Abstract
Heart failure with preserved ejection fraction is a socioeconomic burden in Japan as well as other developed countries. Diuretics are widely used to attenuate symptoms and signs of congestion in both heart failure with preserved and reduced ejection fraction, although their effects on long-term prognosis of both phenotypes of heart failure have not been demonstrated because of an ethical difficulty in designing a randomized and prospective clinical trial. Guidelines do not provide any guidance on therapy choice, and physicians blindly choose furosemide among loop diuretics in current clinical settings. However, several clinical studies have suggested that the effects of loop diuretics are not consistent, and that furosemide is not necessarily preferable as compared with other loop diuretics. We should pay attention to the choice of loop diuretics. Regarding the improvement of long-term prognosis, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, mineralocorticoid receptor blocker and β-blocker are proven effective for heart failure with reduced ejection fraction. However, none of these drugs have improved prognosis of heart failure with preserved ejection fraction in clinical trials. Observational studies and subanalysis of clinical trials suggest the benefits of these drugs in this phenotype of heart failure. All of clinical trials and observational studies present facts to us, and let us recognize that “one size fits all approach” may be a cause for a lack of evidence about the therapeutic strategy of heart failure with preserved ejection fraction until now. We have to make efforts to clarify characteristics of patients with heart failure and preserved ejection fraction to whom the administration of each drug provides benefits or do not.
Keywords: Diastole, Heart failure, Pharmacological intervention
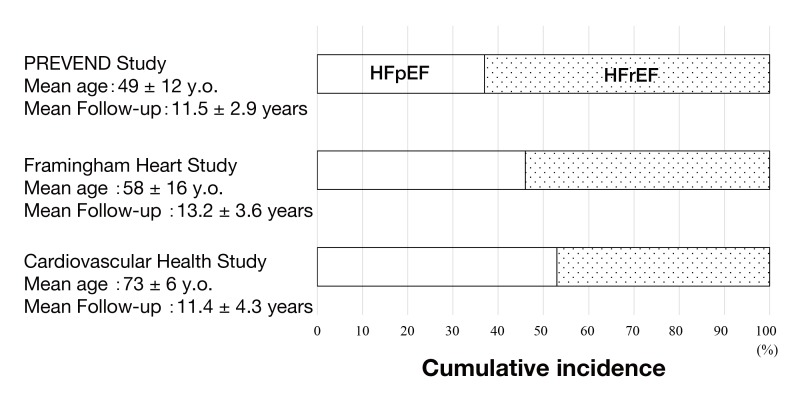
About a half of patients with heart failure (HF) have normal or near-normal ejection fraction in the current era,1, 2 and this phenotype of HF is termed heart failure with preserved ejection fraction (HFpEF). The prevalence of HFpEF particularly increases with aging (Fig. 1).3
Fig. 1.
Difference of cumulative incidence of HFpEF and HFrEF among cohort studies. With an increase in age of study subjects, incidence of HFpEF enhances. (Figure was produced based on data of reference 3.)
HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PREVEND study, Prevention of Renal and Vascular Endstage Disease study; y.o., years old.
The accumulated evidence about the effects of therapeutic interventions on HF with reduced ejection fraction (HFrEF) has resulted in the improvement of its prognosis, although not satisfactorily.4 In contrast, there has been no established therapeutic strategy to be efficacious against HFpEF. Its prognosis has not changed over the past decades and is currently as poor as that of HFrEF.4 Therefore, it is an exigent issue to find out effective therapeutic intervention for HFpEF.
CHOICE OF DIURETICS FOR HFpEF
Diuretics improve symptoms of HF patients,5 and are widely used irrespective of left ventricular ejection fraction. Loop diuretics are most widely used, but little attention is paid to the difference in their pharmacokinetics. Our experimental study showed azosemide, a long-acting loop diuretic, provided better prognosis in HFrEF model rats compared with furosemide, a short-acting and most widely used diuretic, and this result was partly explained by less reflex increase in cardiac sympathetic neuronal activity in the azosemide group.6 This finding was confirmed by a Japanese clinical trial, J-MELODIC study which reported that azosemide, compared with furosemide, reduced the risk of cardiovascular death or unplanned hospitalization for worsening HF in patients with HF.7 This study included both of HFrEF and HFpEF, and more than a half of patients were HFpEF. The subgroup analysis showed that the results were consistent across left ventricular ejection fraction.
Torasemide, another long-acting loop diuretic with anti-aldosterone effects, is likely to provide beneficial effects over furosemide in HF patients.8 There are only studies with a small number of patients, and no study investigated the effects of torasemide on HFpEF.
Physicians usually choose furosemide among loop diuretics for patients with HF in current clinical settings. Guidelines recommend use of diuretics to improve symptoms and signs associated with congestion, but do not provide any guidance on therapy choice. The previous our and other studies have strongly suggested that the choice of loop diuretics affects clinical outcomes in the treatment of HFpEF.
THE EFFECTS OF ANGIOTENSIN CONVERTING ENZYME INHIBITOR (ACEI) AND ANGIOTENSIN RECEPTOR BLOCKER (ARB) IN HFpEF
Previous clinical trials have demonstrated the beneficial effects of ACEI and ARB in HFrEF, and its principal mechanism is to prevent structural remodeling such as myocardial hypertrophy, fibrosis and ventricular dilatation. Myocardial hypertrophy and fibrosis are also observed in HFpEF patients9, 10 and are considered to play crucial roles in the development of signs and symptoms of HF. Our experimental studies have shown that the administration of ACEI and/or ARB provides beneficial effects on HFpEF model rats.11–13
Clinical studies have not reached a consensus. Clinical trials to assess the effects of ACEI and ARB on clinical outcomes of HFpEF (Table 1) have failed to reveal its benefits.14–16 However, an observational study with a larger number of patients demonstrated the prescription of ACEI/ARB resulted in the reduction of mortality of HFpEF patients.17 There are differences in patients’ characteristics between clinical trials and observational studies. In addition, the subanalysis of a clinical trial, I-Preserve trial, reported the beneficial effects of irbesartan in HFpEF patients with low, but not high, values of baseline plasma amino-terminal pro-brain natriuretic peptide.18 These experimental and clinical studies suggest that there are a subgroup of HFpEF patients who benefit from ACEI or ARB, and we have to abolish “one size fits all approach” in searching appropriate treatment strategy for HFpEF.
Table 1.
Clinical trials to investigate the effects of pharmacological intervention on clinical outcomes of HFpEF
| Trial | Drug | Primary endpoint |
| PEP-CHF14 | ACEI | all cause death or HF hospitalization |
| CHARM-Preserved16 | ARB | CV death or HF hospitalization |
| I-Preserve15 | ARB | all cause death or CV hospitalization |
| TOP-CAT26 | MRB | CV death, aborted cardiac arrest, or HF hospitalization |
| J-DHF34 | β-blocker | CV death and HF hospitalization |
| DIG ancillary46 | Digitalis | HF mortality or HF hospitalization |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CV, cardiovascular; HF, heart failure; MRB, mineralocorticoid receptor blocker.
THE EFFECTS OF MINERALOCORTICOID RECEPTOR BLOCKER (MRB) IN HFpEF
Previous clinical trials have demonstrated the beneficial effects of MRB in HFrEF. Our experimental study showed the administration of MRB was effective in the animal model for HFpEF.19 Although a principal ligand of the mineralocorticoid receptor is thought to be aldosterone, both aldosterone and cortisol/corticosterone have the affinity to the mineralocorticoid receptor.20 The selective activation of the mineralocorticoid receptor by aldosterone is maintained through conversion of active cortisol/corticosterone to inactive cortisone/11-dehydrocorticosterone by 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) in the tissues; however, 11β-HSD2 is minimally detectable in the cardiac tissue.19 Our experimental studies have shown that glucocorticoid induces myocyte hypertrophy and enhances collagen production in fibroblasts.21, 22 The glucocorticoid-induced enhancement of collagen production is likely through the interaction of elongation factor eleven-nineteen lysine-rich leukemia, a co-activator of the mineralocorticoid receptor, with the mineralocorticoid receptor.22 Guder et al. reported that higher serum levels of both cortisol and aldosterone were independent predictors of increased mortality risk in HFrEF patients.23 Therefore, we have speculated that both glucocorticoid and mineralocorticoid play important roles in the development of HF, partly through the activation of the mineralocorticoid receptor-mediated pathway.
Several clinical studies have reported that MRB improves left ventricular diastolic function and suppresses markers of collagen turnover in HFpEF patients.24, 25 In spite of these experimental and clinical studies, the treatment with MRB, spironolactone, did not significantly reduce the incidence of the primary composite outcome of death from cardiovascular causes, aborted cardiac arrest, or hospitalization for heart failure in a large clinical trial, TOPCAT.26 However, hospitalization for heart failure was significantly reduced by spironolactone in this trial. HFpEF patients are elderly, and their quality of life should be underscored besides mortality. It is well known that repeated hospitalization result in the impairment of functional status of heart failure patients. We need to reconsider what is an appropriate endpoint for clinical studies to establish therapeutic strategies for HFpEF.27
THE EFFECTS OF β-BLOCKER IN HFpEF
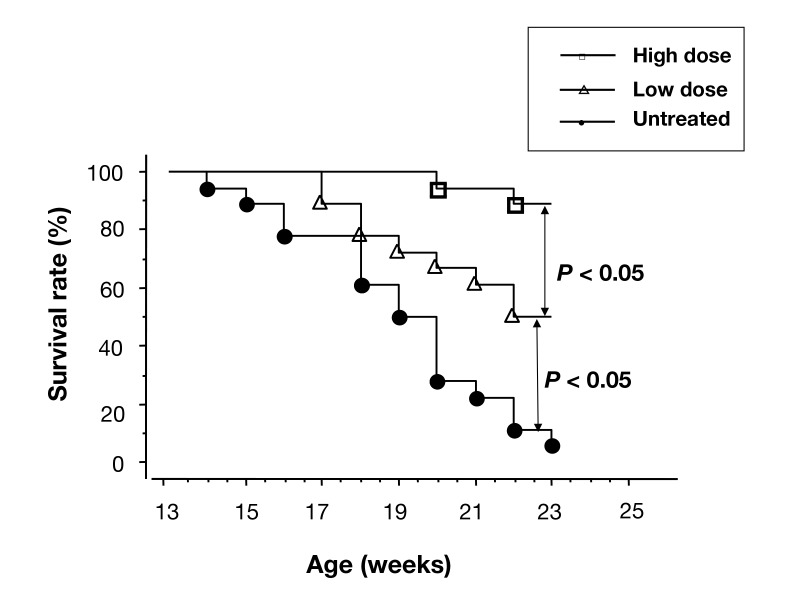
It is well known that β-blocker significantly reduces mortality and morbidity of HFrEF patients, although the mechanisms of its benefits are not well clarified. Our clinical study showed that β-blocker improved LV diastolic function and symptoms of HFrEF patients even without the changes in LV systolic function.28 Our and other experimental studies demonstrated that the administration of β-blocker in the HFpEF model rats improved their survival rate.29, 30 In addition, the improvement of survival rate was dose-dependent (Fig. 2).30
Fig. 2.
Kaplan-Meier survival curves of the HFpEF model rats of an untreated group, a group treated with low dose of bisoprolol (12.5 mg/kg/day) and a group treated with high dose of bisoprolol (250 mg/kg/day). The survival rate was improved by the administration of bisoprolol, and the effects of bisoprolol were dose-dependent.
Copyright © 2014 John Wiley and Sons. All rights reserved. (Permission granted from the publisher of reference 30.)
HFpEF, heart failure with preserved ejection fraction.
In contrast to HFrEF, there have been only a few clinical trials to investigate the effects of β-blocker on HFpEF. The subanalysis of SENIORS trial showed that the benefits of nebivolol were similar between HF patients with EF > and ≤ 35%.31 SWEDIC study compared the changes in several Doppler echocardiographic indices derived from the transmitral and pulmonary venous flow velocity patterns between HFpEF patients treated with and without carvedilol to assess the effects of carvedilol on left ventricular diastolic function.32 Although the treatment with carvedilol increased a ratio of peak mitral E wave velocity to peak mitral A wave velocity (E/A), the increase in E/A can be induced by both improvement and worsening of left ventricular diastolic function,33 and it was difficult to make a conclusive remark from these data.
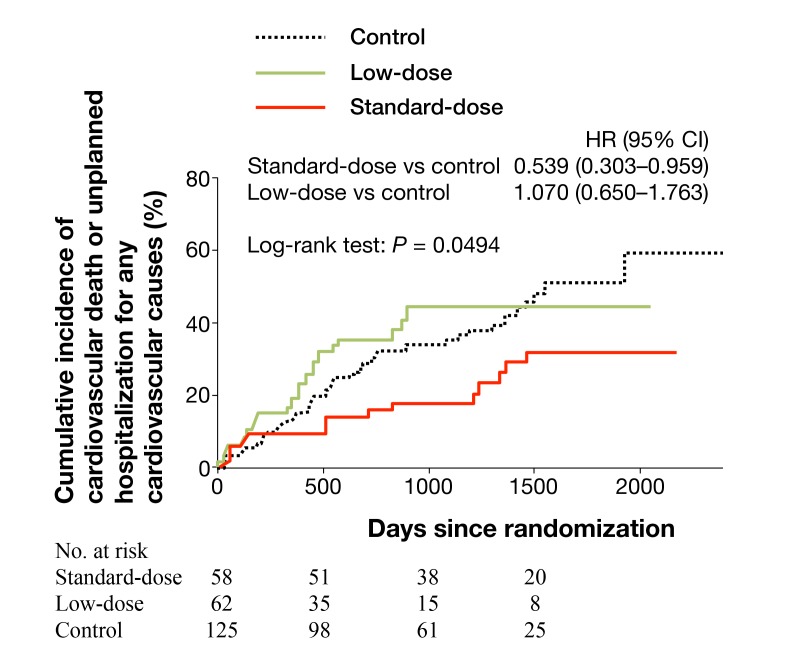
J-DHF investigated the effects of β-blocker on clinical outcomes in HFpEF patients in Japan.34 There was no significant difference in the incidence of the primary endpoint (cardiovascular death or unplanned hospitalization for HF) and another composite endpoint (cardiovascular death or unplanned hospitalization for any cardiovascular causes) between two groups treated with and without carvedilol. However, the subanalysis revealed that the prescription of carvedilol at standard doses for HF, but not at low doses, was associated with the significant reduction of the incidence of cardiovascular death or unplanned hospitalization for any cardiovascular causes (Fig. 3).
Fig. 3.
Kaplan-Meier curves showing the time to first occurrence of the prespecified outcome, cardiovascular death or unplanned hospitalization for any cardiovascular causes. The carvedilol group was further divided into a group treated with carvedilol > 7.5mg/day (n = 58, standard-dose group) and carvedilol ≤ 7.5mg/day (n = 62, low-dose group). Control group consists of HFpEF patients treated without β-blocker.
Copyright © 2014 John Wiley and Sons. All rights reserved. (Permission granted from the publisher of reference 34.)
CI, confidence interval; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; No., number.
Currently, there are no other prospective and randomized trials to investigate the effects of β-blocker on HFpEF, and observational studies have reached inconsistent results. The meta-analysis of 12 previous studies reported that β-blocker provides beneficial effects on HFpEF,35 which is consistent with our observational study.36 In particular, two studies indicated the dose-dependent benefits of β-blocker as J-DHF study did.37, 38 Shah et al. demonstrated that benefits of β-blocker emerged at follow-up for 3 years but not for 1 year in patients with HFpEF.39 On the other hand, there are studies which concluded a lack of beneficial effects of β-blocker on HFpEF.40–45 However, all of these studies have at least one of the following limitations; the number of the subjects was small, or no attention was paid to the effects of the dose or the duration of β-blocker therapy in the data analysis.
OTHER DRUGS AVAILABLE IN JAPAN
DIG ancillary trial reported that digoxin did not reduce mortality and all-cause or cardiovascular hospitalizations of HFpEF patients with sinus rhythm receiving ACEI and diuretics.46 The subanalysis of a clinical trial, GISSI-HF study, showed a lack of benefits of statin in HFpEF,47 but, several observational studies have reported beneficial effects of statins.48, 49 Nitrates are prescribed for symptom relief in HF patients, and this may be attributable to the reduction of preload of the left ventricle. However, NEAT-HFpEF study showed no significant differences in the 6-minute walk distance, quality-of-life scores, or N-terminal pro-brain natriuretic peptide (NT-proBNP) levels between the HFpEF patients treated with and without isosorbide mononitrate for 6 weeks.50 Phosphodiesterase-5 inhibitors are expected to reduce pulmonary artery pressure and to attenuate the severity of HF, however, RELAX trial showed administration of sildenafil for 24 weeks did not improve exercise capacity or clinical status.51 Both of isosorbide mononitrate and sildenafil were expected to provide beneficial effects through activation of cyclic guanosine monophosphate/protein kinase G (cGMP/PKG) signaling pathway. Although these clinical trials yielded disappointing outcomes, they only observed the short-term effects of each drug and did not assess the effects on clinical outcomes.
Recent small clinical trials suggested the beneficial effects of sacubitril/valsartan (LCZ 696, angiotensin receptor neprilysin inhibitor),52 ivabradine,53 or ranolazine54 for HFpEF. Some of these new agents are currently under a clinical trial for HFrEF in Japan, and clinical trials with larger number of HFpEF patients are ongoing in the world.
CONCLUSIONS
Currently, there is no therapeutic intervention proven to improve the prognosis of HFpEF by clinical trials, and observational studies have demonstrated opposite results about the effects of some drugs on HFpEF. The bias of the study subjects is always argued against observational studies. However, the study subjects of the clinical trial do not necessarily represent patients’ characteristics in the clinical settings, because many exclusion criteria are set. Patients with HFpEF frequently have comorbidities and infringe on exclusion criteria. Therefore, the characteristics of HFpEF patients in clinical settings are consistent with those of observational studies rather than the clinical trials. HFpEF is a generic term of heterogeneous pathophysiology and is not a sole disease. All of clinical trials and observational studies present facts to us, and let us recognize that “one size fits all approach” may be a cause for inconsistent results of previous studies and for a lack of evidence about the therapeutic strategy of HFpEF until now. We have to make efforts to clarify characteristics of patients with HFpEF to whom the administration of each drug provides benefits or do not.
Conflicts of Interest: Dr. Yamamoto has received speakers’ bureau/honorarium from Otsuka Pharmaceutical, Ono Pharmaceutical, Mitsubishi Tanabe Pharma, Toa Eiyo, Takeda Pharmaceutical, Medtronic, Bristol-Myers Squibb, Pfizer, research funds from St. Jude Medical Japan, Otsuka Pharmaceutical, Daiichi-Sankyo, Ono Pharmaceutical, Biotronik Japan, Japan Lifeline, Astellas, Sanwa Kagaku Kenkyusho, Boehringer Ingerlheim, Abbott Vascular Japan, Bayer Yakuhin, Teijin Pharma, Mitsubishi Tanabe Pharma, Novartis Pharma K.K., Fukuda Denshi, Taisho Toyama Pharmaceutical, Fukuda Life Tec, and consultation fees from Novartis Pharma K.K.
REFERENCES
- 1. Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sato N, Kajimoto K, Keida T, Mizuno M, Minami Y, Yumino D, et al. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J. 2013;77:944-51. [DOI] [PubMed] [Google Scholar]
- 3. Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, et al. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9:e003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-9. [DOI] [PubMed] [Google Scholar]
- 5. Faris R, Flather M, Purcell H, Henein M, Poole-Wilson P, Coats A. Current evidence supporting the role of diuretics in heart failure: a meta analysis of randomised controlled trials. Int J Cardiol. 2002;82:149-58. [DOI] [PubMed] [Google Scholar]
- 6. Yoshida J, Yamamoto K, Mano T, Sakata Y, Nishio M, Ohtani T, et al. Different effects of long- and short-acting loop diuretics on survival rate in Dahl high-salt heart failure model rats. Cardiovasc Res. 2005;68:118-27. [DOI] [PubMed] [Google Scholar]
- 7. Masuyama T, Tsujino T, Origasa H, Yamamoto K, Akasaka T, Hirano Y, et al. Superiority of long-acting to short-acting loop diuretics in the treatment of congestive heart failure. Circ J. 2012;76:833-42. [DOI] [PubMed] [Google Scholar]
- 8. Buggey J, Mentz RJ, Pitt B, Eisenstein EL, Anstrom KJ, Velazquez EJ, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borbely A, van der Velden J, Papp Z, Bronzwaer JGF, Edes I, Stienen GJM, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774-81. [DOI] [PubMed] [Google Scholar]
- 10. van Heerebeek L, Borbely A, Niessen HWM, Bronzwaer JGF, van der Velden J, Stienen GJM, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966-73. [DOI] [PubMed] [Google Scholar]
- 11. Sakata Y, Masuyama T, Yamamoto K, Doi R, Mano T, Kuzuya T, et al. Renin angiotensin system-dependent hypertrophy as a contributor to heart failure in hypertensive rats: different characteristics from renin angiotensin system-independent hypertrophy. J Am Coll Cardiol. 2001;37:293-9. [DOI] [PubMed] [Google Scholar]
- 12. Sakata Y, Yamamoto K, Mano T, Nishikawa N, Yoshida J, Miwa T, et al. Temocapril prevents transition to diastolic heart failure in rats even if initiated after appearance of LV hypertrophy and diastolic dysfunction. Cardiovasc Res. 2003;57:757-65. [DOI] [PubMed] [Google Scholar]
- 13. Yoshida J, Yamamoto K, Mano T, Sakata Y, Nishikawa N, Nishio M, et al. AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failure. Hypertension. 2004;43:686-91. [DOI] [PubMed] [Google Scholar]
- 14. Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338-45. [DOI] [PubMed] [Google Scholar]
- 15. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456-67. [DOI] [PubMed] [Google Scholar]
- 16. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777-81. [DOI] [PubMed] [Google Scholar]
- 17. Lund LH, Benson L, Dahlstrom U, Edner M. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA. 2012;308:2108-17. [DOI] [PubMed] [Google Scholar]
- 18. Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail. 2011;4:569-77. [DOI] [PubMed] [Google Scholar]
- 19. Ohtani T, Ohta M, Yamamoto K, Mano T, Sakata Y, Nishio M, et al. Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: Beneficial effects of mineralocorticoid receptor blocker. Am J Physiol Regul Integr Comp Physiol. 2007;292:R946-54. [DOI] [PubMed] [Google Scholar]
- 20. Binart N, Lombes M, Rafestin-Oblin M, Baulieu E. Characterization of human mineralocorticosteroid receptor expressed in the baculovirus system. Proc Natl Acad Sci U S A. 1991;88:10681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohtani T, Mano T, Hikoso S, Sakata Y, Nishio M, Takeda Y, et al. Cardiac steroidogenesis and glucocorticoid in the development of cardiac hypertrophy during the progression to heart failure. J Hypertens. 2009;27:1074-83. [DOI] [PubMed] [Google Scholar]
- 22. Omori Y, Mano T, Ohtani T, Sakata Y, Takeda Y, Tamaki S, et al. Glucocorticoids Induce Cardiac Fibrosis via Mineralocorticoid Receptor in Oxidative Stress: Contribution of Elongation Factor Eleven-Nineteen Lysine-Rich Leukemia (ELL). Yonago Acta Med. 2014;57:109-16. [PMC free article] [PubMed] [Google Scholar]
- 23. Guder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, et al. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754-61. [DOI] [PubMed] [Google Scholar]
- 24. Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM-PEF). J Card Fail. 2011;17:634-42. [DOI] [PubMed] [Google Scholar]
- 25. Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558-65. [DOI] [PubMed] [Google Scholar]
- 26. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383-92. [DOI] [PubMed] [Google Scholar]
- 27. Butler J, Hamo CE, Udelson JE, Pitt B, Yancy C, Shah SJ, et al. Exploring New Endpoints for Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9:e003358. [DOI] [PubMed] [Google Scholar]
- 28. Tamaki S, Sakata Y, Mano T, Ohtani T, Takeda Y, Kamimura D, et al. Long-term beta-blocker therapy improves diastolic function even without the therapeutic effect on systolic function in patients with reduced ejection fraction. J Cardiol. 2010;56:176-182. [DOI] [PubMed] [Google Scholar]
- 29. Kobayashi M, Machida N, Mitsuishi M, Yamane Y. Beta-blocker improves survival, left ventricular function and myocardial remodeling in hypertensive rats with diastolic heart failure. Am J Hypertens. 2004;17:1112-9. [DOI] [PubMed] [Google Scholar]
- 30. Nishio M, Sakata Y, Mano T, Ohtani T, Takeda Y, Miwa T, et al. Beneficial effects of bisoprolol on the survival of hypertensive diastolic heart failure model rats. Eur J Heart Fail. 2008;10:446-53. [DOI] [PubMed] [Google Scholar]
- 31. van Veldhuisen DJ, Cohen-Solal A, Bohm M, Anker SD, Babalis D, Roughton M, et al. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure). J Am Coll Cardiol. 2009;53:2150-8. [DOI] [PubMed] [Google Scholar]
- 32. Bergstrom A, Andersson B, Edner M, Nylander E, Persson H, Dahlstrom U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC). Eur J Heart Fail. 2004;6:453-61. [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto K, Redfield MM, Nishimura RA. Analysis of left ventricular diastolic function. Heart. 1996;75(Suppl 2):27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto K, Origasa H, Hori M. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail. 2013;15:110-8. [DOI] [PubMed] [Google Scholar]
- 35. Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J, et al. Effects of beta-blockers on heart failure with preserved ejection fraction: a meta-analysis. PLoS One. 2014;9:e90555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yanagihara K, Kinugasa Y, Sugihara S, Hirai M, Yamada K, Ishida K, et al. Discharge use of carvedilol is associated with higher survival in Japanese elderly patients with heart failure regardless of left ventricular ejection fraction. J Cardiovasc Pharmacol. 2013;62:485-90. [DOI] [PubMed] [Google Scholar]
- 37. Dobre D, van Veldhuisen DJ, DeJongste MJ, Lucas C, Cleuren G, Sanderman R, et al. Prescription of beta-blockers in patients with advanced heart failure and preserved left ventricular ejection fraction. Clinical implications and survival. Eur J Heart Fail. 2007;9:280-6. [DOI] [PubMed] [Google Scholar]
- 38. El-Refai M, Peterson EL, Wells K, Swadia T, Sabbah HN, Spertus JA, et al. Comparison of beta-blocker effectiveness in heart failure patients with preserved ejection fraction versus those with reduced ejection fraction. J Card Fail. 2013;19:73-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah R, Wang Y, Foody JM, et al. Effect of statins, angiotensin-converting enzyme inhibitors, and beta blockers on survival in patients >or=65 years of age with heart failure and preserved left ventricular systolic function. Am J Cardiol. 2008;101:217-22. [DOI] [PubMed] [Google Scholar]
- 40. Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure. Circulation. 2005;112:357-63. [DOI] [PubMed] [Google Scholar]
- 41. Grigorian Shamagian L, Roman AV, Ramos PM, Veloso PR, Bandin Dieguez MA, Gonzalez-Juanatey JR. Angiotensin-converting enzyme inhibitors prescription is associated with longer survival among patients hospitalized for congestive heart failure who have preserved systolic function: a long-term follow-up study. J Card Fail. 2006;12:128-33. [DOI] [PubMed] [Google Scholar]
- 42. Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farasat SM, Bolger DT, Shetty V, Menachery EP, Gerstenblith G, Kasper EK, et al. Effect of Beta-blocker therapy on rehospitalization rates in women versus men with heart failure and preserved ejection fraction. Am J Cardiol. 2010;105:229-34. [DOI] [PubMed] [Google Scholar]
- 44. Tehrani F, Phan A, Chien CV, Morrissey RP, Rafique AM, Schwarz ER. Value of medical therapy in patients >80 years of age with heart failure and preserved ejection fraction. Am J Cardiol. 2009;103:829-33. [DOI] [PubMed] [Google Scholar]
- 45. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Dosing of beta-blocker therapy before, during, and after hospitalization for heart failure (from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure). Am J Cardiol. 2008;102:1524-9. [DOI] [PubMed] [Google Scholar]
- 46. Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231-9. [DOI] [PubMed] [Google Scholar]
- 48. Nochioka K, Sakata Y, Miyata S, Miura M, Takada T, Tadaki S, et al. Prognostic impact of statin use in patients with heart failure and preserved ejection fraction. Circ J. 2015;79:574-82. [DOI] [PubMed] [Google Scholar]
- 49. Alehagen U, Benson L, Edner M, Dahlstrom U, Lund LH. Association Between Use of Statins and Mortality in Patients With Heart Failure and Ejection Fraction of ≥50. Circ Heart Fail. 2015;8:862-70. [DOI] [PubMed] [Google Scholar]
- 50. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015;373:2314-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2013;309:1268-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387-95. [DOI] [PubMed] [Google Scholar]
- 53. Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH, et al. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol. 2013;62:1330-8. [DOI] [PubMed] [Google Scholar]
- 54. Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115-22. [DOI] [PubMed] [Google Scholar]