Abstract
Background
Previously, we had performed a clinical study using human leukocyte antigen (HLA)-A24-binding peptide vaccines containing a combination of novel cancer-testis antigens and anti-angiogenic peptides derived from DEPDC1, URLC10, FOXM1, KIF20A and VEGFR1 for advanced gastric cancer (AGC) patients who were refractory to chemotherapy. We applied the cocktail vaccine to the combination therapy with S-1 for patients with AGC as a post-operative adjuvant therapy and performed this clinical pilot study.
Methods
AGC patients who had curative surgery and were classified as pathologically stage III were enrolled. At each 6-week treatment cycle, patients received weekly subcutaneous administration of the cocktail vaccine with 5 continuous injections and one break for the first 4 cycles and with bi-weekly injections for the following 4 cycles. S-1 (80 mg/m2) was administered orally for 4 weeks with 2-week rest for all 8 cycles. The primary endpoint was the safety of the combination therapy and the secondary was the relative dose intensity for S-1.
Results
Fourteen patients were enrolled. Six patients with HLA-A*2402 had received S-1 plus the cocktail vaccine as an adjuvant therapy and the remaining 8 had S-1 monotherapy for eight cycles. Five out of 6 patients subjected to the combination group completed the therapy and one patient discontinued because of Grade 3 injection-site reaction. No adverse events of grade 3 or higher were observed except injection-site reactions shown in 5 out of 6 patients who had vaccine therapy. The mean and median relative dose intensities for S-1 were 75.5% and 88% in the combination group and 67% and 80.5% in S-1.
Conclusion
The vaccine therapy combined with S-1 was manageable and safe adjuvant therapy for stage III gastric cancer. Furthermore, the optimal relative dose intensity of S-1 was achieved in combination group, although the injection-site reaction should be considered.
Keywords: adjuvant therapy, gastric cancer, immunotherapy, peptide vaccine, S-1
Gastric cancer is the fifth most common malignancy and the third leading cause of cancer-related death worldwide.1 In Japan, gastric cancer is the third-leading cause of death for both men and women.2 The only curative treatment for gastric cancer is radical gastrectomy with regional lymph node dissection. However, recurrence rates of advanced gastric cancer (AGC) are still high (40–80%)3, 4 and the adjuvant chemotherapy have been proposed even after curative resection to improve patient survival. Meta-analyses have shown that adjuvant chemotherapy is effective and provides a significant survival benefit for patients with advanced gastric cancer.5–10 Postoperative adjuvant chemotherapy with S-1 for stage II or III AGC prolonged overall survival and relapse-free survival with the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC).11 According to the multicenter trial, adjuvant chemotherapy with S-1 for gastric cancer of stage II or III has been considered as a standard treatment in Japan. However, the efficacy of S-1 is still limited, with a 5-year overall survival rate of 67.1% for patients with stage IIIA disease and 50.2% for patients with stage IIIB disease.11 Also, the treatment benefit of S-1 adjuvant chemotherapy seems to be smaller in stage IIIA or stage IIIB disease than in stage II disease comparing to the surgery alone in ACTS-GC study.11 Thus, the treatment for patients with stage III disease needs further improvement. Several studies have been conducted to explore combination chemotherapy as adjuvant chemotherapy for gastric cancer. The CLASSIC study compared surgery alone and surgery plus adjuvant therapy with capecitabine and oxaliplatin in patients who had undergone curative resection of stage II, IIIA, or IIIB gastric cancer with D2 lymph node dissection. The 3-year disease-free survival as the primary endpoint was significantly improved with capecitabine and oxaliplatin therapy.12 In Japan, a feasibility study was conducted to investigate the tolerability of adjuvant therapy with S-1 and cisplatin (SP). In that study, because of toxic effects observed mainly during the first cycle of SP therapy, the treatment completion rate was lower than expected. Therefore, the treatment schedule was revised during the study period to include S-1 monotherapy in the first cycle.13
After identification of tumor associated antigens, such as the melanoma-associated antigen (MAGE) family in 1991, cancer immunotherapy has become a promising approach to fight cancer with minimum toxicity.14, 15 Recently, several clinical trials using peptide vaccine therapy targeting cancer-specific antigen peptides have been performed in the world and suggested improvement in patient survival.16–18 We previously performed a clinical trial using human leukocyte antigen (HLA)-A24-binding peptide vaccines containing a combination of novel cancer-testis antigens and anti-angiogenic peptides for AGC. In the study, 35 AGC patients who had shown resistance to the standard therapy were enrolled and were treated with vaccine therapy targeted for multiple peptides derived from DEPDC1, URLC10, FOXM1, KIF20A and VEGFR1. In conclusion, the peptide cocktail vaccine therapy was found to be safe and is expected to induce specific T cell responses frequently in patients with AGC.19
In this study, we conducted a pilot study to evaluate the feasibility of the peptide cocktail vaccine combined with S-1 in the adjuvant treatment for curatively resected AGC patients.
MATERIALS AND METHODS
Patient eligibility
Patients who had curative gastrectomy with D2 lymphadenectomy and whose pathological stage was classified as Stage IIIA, IIIB, or IIIC were enrolled in this trial at the Department of Surgery, Osaka Medical Center for Cancer and Cardiovascular Diseases. The following were the other main inclusion criteria: i) Eastern Cooperative Oncology (ECOG) performance status of 0–1; ii) age between 20 years and 79 years; iii) adequate bone-marrow, cardiac, pulmonary, hepatic and renal functions including leukocyte count 3,000-10,000/mm3, platelet count > 75,000/mm3, hemoglobin level > 8.0 g/dL, aspartate aminotransferase and alanine aminotransferase < 100 U/L, total bilirubin < 1.5, and creatinine: no more than 1.5 mg/dL; iv) signed informed consent was obtained. The main exclusion criteria were: i) the presence of another serious disease such as uncontrolled diabetes, hepatic disorder, cardiac disease, or hemorrhage/bleeding; ii) pregnant or breast-feeding woman; iii) patients who planned to become pregnant during the study period; iv) symptomatic infectious disease; v) need for concurrent treatment with steroids or immunosuppressive agents; vi) uncontrolled other malignant disease; vii) unhealed wound; viii) intestinal obstruction or interstitial pneumonia; ix) decision of unsuitableness by the principal investigator or physician in charge.
Study design
This study was a pilot study on the combination of S-1 chemotherapy and cancer vaccine therapy as a post-operative adjuvant setting. All enrolled patients were tested HLA-A typing as a screening for enrollment and those with HLA-A*2402 received the combination therapy. Patients with other HLA-A genotypes had a standard chemotherapy with S-1 as a control. The HLA-A*2402 restricted epitope peptide cocktail containing peptides for FOXM1, URLC10, KIF20, DEPDC1 and VFGFR1 each at a dose of 1 mg were prepared in incomplete Freund’s adjuvant (Montanide ISA-51VG, SEPPIC, Paris, France) and injected subcutaneously in the inguinal region of the patients weekly. One treatment cycle consisted of 5 injections on days 1, 8, 15, 22, and 29 with one week rest. The primary endpoint was the safety of this combination therapy containing the peptide cocktail vaccine. The secondary endpoint was the relative dose intensity for S-1 in the combination therapy. Toxicities were assessed by the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE ver. 4.0).
This trial was approved by the Ethics Committees of Osaka Medical Center for Cancer and Cardiovascular Diseases (approval number 1209055041) and carried out in accordance with the Helsinki declaration on experimentation on human subjects.
Peptides
HLA-A*2402-restricted FOXM1-262 (IYTWIEDHF), URLC10-177 (RYCNLEGPPI)20, DEPDC1-294 (EYYELFVNI)21, KIF20A-66 (KVYLRVRPLL)22 and VEGFR1-1084 (SYGVLLWEIF)23 peptides were synthesized by the American Peptide Company (Sunnyvale, CA) according to a standard solid-phase synthesis method and purified by reversed-phase high-performance liquid chromatography (HPLC).19 The purity (> 90%) and identity of the peptides were determined by analytical HPLC and mass spectrometry, respectively.
RESULTS
Patient characteristics
Fourteen patients were enrolled in this trial between October 2012 and Jun 2014. Table 1 shows the patient characteristics at study entry. Six patients with HLA-A*2402 genotype were allocated to the combination therapy of vaccine plus S-1 and the remaining 8 including 1 patient with HLA-A*2402 genotype were to S-1 mono-therapy. The patient with HLA-matched genotype refused to have vaccine therapy and was converted to S-1 monotherapy. They included 11 males and 3 females. All patients were performed with curative surgery including total gastrectomy in 5 patients and partial gastrectomy in 9. All patients were classified as pathologically stage III after surgery.
Table 1.
Patient characteristics enrolled in this trial
| Total | S-1 + vaccine | S-1 | |||
| n = 14 | n = 6 | n = 8 | |||
| Age (years) | 58.5 (40–71) | 61.5 (54–71) | 55 (40–71) | ||
| Gender (Male/Female) | 11/3 | 6/0 | 5/3 | ||
| Performance Status (0/1) | 0/14 | 0/6 | 0/8 | ||
| HLA-A*2402 (+/–) | 7/7 | 6/0 | 1/7 | ||
| Surgery (Total/Partial) | 5/9 | 3/3 | 2/6 | ||
| pStage (IIIA/IIIB/IIIC) | 5/7/2 | 1/3/2 | 4/4/0 | ||
| pT stage (T2/T3/T4a/T4b) | 1/10/2/1 | 0/3/2/1 | 1/7/0/0 | ||
| pN stage(N0/N1/N2/N3a/N3b) | 1/0/4/6/3 | 1/0/1/2/2 | 0/0/3/4/1 | ||
| Number of LN metastasis | 7 (0–32) | 11 (0–23) | 7 (4–32) |
HLA, human leukocyte antigen; LN, lymph node; pN, pathological Nodal stage; pStage, pathological Stage; pT, pathological T stage.
Toxicity
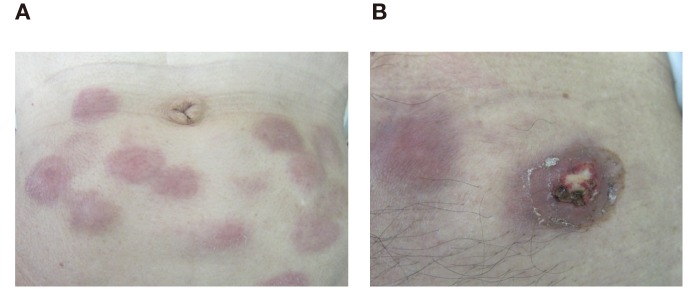
Table 2 lists the toxicity profile recorded during the adjuvant therapy after surgery. The therapy was well-tolerated without any severe adverse events associated with the therapy except for 5 patients with the combination therapy who showed grade 3 injection-site reaction. Representative injection-site reactions are shown in Fig. 1. The Grade 2 skin reaction is shown in Fig. 1A and the Grade 3 skin ulceration is shown in Fig. 1B.
Table 2.
Toxicity profile
| S-1 + vaccine | S-1 | ||||||||
| n = 6 | n = 8 | ||||||||
| Grade | Grade | ||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| Injection-site reaction | 0 | 1 | 5 | / | – | – | – | / | |
| Leukopenia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Neutropenia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Diarrhea | 2 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | |
| Anorexia | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | |
| Fatigue | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Stomatitis | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | |
| Increase in AST/ALT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Increase in creatine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Fever | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Flu-like symptoms | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Fig. 1.
Representative injection-site reactions in the combination therapy group.
A: Grade 1 skin redness was observed. B: Grade 3 skin ulceration was observed.
Feasibility
Table 3 summarized the therapy intensities in both groups. The mean and median number of vaccine injection was 26.5 and 29 in the combination therapy group. One patient discontinued the vaccine therapy after 2 cycles (10 injections) due to Grade 3 skin reaction and discontinued S-1 therapy after 4 cycles due to patient refuse. The relative dose intensity for one-year S-1 was satisfactory in both groups. The mean (median) relative dose intensity for S-1 were 75.5% (88%) in the combination therapy group and 67% (80.5%) in the S-1 monotherapy group. One patient discontinued S-1 due to para-aortic lymph node recurrence within one year and one patient did due to patient refuse in S-1-monotherapy group.
Table 3.
Clinical and immunological outcomes
| S-1 + vaccine | S-1 | |
| n = 6 | n = 8 | |
| Number of vaccine injection | 26.5 (29) | 0 |
| Mean (Median) | ||
| Relative dose intensity for S-1 | 75.5% | 67% |
| Mean (Median) | (88%) | (80.5%) |
DISCUSSION
The prognosis of pathologically stage III patients with curative gastrectomy is not satisfactory even having adjuvant therapy with S-1.11 Therefore, the combination chemotherapy has been developed and several clinical trials have been performed.12, 13, 24 However, toxicities of the combination chemotherapy reduced the completion rate of the therapy and some modifications of the treatment schedule and optimal management of adverse events were needed to increase the completion rate.13, 24 Therefore, less toxic and more effective adjuvant treatment should be developed for such kind of AGC patients.
Previously, we developed a cancer vaccine therapy with multiple peptides specific for AGC and we applied it for AGC patients who had shown resistance to the standard therapy as a monotherapy.19 According to the study, the cocktail vaccine using a combination of multiple peptides (DEPDC1, FOXM1, KIF20, URLC10, and VEGFR1) were well tolerated for AGC patients. Furthermore, specific cytotoxic T lymphocyte (CTL) responses for these five peptide antigens were frequently observed in the peripheral blood of patients and patients who showed the specific CTL responses induction tended to have better prognosis than those with no CTL induction.
In this study, we applied the cocktail vaccine combined with S-1 to the post-operative adjuvant therapy for pathologically stage III AGC patients. This study was a pilot study to examine the safety and feasibility of the combination therapy as a post-operative adjuvant treatment. The combination therapy was safe and feasible in the completion rate of S-1 and vaccine, although the injection-site reaction should be cared during treatment. In our previous study, specific CTL responses against 5 peptides in HLA-matched AGC patients were frequently observed: 55% of patients showed specific response against VEGFR1, 60% against DEPDC1 and KIF20A, 90% against URLC10 and 100% against FOXM1. Injection-site reaction was shown in 26 (67%) out of 24 patients with HLA-A*2402. In this study, 5 (83%) out of 6 patients with HLA-matched genotype showed injection-site reaction. Therefore, although immunological responses against peptides had not been examined in this study, high frequent immunological responses might be induced in patients having adjuvant peptide vaccines even combined with S-1 chemotherapy. Miyazawa et al. conducted phase II clinical trial using the peptide vaccines including KIF20A (OCV-C01) and chemotherapy with gemcitabine for surgically resected pancreatic cancer as post-operative adjuvant treatment.25 The study demonstrated that the combination therapy was tolerable and patients with KIF20A-specific CTL responses had shown significantly better disease-free survival than those without responses. In conclusion, the combination therapy of OCV-C01 and gemcitabine were promising, however, the clinical benefit of the therapy should be demonstrated by a randomized controlled trial. The adjuvant vaccine combined with chemotherapeutic agent might be a promising strategy for surgically resected solid malignancies.
There are several limitations in this study. First, we haven’t performed dose escalation testing for peptide dose used in the combination therapy with S-1 and we have used the same dose of our previous study with the same cocktail vaccine used for AGC as a mono-therapy. The “3+3 design” should have been used for decision of optimal dose based upon the dose limiting toxicity; however, in cancer peptide vaccine therapy, it is difficult to identify the maximum tolerated dose because of the safety profile of vaccine therapy. Second, this study is an early exploratory study and showed no clinical benefit of the combination therapy.
In conclusion, the peptide cocktail vaccine combined with S-1 was tolerable and further clinical studies are essential to demonstrate the clinical benefit of this treatment.
The authors declare no conflict of interest.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86. [DOI] [PubMed] [Google Scholar]
- 2. Ministry of Health, Labor and Welfare. Summary of vital statics 2015 [Internet]. Tokyo: Ministry of Health, Labor and Welfare; [Updated 2015 Mar 30]. Available from: http://www.mhlw.go.jp/english/database/db-hw/populate/index.html. [Google Scholar]
- 3. Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol. 2006;12:3237-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gunderson LL. Gastric cancer-patterns of relapse after surgical resection. Semin Radiat Oncol. 2002;12:150-61. [DOI] [PubMed] [Google Scholar]
- 5. Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomized trials. Eur J Cancer. 1999;35:1059-64. [DOI] [PubMed] [Google Scholar]
- 6. Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, et al. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441-7. [DOI] [PubMed] [Google Scholar]
- 7. Janunger KG, Hafström L, Nygren P, Glimelius B; SBU-group. Swedish Council of Technology Assessment in Health Care, A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001;40:309-26. [DOI] [PubMed] [Google Scholar]
- 8. Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente). Ann Oncol. 2000;11:837-43. [DOI] [PubMed] [Google Scholar]
- 9. Panzini I, Gianni L, Fattori PP, Tassinari D, Imola M, Fabbri P, et al. Adjuvant chemotherapy in gastric cancer: a meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori. 2002;88:21-7. [PubMed] [Google Scholar]
- 10. Pignon JP, Ducreux M, Rougier P. Meta-analysis of adjuvant chemotherapy in gastric cancer: a critical reappraisal. J Clin Oncol. 1994;12:877-8. [DOI] [PubMed] [Google Scholar]
- 11. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-20. [DOI] [PubMed] [Google Scholar]
- 12. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-96. [DOI] [PubMed] [Google Scholar]
- 13. Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, et al. Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol. 2011;67:1423-8. [DOI] [PubMed] [Google Scholar]
- 14. Boon T, De Plaen E, Lurquin C, Van den Eynde B, van der Bruggen P, Traversari C, et al. Identification of tumour rejection antigens recognized by T lymphocytes. Cancer Sur. 1992;13:23-37. [PubMed] [Google Scholar]
- 15. Rosenberg SA, Yang , Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22. [DOI] [PubMed] [Google Scholar]
- 17. Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber JS, O’Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950-6. [DOI] [PubMed] [Google Scholar]
- 19. Fujiwara Y, Okada K, Omori T, Sugimura K, Miyata H, Ohue M, et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int J Oncol. 2017;50:1655-62. [DOI] [PubMed] [Google Scholar]
- 20. Iinuma H, Fukushima R, Inaba T, Tamura J, Inoue T, Ogawa E, et al. Phase I clinical study of multiple epitope peptide vaccine combined with chemoradiation therapy in esophageal cancer patients. J Transl Med. 2014;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Obara W, Ohsawa R, Kanehira M, Takata R, Tsunoda T, Yoshida K, et al. Cancer peptide vaccine therapy developed from oncoantigens identified through genome-wide expression profile analysis for bladder cancer. Jpn J Clin Oncol. 2012;42:591-600. [DOI] [PubMed] [Google Scholar]
- 22. Asahara S, Takeda K, Yamao K, Maguchi H, Yamaue H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J Transl Med. 2013;11:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masuzawa T, Fujiwara Y, Okada K, Nakamura A, Takiguchi S, Nakajima K, et al. Phase I/II study of S-1 plus cisplatin combined with peptide vaccines for human vascular endothelial growth factor receptor 1 and 2 in patients with advanced gastric cancer. Int J Oncol. 2012;41:1297-304. [DOI] [PubMed] [Google Scholar]
- 24. Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, et al. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer. 2017;20:175-81. [DOI] [PubMed] [Google Scholar]
- 25. Miyazawa M, Katsuda M, Maguchi H, Katanuma A, Ishii H, Ozaka M, et al. Phase II clinical trial using novel peptide cocktail vaccine as a postoperative adjuvant treatment for surgically resected pancreatic cancer patients. Int J Cancer. 2017;140:973-82. [DOI] [PubMed] [Google Scholar]