Over the past decade, the field of immunometabolism has expanded our understanding of the various means by which cells modulate metabolism to achieve the effector functions necessary to fight infection or maintain homeostasis. Harnessing these metabolic pathways to manipulate inappropriate immune responses as a therapeutic strategy in cancer and autoimmunity has received increasing scrutiny by the scientific community. Fine tuning immunometabolism to provide the desired response, or prevent a deleterious response, is an attractive alternative to chemotherapy or overt immunosuppression. The various metabolic pathways used by immune cells in rheumatoid arthritis, systemic lupus erythematosus and osteoarthritis offer numerous opportunities for selective targeting of specific immune cell subsets to manipulate cellular metabolism for therapeutic benefit in these rheumatologic diseases.
Inflammatory and autoimmune diseases are driven by the activation and effector functions of both innate and adaptive immune cells. In addition to neutrophils and other cells involved in acute inflammation, macrophages and dendritic cells are activated to promote T and B lymphocyte responses in rheumatologic diseases such as systemic lupus erythematosus (SLE)1 and rheumatoid arthritis (RA)2. Osteoarthritis (OA), although generally considered non-inflammatory, can present with an inflammatory phenotype and the inflammatory processes involved in this disease are increasingly recognized3. In each of these diseases, inflammatory cytokines stimulate immune cells4 or monocyte-to-osteoclast differentiation to promote autoimmunity or bone resorption and degradation5,6. Although these rheumatologic diseases have unique characteristics, in each setting haematopoietic cells must be stimulated to gain effector functions and differentiate. The signalling and gene expression changes that accompany these cellular activation and differentiation events have been well studied, but it is now apparent that the metabolism of disease-effector cells is also tightly regulated6–9. Each inflammatory cell, and even anti-inflammatory cell, undergoes metabolic reprogramming upon activation and these changes are essential for disease. Therefore, targeting the metabolic pathways involved offers a new avenue for potential treatment of rheumatologic diseases. Because immunological functions are associated with specific metabolic programmes, this approach affords the particularly attractive possibility that inhibiting the appropriate pathway could lead to selective, cell-specific blockade. In this Perspectives article, we discuss the various metabolic pathways used by immune cells to attain optimal responses and explore the possibility and key principles of manipulating these pathways for therapeutic benefit in rheumatologic diseases, with a focus on RA, SLE and OA.
Cellular metabolic reprogramming
Activation of immune cells leads to changes in metabolic pathways
Resting lymphocytes, macrophages and dendritic cells all use catabolic metabolic pathways that switch to anabolic programmes after activation by antigens, cytokines or stimulation of innate pattern-recognition receptors by pathogen-associated or damage-associated molecular patterns (PAMPs and DAMPs, respectively)7. This switch supports resting cell survival and immune surveillance as well as growth and effector function of stimulated cells. Resting T cells take up glucose, amino acids and lipids at a low rate and flux these fuels through glycolysis, glutaminolysis and fatty acid oxidation to maximize mitochondrial oxidative metabolism7. This mode of metabolism generates maximal ATP and is associated with a long T cell lifespan10–13. Given the need to maintain osmolarity through the sodium–potassium ATPase and the energy demands of rapid chemotaxis and cytoskeletal remodelling during this surveillance mode of resting lymphocytes14, it is not surprising that metabolism in resting immune cells is programmed to actively support the most efficient ATP-generating processes.
Lymphocyte stimulation leads to abrupt changes in metabolic pathways in these cells. Stimulation of T cells through the T cell receptor in conjunction with co-stimulation leads to a sharp increase in glycolysis and glutaminolysis15–17 (FIG. 1). Simultaneously, activated T cells decrease mitochondrial fatty acid oxidation in order to conserve lipids for new membrane synthesis18,19. Co-stimulatory signals have key roles in this transition; CD28 augments glucose uptake and glycolysis in activated T cells16, whereas inhibitory receptors, such as cytotoxic T-lymphocyte protein 4 (CTLA4) and programmed cell death protein 1 (PD-1), can decrease glycolysis and instead promote mitochondrial fatty acid oxidation15,20–22. In part, these regulators act through control of signalling via phosphatidyl-inositol 3-kinase (PI3K), AKT and mechanistic target of rapamycin (mTOR)23. Resting B cells undergo a similar metabolic shift upon activation. Stimulation of B cells through antigen receptors or Toll-like receptors (TLRs) leads to upregulation of the glucose transporter GLUT1 and glycolysis24,25. As in T cells, this metabolic reprogramming is dependent on mTOR signalling, as deficiency of regulatory-associated protein of mTOR (RAPTOR) and mTOR complex I (mTORC1) or alteration of the PI3K pathway disrupts B cell development and activation, and can impair class-switching in germinal centres26–28. Ultimately, as an immune response ceases, memory lymphocytes revert to oxidative pathways that are essential to enabling persistence of memory and robust secondary responses10,12. Memory lymphocytes can, however, retain enhanced metabolic features that facilitate rapid and strong secondary responses29,30.
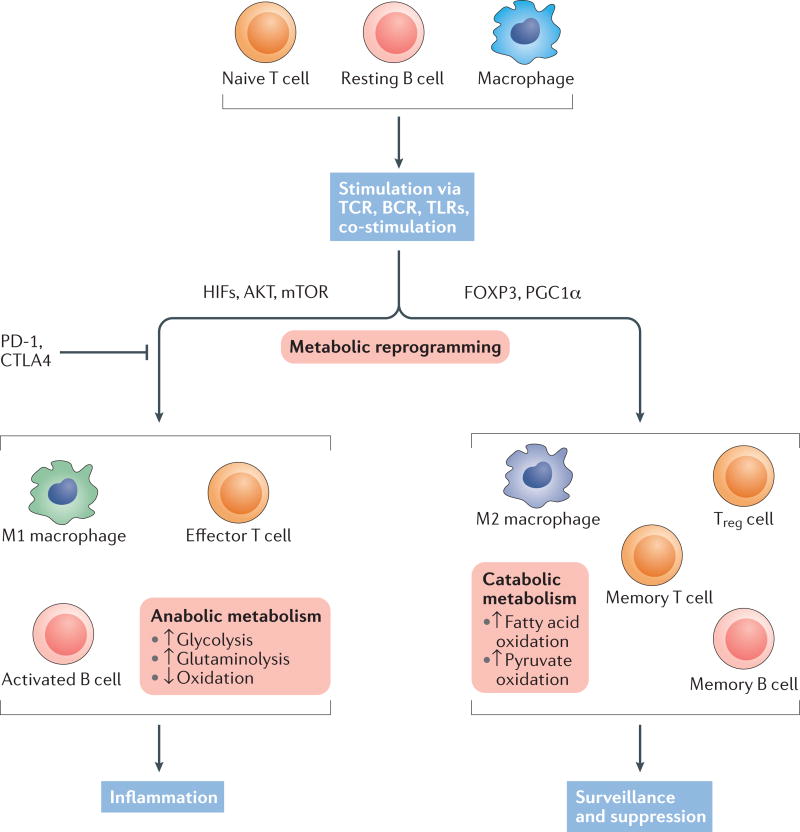
Figure 1. Metabolic reprogramming of immune cell populations matches immunological function.
Naive T cells, resting B cells and macrophages utilize a catabolic and oxidative metabolic programme. After stimulation via antigen receptor with co-stimulation or through pattern-recognition receptors such as Toll-like receptors (TLRs), these immune cells undergo metabolic reprogramming. Effector lymphocytes or inflammatory macrophages induce an anabolic meta bolic programme with highly increased nutrient uptake for glycolysis and glutamine metabolism. Regulatory cells or alternatively activated macrophages, by contrast, primarily utilize a programme of lipid and pyruvate oxidation. These programmes are important to the function of each subset; if the cellular metabolism does not match the cell fate, immune cells will fail to gain appropriate functional capacity. BCR, B cell receptor; CTLA-4, cytotoxic T lymphocyte protein 4; HIF, hypoxia-inducible factor; mTOR, mechanistic target of rapamycin; PD-1, programmed cell death protein 1; PGCla, peroxisome proliferator-activated receptor γ co-activator 1-α; TCR, T cell receptor; Treg cell, regulatory T cell.
Dendritic cells and macrophages differ from lymphocytes in that proliferation is not as important a cellular goal following activation. The ability to mature and gain effector function (including the differentiation of monocytes into osteoclasts) is, however, essential for these cells. Macrophages and dendritic cells are activated in response to PAMPs and DAMPs, including TLRs, and this activation increases glycolysis to promote inflammatory function and maturation31–35. TLR signalling through serine/threonine-protein kinase TBK1 leads to AKT activation and mTORC1 signalling to promote this glycolytic switch36–38. Increased glycolysis both promotes inflammation and can enhance ‘trained immunity’, a process that, although not specific in the same way as adaptive immune responses, can lead to improved secondary innate responses32,39. In addition to enabling enhanced biosynthesis of effector molecules and cytokines, this metabolic reprogramming supports the growth of essential cell structures, such as the endoplasmic reticulum and Golgi37, which have critical roles in the cell biology of effector function.
Metabolic programmes are specific for immune cell subsets and functions
A critical aspect of the metabolic reprogramming events described above is that they are not uniform in a given cell type, but instead utilize specific pathways that are essential for particular cell subsets and functions (FIG. 1). This specificity was first demonstrated in classical ‘M1’ macrophages and alternatively activated ‘M2’ macrophages, in which activation with IL-4 led to a peroxisome proliferator-activated receptor γ co-activator 1-β (PGC1β)-dependent increase in oxidative metabolism that contrasted with the more glycolytic metabolism of macrophages activated by IFNγ and the TLR4 ligand lipopolysaccharide40. Indeed, these metabolic pathways were linked to the functions of the cells, as promoting increased glucose uptake by GLUT1 expression enhanced proinflammatory macrophage activity35, whereas promoting mitochondrial lipid oxidative pathways stimulated anti-inflammatory macrophage function40,41.
Although OA is characterized by subchondral sclerosis42, inflammation and innate immunity can contribute substantially to disease pathogenesis3. The differentiation of monocytes/macrophages into osteoclasts that contribute to inflammation in OA also depends on specific metabolic programmes. In particular, hypoxia and the hypoxia-inducible factors HIF-1α and HIF-2α promote osteoclast differentiation43,44. Increased lactic acid, which can suppress glycolysis, also promotes generation of osteoclasts45. Together, these findings support the model that different macrophage-derived subsets have distinct metabolic programmes that promote, and are intimately linked to, cell function and fate.
Subsequent to these early studies in macrophages, T cell subsets were also found to utilize distinct metabolic programmes46, with particular differences noted between regulatory T (Treg) cells and CD4+ effector subsets, including type 1 T helper (TH1), TH2, and TH17 cells19,46,47. Effector T cells are largely glycolytic downstream of mTOR signals48 that differentially affect specific CD4 subsets through mTORC1 or mTORC2 (REF. 49), whereas Treg cells preferentially utilize a mitochondrial oxidative metabolism consisting of lipid and pyruvate oxidation19,46,47. Indeed, whereas lipid synthesis is required for TH17 cells, and overproduction of lipids can lead to T cell phenotypes associated with autoimmunity50, lipid oxidation promotes Treg cell differentiation51. This alternative metabolic programme is regulated by the Treg cell transcription factor FOXP3 (REFS 52,53) as well as by PGC1α and hSIRT3 (also known as NAD-dependent protein deacetylase sirtuin-3, mitochondrial)54. In vivo, effector T cells depend on GLUT1 (REF. 55) as well as the amino acid transporters solute carrier family 1 member 5 (SLC1A5, also known as ASCT2 or neutral amino acid transporter B(0))56 and solute carrier family 7 member 5 (SLC7A5, also known as large neutral amino acids transporter small subunit 1 or LAT1)57, whereas Treg cells can function independently of these transporters55–57. Treg cells can, however, initiate glycolysis, in a manner dependent on mTORC1 activation for proliferation58–60 following activating or inflammatory signals52. Increased glycolysis in Treg cells augmented proliferation but also reduced the suppressive capacity of these cells52. This switch between maximal Treg cell proliferation or suppressive capacity was controlled in part by the PI3K–AKT–mTORC1 pathway, and constitutive activation of AKT or mTORC1 led to accumulation of poorly suppressive Treg with low phenotypic stability52,61–63. Tight regulation of mTOR activity is thus required for Treg cell function. In other CD4+ T cell subsets, such as T follicular helper cells, metabolism seems to be more balanced and relies on both glycolysis and oxidative phosphorylation64,65. Metabolism in macrophages and dendritic cells is also regulated by mTORC1 and mTORC2 signalling66,67. In particular, signalling through mTORC1 can promote glycolysis, which can enhance M1 macrophage activation35,41,66, whereas M2 macrophages utilize oxidative metabolism that is regulated by signal transducer and activator of transcription 6 (STAT6) and PGC1β40. Inhibition of mTOR kinase can, therefore, alter macrophage metabolism and might affect macrophage subsets.
Immunometabolism in disease
Chronic encounters with autoantigens and inflammatory signals can sharply alter immunometabolism in ways that differ from the response to acute stimulation. Indeed, chronic viral infections diminished glucose metabolism in T cells68. Alterations in immunometabolism in inflammatory diseases reveals insight into disease processes and potential therapeutic targets.
Systemic lupus erythematosus
Metabolomics analyses of sera from patients with SLE have revealed a variety of considerable alterations in metabolites and metabolic pathways that correlate with disease activity and manifestations69–71. Although serum metabolites can be affected by multiple cell types and tissues, several metabolic pathways have been shown to differ between T cells of healthy individuals and patients with SLE, and between healthy and lupus-prone animals. Mitochondrial glucose oxidation can be increased72 and mitochondria have been shown to be hyperpolarized in chronically activated T cells in SLE73,74. Persistent mitochondrial hyperpolarization leads to production of reactive oxygen species (ROS), which can sensitize T cells to necrosis, leading to the release of self-antigens and perpetuation of the autoimmune response75. The Sle1c locus conferred chronic CD4+ T cell activation in the NZB mouse model of lupus76. This locus can be further divided, and the Sle1c2 susceptibility locus contains only two genes, one of which, Esrrg, encodes oestrogen-related receptor γ (ERRγ), a nuclear receptor that regulates oxidative phosphorylation and mitochondrial function. Studies by Perry et al. in CD4+ T cells from mice expressing the Sle1c2 locus showed decreased mitochondrial mass and chronic mitochondrial hyperpolarization compared with wild-type CD4+ T cells77. Interestingly, B6.Sle1c2 CD4+ T cells produced more IFNγ than controls. Increased proliferation and activation of B6.Sle1c2 CD4+ T cells could be attributable to decreased expression of ERRγ — in breast cancer cells, a decrease in levels of ERRγ led the cells to undergo aerobic glycolysis and expend ATP78. Although Perry et al.77 did not demonstrate that decreased Essrg expression in Sle1c2 CD4+ T cells, or the effects of this decrease on mitochondrial function, were directly responsible for increased TH1 skewing, studies have shown that increased glycolysis due to overexpression of GLUT1 in CD4+ T cells increases IFNγ production16. Most importantly, the studies in B6.Sle1c2 mice further confirm a role for mitochondrial metabolism in rheumatologic diseases and suggest that altered T cell metabolism is, in part, genetically programmed.
In addition to changes in glucose metabolism, CD4+ T cells from patients with SLE also display defects in lipid metabolism. T cells from these patients show increased levels of glycosphingolipids and cholesterol, as well as increased expression of the nuclear receptor oxysterols receptor LXRβ (also known as liver X receptor β), which has a role in cellular lipid metabolism and trafficking79,80. Treatment of CD4+ T cells from patients with SLE with an LXR antagonist led to decreased glycosphingolipid production, and blockade of glycosphingolipid biosynthesis in these cells restored normal T cell function50.
Whole-body metabolism can also be affected in SLE, which could influence autoimmunity. Although the underlying mechanisms are poorly understood, patients with SLE had significantly elevated fasting levels of insulin, indicating a predilection for insulin resistance and metabolic disease81. This phenomenon was recapitulated in a mouse model of lupus whereby B6.Sle1.Sle2.Sle3 mice spontaneously developed glucose intolerance without being fed a high-fat diet82. Whereas immune dysfunction might contribute directly to the sequelae of metabolic syndrome, such as atherosclerosis83, altered metabolic hormones and lipids can also modulate immunity, promoting B cell dysfunction82 and effector T cell differentiation and function84–86.
Rheumatoid arthritis
Chronic stimulation and the synovial microenvironment alters T cell metabolism in RA. T cells of patients with RA have reduced expression of 6-phosphofructo 2-kinase/fructose-2, 6-bisphosphatase 3 (PFKFB3)87. This enzyme is a key regulator of fructose-2, 6-bisphospate, the allosteric activator of phospho fructokinase, and lower PFKFB3 will lower glycolysis while increasing flux to the pentose phosphate pathway and generation of NADPH7,87. Elevated NADPH can neutralize ROS, which, although damaging at high concentrations, are otherwise essential to promote T cell activation88. Indeed, restoration of T cell ROS could suppress synovial inflammation89. In addition to direct changes in T cells, the hypoxic environment in the RA synovium90 creates a situation similar to the chronic mitochondrial hyper-polarization seen in SLE. The formation of the synovial pannus restricts the availability of oxygen to infiltrating immune cells, which might contribute to altered glucose and mitochondrial metabolism90.
Osteoarthritis
Altered metabolism contributes to OA but the underlying mechanisms are less firmly established than in SLE or RA. Nevertheless, increased glucose uptake, as determined by 18F-fluorodeoxyglucose PET imaging, correlated with OA progression91. The hypoxic environment of the OA synovium might promote osteoclast differentiation and function9. Furthermore, metabolic syndrome can exacerbate OA92, and advanced glycation end products (AGEs) can activate the AGE-specific receptor (RAGE) to impair osteoblast growth and function and promote receptor activator of NF-κB ligand (RANKL, also known as TNF ligand superfamily member 11) and osteoclastogenesis92,93. Indeed, chondrocyte-synthesized RANKL might promote bone destruction in OA94. The role for mitochondria in osteoclast differentiation was established by genetic deletion of a component of electron transport complex I, Ndufs4, in mice. Deletion of Ndufs4 led to greater differentiation of precursor cells into macrophages rather than osteoclasts95, supporting a model in which mitochondrial oxidative metabolism promotes osteoclastogenesis. This balance is complicated, with oxidative metabolism seemingly important for osteoclast differentiation and glycolysis seemingly important for bone resorption96.
Targeting immunometabolism
Rationale for targeting immunometabo-lism in rheumatologic diseases
Given the metabolic changes associated with immune cell activation and function, as well as the altered metabolism of T cells, macrophages and dendritic cells in rheumatologic diseases, a key question is to what extent is it possible to target metabolism with new therapies? The observation of aerobic glycolysis (the Warburg effect) in cancer cells has led to cell metabolism being considered an attractive potential target for cancer treatment for a number of years97. However, the effects of strategies directly inhibiting metabolic pathways have been disappointing or generally modest97. One very important difference between successful cancer therapies and successful therapies to control inflammatory diseases is that cancer cells must be fully eliminated, whereas simply halting effector function would be sufficient in immunologic diseases. When targeting immunometabolism in autoimmunity, therefore, blocking a metabolic pathway to the extent that apoptosis is induced is not necessary98. Rather, it is essential only to impair a pathway sufficiently so as to alter specific cell functions. A variety of pathways could, in principle, be targeted to modulate an immune response. Effector T cells, for example, require high rates of glycolysis and amino acid uptake, whereas Treg cells are less dependent on or can even be independent of these pathways55–57. Therefore, it is reasonable to hypothesize that inhibition or modulation of glycolytic pathways could shift the balance of effector and regulatory T cell subsets to provide a favourable outcome in autoimmune disorders. Each of these pathways has multiple metabolic steps and specific enzymes or nutrient transporters amenable to pharmacologic intervention.
Principles of targeting immunometabolism in rheumatologic diseases
Several key principles will dictate approaches to pharmacologic modulation of immunometabolism in rheumatologic diseases (BOX 1). For example, unlike kinase signalling pathways, metabolic pathways are not generally amplificatory and weak inhibitors might be most useful. With kinases, the potential for exponential expansion of signalling cascades typically makes it essential to inhibit the vast majority of kinase activity to elicit a functional effect. Metabolic pathways, by contrast, are limited by the levels of metabolites and conservation of mass. Thus, modest inhibition of a kinase might achieve little, but modest inhibition of a metabolic pathway could have a strong effect. This paradigm is evident in the action of metformin, a weak inhibitor of mitochondrial electron transport complex I99 that can nonetheless leads to multiple effects that modify cell function and survival, including reducing TH17 cells and osteoclasts in a model of RA100 and promoting Treg cell differentiation46,100. It stands to reason that this treatment strategy would also be beneficial in other autoimmune disorders characterized by effector T cell dysregulation, such as SLE. Additionally, specificity of a therapeutic approach targeting metabolic pathways can arise not only from restricted expression of the target, but from the dependence of specific cell populations on specific metabolic pathways. Ideally, a pharmacologic target would be selectively expressed only in the target cell type. However, an equivalent outcome can be achieved if the drug target is only essential in a specific population of cells. This seems to be the case for many potential targets in immunometabolism. Such a strategy could be employed by inhibiting HIF-1α to block the development of TH17 cells and promote Treg cell differentiation in RA and OA. HIF-1α is specifically required for glycolysis in TH17 cells, and does not play a part in other T cell subsets. Thus, although fundamental metabolic pathways might be shared, the selective reliance of immune cell subsets or populations on specific metabolic programmes renders those cell populations susceptible to inhibition.
Box 1. Key principles in immunometabolism pharmacology.
Specificity
A critical goal in targeting any pathway is specificity for a population of cells that drives the disease phenotype. Because metabolic pathways are, in principle, shared between all cells, target specificity is a concern when developing new therapies. However, despite potentially shared expression of enzymes, specificity arises from the requirements of immune cells to maintain high metabolic fluxes through specific pathways to elicit specific functions.
Redundancy
Typically, multiple isoforms of each enzyme or multiple transporters for each nutrient exist. Only specific cell populations rely on a given enzyme isoform or transporter, so inhibition of these proteins will affect only that particular population of cells.
Plasticity
Metabolic pathways can adapt to shifts in nutrient availability. Thus, blockade of a specific pathway can simply elicit plasticity and many cells can adjust to bypass the block or to utilize a different pathway. However, these changes in the cellular metabolic programme can modify the function of immune cells. A shift in pathways that might be insufficient to induce apoptosis or block proliferation might nevertheless shift the fate of a T cell or macrophage to reduce or modify inflammatory function.
Partial inhibition
Because metabolic pathways are limited by conservation of mass and, unlike kinase signalling cascades, do not generally amplify, a partial inhibition can lead to a large functional effect.
Durability of response
Concerns of adverse effects will be reduced if the fate of immune cells is shifted so as to elicit durable responses to time-limited or episodic treatment.
Several strategies might be used to modulate immunometabolism in rheumatologic diseases. In addition to targeting key metabolic regulatory signalling pathways, such as the mTOR pathway48,49, or direct inhibition of metabolic events, such as nutrient uptake or enzyme function, metabolic pathways could be modulated at bifurcation points in order to shift metabolic flux from one pathway to another. Pyruvate metabolism might provide such a target. Two of the major fates of pyruvate are conversion to lactate by lactate dehydrogenase (LDH) or uptake into mitochondria to generate acetyl-CoA for oxidation by pyruvate dehydrogenase (PDH). Inflammatory effector T cells favour pyruvate conversion to lactate, whereas Treg cells favour pyruvate oxidation19. The flux of pyruvate towards lactate or acetyl-CoA can be regulated by PDH kinase (PDHK) phosphorylation and the inhibition of PDH. Thus, effector T cells utilize PDHK to maintain LDH-mediated conversion of pyruvate to lactate. Inhibition of PDHK relieves PDH inhibition to promote pyruvate conversion to acetyl-CoA and impairs effector T cell function while promoting Treg cell differentiation. This strategy has shown promise in relieving inflammation and promoting Treg cells in models of disease including collagen-induced arthritis101, asthma102, alloreactivity103 and experimental autoimmune encephalitis (EAE)19.
Immunometabolic therapeutic targets
There are many potential targets from which to choose to modulate autoimmunity and improve rheumatologic disease outcomes. Some metabolic processes are already targeted by standard of care treatments for these diseases. Methotrexate, for instance, has many modes of action, including potential inhibition of Janus kinase (JAK)– STAT signalling104. Inhibition of one-carbon metabolism (a network of pathways involved in amino acid metabolism and nucleotide synthesis) by methotrexate might also have important inhibitory functions on cell growth, redox balance and epigenetics105. Other key areas could also provide focal points for new drug development (FIG. 2); indeed, several examples now exist in which pharmacologic targeting of metabolism has had protective effects against immune-mediated diseases. In an important proof-of-principle study, inhibition of T cell metabolic pathways protected lupus-prone mice from disease: Yin et al. showed that treatment with the non-metabolizable glucose analogue 2-deoxy-d-glucose (2-DG) plus metformin reversed cytokine and autoantibody production in an animal model of lupus106. Furthermore, in vitro production of IFNγ by T cells from patients with SLE was normalized by metformin treatment. The combination of 2-DG and metformin would suppress both glycolysis and mitochondrial metabolism. The extent to which such dual metabolic inhibition might be broadly necessary in the treatment of rheumatologic diseases is unclear, but the metabolic plasticity of T cells might require this approach.
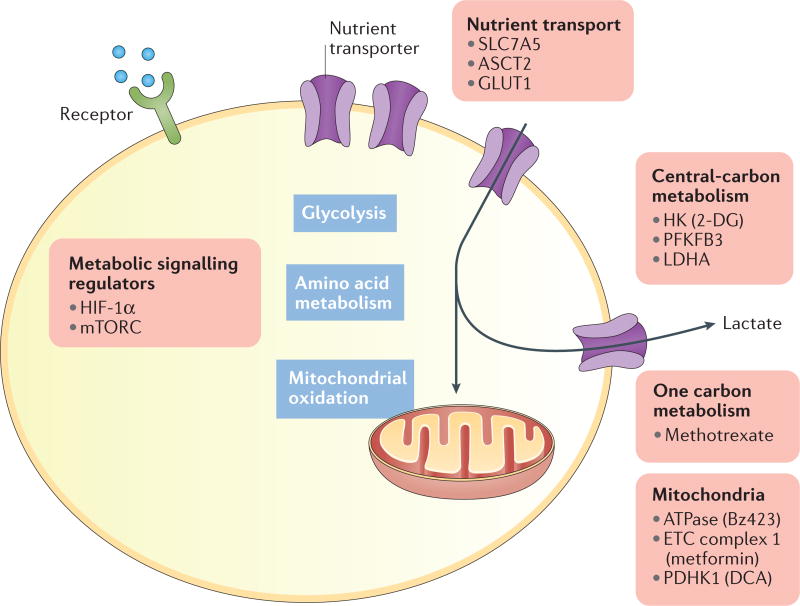
Figure 2. Metabolic processes to target in the treatment of rheumatologic diseases.
Metabolic areas and key current or potential targets for drugs to modify immunometabolism and shift immune cell subsets and fate are indicated. 2-DG, 2-deoxy-d-glucose; ASCT2, solute carrier family 1 member 5; DCA, dichloroacetate; ETC, electron transport chain; GLUT1, glucose transporter 1; HIF-1α, hypoxia-inducible factor 1α; HK, hexokinase; LDHA, lactate dehydrogenase A; mTORC, mechanistic target of rapamycin complex; PDHK1, pyruvate dehydrogenase kinase 1; PFKFB3. 6-Phosphofructo 2-kinase/fructose-2,6-bisphosphatase 3.
Beyond combinations of 2-DG and metformin, targeting amino acid metabolism could prove a promising approach. One potential therapeutic strategy is inhibition of glutamine uptake and metabolism. Glutamine is a non-essential amino acid that is used at high rates to support anabolic metabolism and its uptake is rapidly increased during T cell activation via the transporter SLC1A5 (REFS 56,107). Importantly, SLC1A5 deficiency attenuates TH1 and TH17 responses and prevents the onset of EAE in experimental mouse models56. The amino acid transporter SLC7A5 is also essential for T cell activation57 by supporting amino acid uptake essential for mTORC1 activity. Given the wide role of amino acids in anabolic metabolism and intracellular signalling, mechanisms that regulate these pathways are promising targets for modulation of immune cell function in inflammatory diseases. Strategies to suppress glycolysis, mitochondrial metabolism and amino acid metabolism could have far-reaching applications beyond autoimmunity. A 2015 study demonstrated that the combination of 2-DG and metformin, with the addition of an inhibitor of glutamine metabolism, reduced rejection of skin allografts or heart transplants in mice whereas the individual treatments had minimal effects108.
Regulation of ROS is also critical for immunological function88, and mitochondrial ROS production could be a target. Indeed, the F1F0-ATPase inhibitor Bz-423 (REF. 109) does not block ATP production but rather leads to increased ROS and can protect against lupus and graft-versus-host disease in animal models, in part by inducing lymphocyte apoptosis110,111. PDHK1 can also regulate mitochondrial ROS via regulation of pyruvate flux into the TCA cycle. Indeed, inhibition of PDHK1 led to increased ROS that promoted Treg cells and could protect from EAE19. In addition, the mitochondrial ROS scavenger MitoQ reduced mitochondrial anti-viral signalling (MAVS) activation and attenuated IFNγ production32,112.
A number of other metabolic events have promise as targets in rheumatologic diseases. Given the role of hypoxia in RA and OA, targeting the stability of HIF-1α or HIF-2α and the hypoxic response might offer protection from multiple aspects of joint inflammation113. Similarly, modulators of glycolysis, such as PFKFB3 (REF. 114) or LDH115, can suppress T cell activation or regulate IFNγ production. With these approaches, direct inhibition of a central carbon glucose metabolism pathway raises concerns of broad toxicity. However, in the studies discussed above the effects in vivo were surprisingly modest. This outcome is probably due to the partial inhibitory effect of each of these strategies and the selective dependence on those pathways of metabolically active inflammatory cells.
Challenges and future directions
Immunometabolism offers the opportunity to selectively target specific immune cell subsets by modifying the metabolic pathways essential for their function. This concept represents a paradigm shift away from targeting specific signalling pathways that might be active in a wide range of cells. However, a concern is that although only selected cells might require high fluxes through specific metabolic pathways, the extent to which other cell types might also activate and periodically rely on those same pathways remains unclear. Adverse effects of putative metabolic therapies are, therefore, critical challenges. This is particularly true for chronic diseases, which can require long-term treatment. Proliferative or metabolic tissues, such as the gut, liver, muscle and β cells, could be especially sensitive.
Despite these concerns, metabolic pathways are already being targeted, including by standard-of-care therapies, and some metabolic therapies are already standard of care. Other therapies certainly have metabolic implications that might contribute to their mechanisms of action. Methotrexate, for example, inhibits one-carbon metabolism yet is standard-of-care treatment for RA. Also, metabolic changes following inhibition of mTOR signalling certainly contribute to immune suppression48. A potential benefit of targeting immunometabolism to modulate immunity is that the selective use of pathways by effector or regulatory T cells or macrophages may enable short-term treatments to shift immune cell populations and provide durable protection from inflammation and disease. Thus, a short therapy period could provide benefit and reduce the potential for adverse effects. The immunometabolism field is rapidly evolving and our increasing knowledge of the metabolic pathways that promote effector and regulatory immune cell differentiation or the generation of osteoclasts might now provide rational strategies to exploit the metabolic requirements of each subset.
Acknowledgments
The authors would like to thank members of the Rathmell and Major labs for their contributions and intellectual input. The authors’ work is supported by the Alliance for Lupus Research (J.C.R.), NIH National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK105550 (J.C.R.), the Lupus Research Alliance (A.S.M.), U.S. Department of Veterans Affairs Merit Award I0BX002968 (A.S.M.) and NIH National Heart, Lung, and Blood Institute grant F31 HL128040 (J.P.R.).
Footnotes
Author contributions
All authors researched the data for the article, provided a substantial contribution to discussions of the content and contributed to writing the article and to review and/or editing of the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
Contributor Information
Jillian P. Rhoads, Division of Molecular Pathology, Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, 1161 21st Avenue South, Nashville, Tennessee 37232, USA
Amy S. Major, Division of Molecular Pathology, Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center; the Division of Rheumatology and Immunology, Department of Medicine, Vanderbilt University Medical Center; and the Vanderbilt Center for Immunobiology, Vanderbilt University School of Medicine, 1161 21st Avenue South, Nashville, Tennessee 37232, USA Department for Veterans Affairs, Tennessee Valley Healthcare System, Nashville, Tennessee 37232, USA.
Jeffrey C. Rathmell, Division of Molecular Pathology, Department of Pathology, Microbiology, and Immunology, and the Vanderbilt Center for Immunobiology, Vanderbilt University School of Medicine, 1161 21st Avenue South, Vanderbilt University Medical Center, Nashville, Tennessee 37232, USA
References
- 1.Yildirim-Toruner C, Diamond B. Current and novel therapeutics in the treatment of systemic lupus erythematosus. J. Allergy Clin. Immunol. 2011;127:303–312. doi: 10.1016/j.jaci.2010.12.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahlenberg JM, Fox DA. Advances in the medical treatment of rheumatoid arthritis. Hand Clin. 2011;27:11–20. doi: 10.1016/j.hcl.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirahara K, Schwartz D, Gadina M, Kanno Y, O’Shea JJ. Targeting cytokine signaling in autoimmunity: back to the future and beyond. Curr. Opin. Immunol. 2016;43:89–97. doi: 10.1016/j.coi.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy. 2005;4:325–328. doi: 10.2174/1568010054022015. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K, Takeshita S. The role of osteoclast differentiation and function in skeletal homeostasis. J. Biochem. 2016;159:1–8. doi: 10.1093/jb/mvv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J. Exp. Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowles HJ. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia (Auckl.) 2015;3:73–82. doi: 10.2147/HP.S95960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukumar M, et al. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 2016;23:63–76. doi: 10.1016/j.cmet.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukumar M, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawalekar OU, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Moreau HD, Bousso P. Visualizing how T cells collect activation signals in vivo. Curr. Opin. Immunol. 2014;26:56–62. doi: 10.1016/j.coi.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck MD, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerriets VA, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patsoukis N, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patsoukis N, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat. Rev. Rheumatol. 2016;12:169–182. doi: 10.1038/nrrheum.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caro-Maldonado A, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J. Immunol. 2014;192:3626–3636. doi: 10.4049/jimmunol.1302062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blair D, Dufort FJ, Chiles TC. Protein kinase Cβ is critical for the metabolic switch to glycolysis following B-cell antigen receptor engagement. Biochem. J. 2012;448:165–169. doi: 10.1042/BJ20121225. [DOI] [PubMed] [Google Scholar]
- 26.Cho SH, et al. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature. 2016;537:234–238. doi: 10.1038/nature19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwata TN, et al. Conditional disruption of Raptor reveals an essential role for mTORC1 in B cell development, survival, and metabolism. J. Immunol. 2016;197:2250–2260. doi: 10.4049/jimmunol.1600492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jellusova J, Rickert RC. The PI3K pathway in B cell metabolism. Crit. Rev. Biochem. Mol. Biol. 2016;51:359–378. doi: 10.1080/10409238.2016.1215288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Windt GJ, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl Acad. Sci. USA. 2013;110:14336–14341. doi: 10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gubser PM, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 2013;14:1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 31.Na YR, et al. GM-CSF induces inflammatory macrophages by regulating glycolysis and lipid metabolism. J. Immunol. 2016;197:4101–4109. doi: 10.4049/jimmunol.1600745. [DOI] [PubMed] [Google Scholar]
- 32.Mills EL, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470.e13. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semba H, et al. HIF-1α–PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat. Commun. 2016;7:11635. doi: 10.1038/ncomms11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freemerman AJ, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014;289:7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Everts B, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearce EJ, Everts B. Dendritic cell metabolism. Nat. Rev. Immunol. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng SC, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vats D, et al. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson AR, et al. Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Mol. Metab. 2016;5:506–526. doi: 10.1016/j.molmet.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012;8:665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- 43.Indo Y, et al. Metabolic regulation of osteoclast differentiation and function. J. Bone Miner. Res. 2013;28:2392–2399. doi: 10.1002/jbmr.1976. [DOI] [PubMed] [Google Scholar]
- 44.Morten KJ, Badder L, Knowles HJ. Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J. Pathol. 2013;229:755–764. doi: 10.1002/path.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasi A, et al. Dendritic cell reprogramming by endogenously produced lactic acid. J. Immunol. 2013;191:3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 46.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4 + T cell subsets. J. Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi LZ, et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol. Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boothby M. Signaling in T cells — is anything the m(a)TOR with the picture(s)? F1000Res. 2016;5:191. doi: 10.12688/f1000research.7027.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDonald G, et al. Normalizing glycosphingolipids restores function in CD4+ T cells from lupus patients. J. Clin. Invest. 2014;124:712–724. doi: 10.1172/JCI69571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berod L, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 52.Gerriets VA, et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat. Immunol. 2016;17:1459–1466. doi: 10.1038/ni.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu S, Hubbard B, Shevach EM. Foxp3-mediated inhibition of Akt inhibits Glut1 (glucose transporter 1) expression in human T regulatory cells. J. Leukoc. Biol. 2015;97:279–283. doi: 10.1189/jlb.2AB0514-273RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beier UH, et al. Essential role of mitochondrial energy metabolism in Foxp3+ T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macintyre AN, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakaya M, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinclair LV, et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015;36:3–12. doi: 10.1016/j.it.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Procaccini C, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Rosa V, et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 2015;16:1174–1184. doi: 10.1038/ni.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shrestha S, et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huynh A, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park Y, et al. TSC1 regulates the balance between effector and regulatory T cells. J. Clin. Invest. 2013;123:5165–5178. doi: 10.1172/JCI69751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ray JP, et al. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity. 2015;43:690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng H, et al. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity. 2016;45:540–554. doi: 10.1016/j.immuni.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin. Immunol. 2015;27:286–296. doi: 10.1016/j.smim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weichhart T, Hengstschlager M, Linke M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bengsch B, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8+ T cell exhaustion. Immunity. 2016;45:358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan B, et al. Serum metabolomic profiling in patients with systemic lupus erythematosus by GC/MS. Mod. Rheumatol. 2016;26:914–922. doi: 10.3109/14397595.2016.1158895. [DOI] [PubMed] [Google Scholar]
- 70.Guleria A, et al. NMR based serum metabolomics reveals a distinctive signature in patients with lupus nephritis. Sci. Rep. 2016;6:35309. doi: 10.1038/srep35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu T, et al. Metabolic disturbances associated with systemic lupus erythematosus. PLoS ONE. 2012;7:e37210. doi: 10.1371/journal.pone.0037210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wahl DR, et al. Characterization of the metabolic phenotype of chronically activated lymphocytes. Lupus. 2010;19:1492–1501. doi: 10.1177/0961203310373109. [DOI] [PubMed] [Google Scholar]
- 73.Nagy G, Koncz A, Perl A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+-and redox-dependent production of nitric oxide. J. Immunol. 2003;171:5188–5197. doi: 10.4049/jimmunol.171.10.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagy G, Koncz A, Fernandez D, Perl A. Nitric oxide, mitochondrial hyperpolarization, and T cell activation. Free Radic. Biol. Med. 2007;42:1625–1631. doi: 10.1016/j.freeradbiomed.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gergely P, Jr, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc. Natl Acad. Sci. USA. 2001;98:1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perry DJ, et al. Murine lupus susceptibility locus Sle1c2 mediates CD4+ T cell activation and maps to estrogen-related receptor γ. J. Immunol. 2012;189:793–803. doi: 10.4049/jimmunol.1200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eichner LJ, et al. miR-378* mediates metabolic shift in breast cancer cells via the PGC-1β/ERRγ transcriptional pathway. Cell Metab. 2010;12:352–361. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 79.Bensinger SJ, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kidani Y, Bensinger SJ. LXR and PPAR as integrators of lipid homeostasis and immunity. Immunol. Rev. 2012;249:72–83. doi: 10.1111/j.1600-065X.2012.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tso TK, Huang HY, Chang CK, Liao YJ, Huang WN. Clinical evaluation of insulin resistance and β-cell function by the homeostasis model assessment in patients with systemic lupus erythematosus. Clin. Rheumatol. 2004;23:416–420. doi: 10.1007/s10067-004-0908-5. [DOI] [PubMed] [Google Scholar]
- 82.Gabriel CL, et al. Autoimmune-mediated glucose intolerance in a mouse model of systemic lupus erythematosus. Am. J. Physiol. Endocrinol. Metab. 2012;303:E1313–E1324. doi: 10.1152/ajpendo.00665.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilhelm AJ, Major AS. Accelerated atherosclerosis in SLE: mechanisms and prevention approaches. Int. J. Clin. Rheumtol. 2012;7:527–539. doi: 10.2217/ijr.12.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 2014;192:136–144. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerriets VA, et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. 2016;46:1970–1983. doi: 10.1002/eji.201545861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lourenco EV, Liu A, Matarese G, La Cava A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc. Natl Acad. Sci. USA. 2016;113:10637–10642. doi: 10.1073/pnas.1607101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J. Exp. Med. 2013;210:2119–2134. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sena LA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Z, et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci. Transl Med. 2016;8:331ra38. doi: 10.1126/scitranslmed.aad7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fearon U, Canavan M, Biniecka M, Veale DJ. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 2016;12:385–397. doi: 10.1038/nrrheum.2016.69. [DOI] [PubMed] [Google Scholar]
- 91.Hong YH, Kong EJ. (18F)Fluoro-deoxy-D-glucose uptake of knee joints in the aspect of age-related osteoarthritis: a case-control study. BMC Musculoskelet. Disord. 2013;14:141. doi: 10.1186/1471-2474-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23:1955–1965. doi: 10.1016/j.joca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 93.Franke S, et al. Advanced glycation end products affect growth and function of osteoblasts. Clin. Exp. Rheumatol. 2011;29:650–660. [PubMed] [Google Scholar]
- 94.Martinez-Calatrava MJ, et al. RANKL synthesized by articular chondrocytes contributes to juxta-articular bone loss in chronic arthritis. Arthritis Res. Ther. 2012;14:R149. doi: 10.1186/ar3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin Z, Wei W, Yang M, Du Y, Wan Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 2014;20:483–498. doi: 10.1016/j.cmet.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lemma S, et al. Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 2016;79:168–180. doi: 10.1016/j.biocel.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 97.Vander Heiden MG. Exploiting tumor metabolism: challenges for clinical translation. J. Clin. Invest. 2013;123:3648–3651. doi: 10.1172/JCI72391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O’Sullivan D, Pearce EL. Targeting T cell metabolism for therapy. Trends Immunol. 2015;36:71–80. doi: 10.1016/j.it.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wheaton WW, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Son HJ, et al. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm. 2014;2014:973986. doi: 10.1155/2014/973986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bian L, et al. Dichloroacetate alleviates development of collagen II-induced arthritis in female DBA/1 mice. Arthritis Res. Ther. 2009;11:132. doi: 10.1186/ar2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ostroukhova M, et al. The role of low-level lactate production in airway inflammation in asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L300–L307. doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eleftheriadis T, et al. Dichloroacetate at therapeutic concentration alters glucose metabolism and induces regulatory T-cell differentiation in alloreactive human lymphocytes. J. Basic Clin. Physiol. Pharmacol. 2013;24:271–276. doi: 10.1515/jbcpp-2013-0001. [DOI] [PubMed] [Google Scholar]
- 104.Thomas S, et al. Methotrexate is a JAK/STAT pathway inhibitor. PLoS ONE. 2015;10:e0130078. doi: 10.1371/journal.pone.0130078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shuvalov O, et al. One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget. 2017 doi: 10.18632/oncotarget.15053. http://dx.doi.org/10.18632/oncotarget.15053. [DOI] [PMC free article] [PubMed]
- 106.Yin Y, et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl Med. 2015;7:274ra18. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sener Z, Cederkvist FH, Volchenkov R, Holen HL, Skalhegg BS. T helper cell activation and expansion is sensitive to glutaminase inhibition under both hypoxic and normoxic conditions. PLoS ONE. 2016;11:e0160291. doi: 10.1371/journal.pone.0160291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee CF, et al. Preventing allograft rejection by targeting immune metabolism. Cell Rep. 2015;13:760–770. doi: 10.1016/j.celrep.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnson KM, et al. Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem. Biol. 2005;12:485–496. doi: 10.1016/j.chembiol.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 110.Gatza E, et al. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci. Transl Med. 2011;3:67ra8. doi: 10.1126/scitranslmed.3001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bednarski JJ, et al. Attenuation of autoimmune disease in Fas-deficient mice by treatment with a cytotoxic benzodiazepine. Arthritis Rheum. 2003;48:757–766. doi: 10.1002/art.10968. [DOI] [PubMed] [Google Scholar]
- 112.Buskiewicz IA, et al. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci. Signal. 2016;9:ra115. doi: 10.1126/scisignal.aaf1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hua S, Dias TH. Hypoxia-inducible factor (HIF) as a target for novel therapies in rheumatoid arthritis. Front. Pharmacol. 2016;7:184. doi: 10.3389/fphar.2016.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Telang S, et al. Small molecule inhibition of 6-phosphofructo-2-kinase suppresses T cell activation. J. Transl Med. 2012;10:95. doi: 10.1186/1479-5876-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peng M, et al. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science. 2016;354:481–484. doi: 10.1126/science.aaf6284. [DOI] [PMC free article] [PubMed] [Google Scholar]